Visual Abstract
Organizing pneumonia (OP) is a known noninfectious pulmonary complication following allogeneic hematopoietic cell transplant (HCT) and represents a significant risk factor for nonrelapse mortality in HCT recipients. Unlike bronchiolitis obliterans syndrome, it is not universally acknowledged as a distinctive pulmonary manifestation of chronic graft-versus-host disease (cGVHD) and, therefore, its diagnostic criteria and management approach are lacking. Given its shared similar clinical features and radiological and histologic findings to OP in the non-HCT population, the diagnostic approach and treatment strategy for OP in HCT recipients is largely adapted from the non-HCT population. In this article, we aim to enhance the understanding of OP within the context of cGVHD following HCT and distinguish its clinical features and treatment strategy from non-HCT counterparts, thereby reinforcing its recognition as a pulmonary manifestation of graft-versus-host disease. We will propose the diagnostic criteria and outline our approach in diagnosis and treatment strategy, highlighting the potential challenges that may arise in each process. Finally, we will discuss knowledge gaps in this field and identify the area of need for future research.
Introduction
Organizing pneumonia (OP) is a form of interstitial lung disease that can arise from a variety of causes, including an idiopathic reaction to medications, collagen vascular disease, aspiration or infection, and common variable immunodeficiency.1 In addition to these causes, OP is also a known noninfectious pulmonary complication following allogeneic hematopoietic cell transplant (HCT). Despite the association of OP with an increased morbidity and mortality after HCT, the understanding of its epidemiology, including its association with chronic graft-versus-host disease (cGVHD), is poorly characterized. Unlike bronchiolitis obliterans syndrome (BOS), OP is not recognized as a distinctive manifestation of cGVHD in National Institutes of Health (NIH) cGHVD guidelines.2,3 Regardless of its underlying cause, histologically, OP is characterized by intraluminal granulation tissue, comprising a mixture of fibroblasts and myofibroblasts that fill the distal respiratory bronchioles, extending to the alveolar ducts and alveolar sacs with surrounding inflammatory cells in the lung interstitium.4-6 Consequently, the diagnostic and treatment approaches of OP in HCT recipients have been adapted from OP that occurs in the non-HCT population. At our institution, patients undergoing HCT evaluation or HCT recipients who develop pulmonary issues are referred to a specialized HCT Chest Clinic. As will be delineated in this review, there are clinical features, disease course, and treatment strategies that distinguish the HCT-OP population from its non-HCT counterparts. These differences warrant further exploration and discussion.
For the readers’ clarity, we will use the term HCT-OP to denote OP that occurs in the setting of graft-versus-host disease (GVHD), as opposed to the commonly used cryptogenic OP (COP) or the previously used bronchiolitis obliterans with OP, which is often used to describe OP without a clear antecedent cause. In this review, we aim to share our knowledge of HCT-OP by summarizing our approach to its diagnosis and treatment, highlighting potential challenges faced in each phase of treatment, and addressing knowledge gaps, thereby identifying areas for future research.
Case 1: clinical presentation and diagnosis of HCT-OP
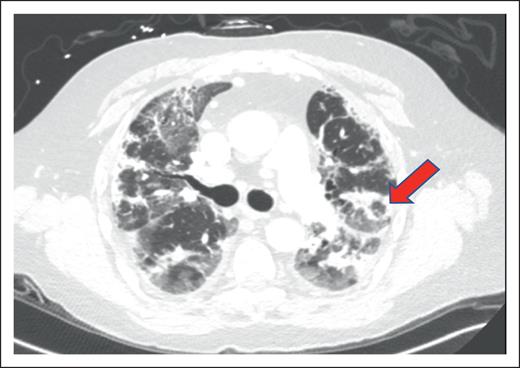
A 73-year-old man with a history of myelodysplastic syndrome, who received a nonmyeloablative allogeneic peripheral blood stem cell transplant (PBSCT), presented with progressive dyspnea, a nonproductive cough, and hypoxemia a year after HCT. Weeks before presentation, the patient was newly diagnosed with cGVHD of the skin. A chest computed tomography (CT) scan showed upper lobe predominant ground-glass opacities in a subpleural and peribronchovascular distribution (Figure 1A). The patient was treated for infectious pneumonia with levofloxacin without improvement. Clinical history did not reveal an antecedent exposure to medications associated with OP. Bronchoscopy with bronchoalveolar lavage (BAL) was performed, and microbiology culture results for bacterial, fungal, and viral pathogens were negative. On the basis of a lack of association with known pulmonary toxic medications or infectious agents, HCT-OP was diagnosed, and prednisone (1 mg/kg per day) was initiated with a slow taper over 4 months. The patient’s clinical symptoms rapidly resolved, including his skin cGVHD, and radiological improvement was achieved (Figure 1B).
Representative coronal images from a noncontrast chest CT scan. (A) CT scan of the chest, demonstrating a right-sided upper lobe–predominant, peripheral ground-glass opacity at the diagnosis of organizing pneumonia. (B) CT images after 4 months of treatment with prednisone, demonstrating resolution of opacities.
Representative coronal images from a noncontrast chest CT scan. (A) CT scan of the chest, demonstrating a right-sided upper lobe–predominant, peripheral ground-glass opacity at the diagnosis of organizing pneumonia. (B) CT images after 4 months of treatment with prednisone, demonstrating resolution of opacities.
Discussion of case 1
This case illustrates the typical presentation of HCT-OP. Typical symptoms of OP include dyspnea, a nonproductive cough, pleuritic chest pain with deep inspiration, and low-grade fevers.7,8 HCT-OP can occur in both adult and pediatric HCT recipients.9-11 HCT-OP often occurs temporally with new onset or an exacerbation of extrapulmonary GVHD, most commonly associated with the skin, gastrointestinal tract, or oral GVHD.5,8,12,13 Infrequently, it can occur while GVHD in other organs is in remission. Most HCT-OP occurs within the first 3 years after HCT, but late occurrences have being described.5,8,14-17 Freudenberger et al described 49 cases of biopsy-proven OP manifesting from a few days to >7 years after HCT.5 Because the symptoms and imaging studies of OP closely resemble those of infectious pneumonia, the diagnosis of HCT-OP is often delayed, potentially contributing to the morbidity and mortality associated with this disease.
Although our understanding of the epidemiology of HCT-OP is limited, in a single case-control study, 49 cases (0.9%) of biopsy-proven OP were found in 5340 patients who received an allogeneic HCT.5 There was a significant association between OP and both acute GVHD (odds ratio, 3.8; 95% confidence interval [CI], 1.2-12.3) and cGVHD (odds ratio, 3.1; 95% CI, 1.1-9.2). Thus, although the pathophysiology for HCT-OP is poorly characterized, the temporal relationship between its occurrence and onset of GVHD is suggestive of similar immune-mediated injury.5,9,12,18-21 Other risk factors for HCT-OP include the stem cell source other than umbilical blood, a myeloablative conditioning regimen, and the use of high-dose irradiation. However, because of the inherent limitations from single-center retrospective studies, these findings have not been consistently observed.12-14,18 Although, at present, there are no defined diagnostic criteria, we propose the following criteria for the diagnosis of HCT-OP:
History of allogeneic HCT.
Presence or history of acute or chronic GVHD in another organ, regardless of disease activity.
Chest CT findings consistent with OP.
A decline in diffusion capacity for carbon monoxide (DLCO) with a restrictive spirometric pattern (Table 1).
Absence of an infectious pathogen in respiratory cultures or absence of clinical or radiological improvement despite broad-spectrum antimicrobial therapy.
Lung biopsy compatible with OP (if available).
The diagnosis of HCT-OP requires a comprehensive clinical evaluation, including exclusion of associated conditions, such as drug-induced pneumonitis. Currently, there are no tests, including pulmonary function testing (PFT), that are specific and capable of distinguishing a drug-induced pneumonitis from OP. Therefore, the diagnosis is established by carefully evaluating the patient’s history for an exposure to a medication known to induce pneumonitis (see www.pneumotox.com). Infectious causes should be vigorously ruled out given the risk of augmenting immunosuppression in the setting of an active infection. Early involvement of a pulmonologist is essential for assisting in the diagnosis and evaluation of pulmonary complications in HCT recipients.22 Flexible bronchoscopy with BAL is a commonly used diagnostic tool in identifying opportunistic infections in the immunocompromised host. Bronchoscopy can be safely performed in the critically ill patient and even those with severe thrombocytopenia.23,24 For a patient who is not suitable for bronchoscopy because of clinical instability, an alternative diagnostic method, such as respiratory culture, respiratory virus polymerase chain reaction (PCR), serum PCR for molds, and other non–culture-based fungal tests (eg, β-D-glucan and Aspergillus galactomannan) should be used. Involvement of an immunocompromised infectious disease specialist to assist with the workup is strongly recommended. We favor the use of both a bronchoscopy study together with noninvasive tests, as both modalities have their own limitations but complement each other when used concurrently.25-27 The yield for bronchoscopy in HCT recipients is often affected by various factors, namely, timing of bronchoscopy, duration of antimicrobial therapy before bronchoscopy, presence of acute GVHD, or neutropenia.28 Noninvasive studies in this scenario can increase diagnostic yield.25 However, there are certain pulmonary complications following HCT where a diagnosis cannot be established by a noninvasive test (eg, diffuse alveolar hemorrhage, infection attributable to other pathogens, such as nontuberculosis mycobacterium, when induced sputum cannot be obtained); in such cases, the benefit of pursuing a bronchoscopy outweighs the potential risk. Lung biopsy is usually deferred because of a high risk of procedural complications observed in the population with cGVHD.3,28-32 We reserve lung biopsy for highly selective cases, for example, instances where (i) the diagnosis remains elusive despite comprehensive assessment; (ii) the patient has not improved clinically despite treatment with corticosteroids; or (iii) malignancy or invasive fungal disease remains high on the differential.
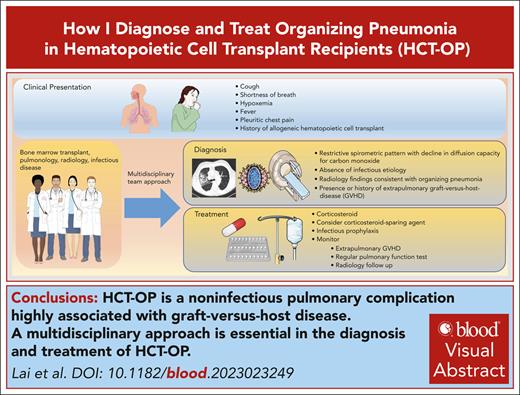
Radiological appearance of HCT-OP can be diverse, ranging from the classic atoll sign (Figure 2) to a combination of nonspecific ground-glass opacities and consolidation in peribronchovascular or subpleural distribution, with or without architectural distortion of the lung, resulting from fibrosis.19 HCT-OP can occur either unilaterally or bilaterally, as a single lesion or with multifocal consolidations without zonal preference.20,33 Rarely, HCT-OP may present as distinct nodular consolidation.34 If the clinical history and radiographic history are highly suggestive of HCT-OP and infectious workup is unrevealing, treatment for HCT-OP should be initiated.
Example of HCT-OP on CT scan of the chest. Noncontrast axial CT image, demonstrating multifocal consolidative and ground-glass pulmonary opacities. The atoll sign (arrowhead) can occasionally be seen and is characterized by central ground-glass opacity surrounded by dense consolidation, representing perilobular involvement, known as the reversed halo sign. Involvement in this case is predominantly peripheral, but HCT-OP can also be peribronchovascular, or occasionally nodular.
Example of HCT-OP on CT scan of the chest. Noncontrast axial CT image, demonstrating multifocal consolidative and ground-glass pulmonary opacities. The atoll sign (arrowhead) can occasionally be seen and is characterized by central ground-glass opacity surrounded by dense consolidation, representing perilobular involvement, known as the reversed halo sign. Involvement in this case is predominantly peripheral, but HCT-OP can also be peribronchovascular, or occasionally nodular.
A restrictive ventilatory pattern and a decline in DLCO are common abnormalities seen on PFT in HCT-OP (Table 1). An obstructive pattern is atypical but has been reported.8,20,35 However, the presence of an obstructive spirometric pattern should prompt the physician to consider BOS, because HCT-OP and BOS are not mutually exclusive diseases, as will be illustrated in a later case in this series.15,17,36 PFTs are used to support the diagnosis of HCT-OP and serve as an important tool in disease surveillance, monitoring treatment efficacy and relapse. However, the results of PFTs may also be impacted by other concomitant lung diseases associated with cGVHD, such as BOS, extrapulmonary restriction attributable to sclerotic skin disease of the torso (known as truncal sclerosis), and infectious pneumonia. For this reason, it is essential that a multidisciplinary approach be undertaken in the management of HCT-OP, including the transplant physician, pulmonologist, infectious diseases specialist, and thoracic radiologist. The utility of PFTs, and their limitations in diagnosis and follow-up, will be discussed in a later section.
Case 2: comparison of HCT-OP vs non–HCT-OP
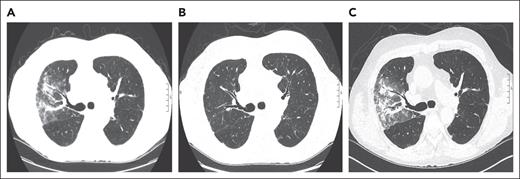
A 70-year-old man with a history of myelofibrosis received a myeloablative allogeneic PBSCT. Three years after HCT, he developed liver cGVHD that was treated with prednisone (80 mg/d or 1 mg/kg per day) and mycophenolate mofetil (1 g twice daily). His liver cGVHD stabilized, and prednisone was tapered to 8 mg/d and mycophenolate mofetil was discontinued over the course of 8 months. Two months later, he started experiencing fever and cough. A CT scan of the chest showed diffuse ground-glass opacities with nodules in the right upper lobe (Figure 3A). Infectious workup, including BAL, was unrevealing, and a diagnosis of HCT-OP was established. His respiratory symptoms and radiographic abnormalities resolved after his prednisone was escalated back to 1 mg/kg and mycophenolate mofetil was reinitiated as a corticosteroid-sparing agent (Figure 3B).
Representative serial axial images of a noncontrast CT scan of the chest for case 2. (A) Axial CT image, showing peribronchovascular consolidations and ground-glass opacities in the right upper lobe. (B) Image showing a complete resolution of radiographic abnormalities after escalating prednisone to 1 mg/kg per day and reinitiation of mycophenolate mofetil. (C) Image showing reappearance of ground-glass opacities with peribronchovascular consolidations in the right upper lobe when the prednisone was tapered to 10 mg/d and positive cytomegalovirus PCR in BAL. Notably, lung findings appear similar to panel A, when his HCT-OP was initially diagnosed.
Representative serial axial images of a noncontrast CT scan of the chest for case 2. (A) Axial CT image, showing peribronchovascular consolidations and ground-glass opacities in the right upper lobe. (B) Image showing a complete resolution of radiographic abnormalities after escalating prednisone to 1 mg/kg per day and reinitiation of mycophenolate mofetil. (C) Image showing reappearance of ground-glass opacities with peribronchovascular consolidations in the right upper lobe when the prednisone was tapered to 10 mg/d and positive cytomegalovirus PCR in BAL. Notably, lung findings appear similar to panel A, when his HCT-OP was initially diagnosed.
The patient remained stable until prednisone was tapered to 10 mg over a period of 4 months, at which point he again developed fever and cough. A CT scan of the chest showed patchy consolidation and ground glass in the right upper lobe, with similar geographic distribution to his previous HCT-OP (Figure 3C). BAL was performed, which was positive for cytomegalovirus (CMV) by PCR, but serum CMV PCR was negative. Lung biopsy was not pursued for a definitive diagnosis of CMV pneumonia given the procedural risk. After a multidisciplinary discussion, ganciclovir was started, and the patient’s symptoms improved while his prednisone remained at 10 mg. Follow-up CT scan of the chest also showed resolution of right upper lobe abnormalities (image not shown).
Discussion of case 2
This case illustrates that HCT-OP can occur not only during new onset of GVHD, but also while tapering immunosuppression used for extrapulmonary cGVHD. Freudenberger et al observed that 22% of biopsy-proven OP cases were associated with corticosteroid tapering.5 As such, PFT should be obtained at the onset of cGVHD, followed by serial PFTs for monitoring, as changes could be an early sign of pulmonary complications (ie, BOS or HCT-OP) during an immunosuppression taper, while the patient remains asymptomatic.37-39 We recommend that PFTs should be performed every 3 to 6 months in the first 1 to 2 years after HCT in an attempt to improve surveillance, particularly in those with risk factors for lung cGVHD, such as those with extrapulmonary cGVHD or an antecedent respiratory tract viral infection. This recommendation is in accordance with NIH guidelines and the NIH 2020 Consensus Development Project on Criteria for Clinical Trials in cGHVD, focusing on early recognition of disease.3,40,41 Unfortunately, adherence to these recommendations is suboptimal as the rate of performing regular PFTs after HCT is low (<40% of HCT recipients had >1 PFT in each year after transplant), and this frequency substantially decreases by the time after transplant.42,43 This may be attributable, in part, to a combination of insufficient physician awareness, patient comorbidities, access difficulties, or the logistical impediment to arranging PFTs. To address these challenges, an increasing number of studies have demonstrated the efficacy of home-based spirometry.38,44 These devices provide acceptable accuracy, allow for telemetric monitoring, and are an attractive solution for fostering early disease detection and intervention, with the potential to improve patient outcomes.38,44,45
Relapse of HCT-OP is common, occurring in 30% to 50% of cases, and may be associated with tapering of immunosuppression, at the onset of new manifestations or during an exacerbation of cGVHD.5,14,46 Our clinical observations, which have been supported by other studies, indicate the risk of HCT-OP relapse is more likely to occur in a person with a history of GVHD.14
The HCT recipient can also develop OP from causes unrelated to GVHD (Table 2). In such instances, evaluation should incorporate a comprehensive diagnostic workup to identify the suspected cause. Respiratory tract viral infections are widely recognized as a cause of OP (Table 2). In these situations, as illustrated in this case, treatment of the underlying viral infection alone may resolve the OP.47,48 If corticosteroids are required to treat virus-induced OP, using available antiviral agents often allows for a more rapid corticosteroid taper, in contrast to the slower tapering approach that we have advised for HCT-OP.
Potential causes of OP in HCT recipients that are unrelated to GVHD
| Causes . | Example . |
|---|---|
| Infection | |
| Bacterial | Chlamydia pneumoniae, Legionella pneumoniae, Mycoplasma pneumoniae |
| Fungi | Aspergillus, Cryptococcus neoformans, Pneumocystis jirovecii |
| Protozoa | Plasmodium vivax, Dirofilaria immitis |
| Viral∗ | Adenovirus,46 cytomegalovirus,47-49 human herpesvirus 6,50 parainfluenza virus,46,47,51 rhinovirus,46 respiratory syncytial virus5 |
| Inhalation injury | |
| Aspiration | Aspiration of gastric content |
| Toxic chemical or substance | Electronic nicotine delivery systems with adulterated products |
| Drug toxicity† | |
| Antimicrobial | Amphotericin, cephalosporins, nitrofurantoin |
| Antiepileptics | Carbamazepine, phenytoin |
| Cardiovascular drug | Amiodarone, β-adrenergic blockers |
| Immunotherapy | Bleomycin, cyclophosphamide, methotrexate, trastuzumab |
| Autoimmune diseases | |
| Connective tissue disease | Rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus |
| Inflammatory bowel disease | Ulcerative colitis, Crohn disease |
| Thyroiditis | |
| Vasculitis | Granulomatosis with polyangiitis |
| Malignancy | |
| Hematological | Diffuse large B-cell lymphoma, leukemia, non-Hodgkin lymphoma |
| Lung cancer | Squamous cell carcinoma, adenocarcinoma |
| Miscellaneous | Chest radiation, lung infarct |
| Causes . | Example . |
|---|---|
| Infection | |
| Bacterial | Chlamydia pneumoniae, Legionella pneumoniae, Mycoplasma pneumoniae |
| Fungi | Aspergillus, Cryptococcus neoformans, Pneumocystis jirovecii |
| Protozoa | Plasmodium vivax, Dirofilaria immitis |
| Viral∗ | Adenovirus,46 cytomegalovirus,47-49 human herpesvirus 6,50 parainfluenza virus,46,47,51 rhinovirus,46 respiratory syncytial virus5 |
| Inhalation injury | |
| Aspiration | Aspiration of gastric content |
| Toxic chemical or substance | Electronic nicotine delivery systems with adulterated products |
| Drug toxicity† | |
| Antimicrobial | Amphotericin, cephalosporins, nitrofurantoin |
| Antiepileptics | Carbamazepine, phenytoin |
| Cardiovascular drug | Amiodarone, β-adrenergic blockers |
| Immunotherapy | Bleomycin, cyclophosphamide, methotrexate, trastuzumab |
| Autoimmune diseases | |
| Connective tissue disease | Rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus |
| Inflammatory bowel disease | Ulcerative colitis, Crohn disease |
| Thyroiditis | |
| Vasculitis | Granulomatosis with polyangiitis |
| Malignancy | |
| Hematological | Diffuse large B-cell lymphoma, leukemia, non-Hodgkin lymphoma |
| Lung cancer | Squamous cell carcinoma, adenocarcinoma |
| Miscellaneous | Chest radiation, lung infarct |
List of virus-induced OPs in HCT populations reported in literature.
Refer to www.pneumotox.com for a comprehensive list.
Interestingly, we have not observed migratory pulmonary infiltrates, which are often described as a feature of COP.1,52,53 Instead, as shown in this case, recurrence occurs in a radiographic distribution akin to the index episode (Figure 3A,C). We have observed this to be the case even when the recurrence of HCT-OP is separated by years from the index occurrence. The exact mechanism is unknown but perhaps is similar to recall radiation pneumonitis.
In contrast to COP, where overall survival is generally favorable and death attributable to respiratory failures is uncommon, a retrospective study done by Nakasone et al found a high rate of respiratory failure (25%) accounted for nonrelapse mortality.12 Similarly, several studies have shown that respiratory failure is the main cause of death in patients with HCT-OP.8,15,54 Nonetheless, most patients treated with corticosteroids have significant clinical and radiographic improvement, which is consistent with our center’s experience. The overall survival rates for HCT-OP at 5 years (70.8% [95% CI, 60%-83.5%]) are comparable to those of BOS (73.8% [95% CI, 66.6%-81.8%]).15 Spontaneous resolution of OP has been reported in COP, but this is rarely seen in HCT-OP.55,56 Other clinical differences between non–HCT-OP and HCT-OP are summarized in Table 3.
Comparison between non–HCT-OP and HCT-OP
| Non–HCT-OP . | HCT-OP . |
|---|---|
| Patient population | |
| Rarely reported in children | Any allogeneic HCT recipient |
| Can be cryptogenic or associated with other processes (infection, drug toxicity, or radiation) | Isolated occurrence without other GVHD in other organs is rare Associated with GVHD of other organs, especially mouth and skin |
| Diagnosis | |
| Lung biopsy is pursued to confirm diagnosis or rule out secondary causes | Lung biopsy is reserved for selective cases given the high risk of postprocedural complications |
| Lower lung zone preference | No zonal preference |
| Recurrence can present with migratory opacities or different radiographic features as to index event | Recurrence tends to occur at the same location with similar radiographic features as to index event |
| Treatment | |
| Spontaneous resolution can be observed | Spontaneous resolution is rare |
| Extended period of corticosteroid for 6-12 mo may be necessary | Extended corticosteroid course >6-12 mo is often required |
| Prognosis | |
| Relapse rate of 13%-58%7 | Relapse rate of 30%-50% |
| Mortality is <10%; death often unrelated to OP | Higher death rate from respiratory failure12 |
| Excellent prognosis, with 5-y survival >90%1 | Prognosis is less favorable20 |
| Corticosteroid-sparing agents∗ | |
| Mycophenolate mofetil, azithromycin, cyclosporine, rituximab | Mycophenolate mofetil, ruxolitinib, cyclosporine |
| Non–HCT-OP . | HCT-OP . |
|---|---|
| Patient population | |
| Rarely reported in children | Any allogeneic HCT recipient |
| Can be cryptogenic or associated with other processes (infection, drug toxicity, or radiation) | Isolated occurrence without other GVHD in other organs is rare Associated with GVHD of other organs, especially mouth and skin |
| Diagnosis | |
| Lung biopsy is pursued to confirm diagnosis or rule out secondary causes | Lung biopsy is reserved for selective cases given the high risk of postprocedural complications |
| Lower lung zone preference | No zonal preference |
| Recurrence can present with migratory opacities or different radiographic features as to index event | Recurrence tends to occur at the same location with similar radiographic features as to index event |
| Treatment | |
| Spontaneous resolution can be observed | Spontaneous resolution is rare |
| Extended period of corticosteroid for 6-12 mo may be necessary | Extended corticosteroid course >6-12 mo is often required |
| Prognosis | |
| Relapse rate of 13%-58%7 | Relapse rate of 30%-50% |
| Mortality is <10%; death often unrelated to OP | Higher death rate from respiratory failure12 |
| Excellent prognosis, with 5-y survival >90%1 | Prognosis is less favorable20 |
| Corticosteroid-sparing agents∗ | |
| Mycophenolate mofetil, azithromycin, cyclosporine, rituximab | Mycophenolate mofetil, ruxolitinib, cyclosporine |
Refer to Table 4 for detail.
Case 3: treatment of HCT-OP
A 67-year-old man with a history of myelodysplastic syndrome received a nonmyeloablative allogeneic HCT and had a posttransplant course complicated by oral cGHVD. At the onset of his oral cGHVD, he complained of a nonproductive cough and right-sided pleuritic chest pain. A chest CT scan showed ground-glass opacities, predominantly in the right lower lobe. Infectious workup, including BAL, was unrevealing, except for a BAL cell count showing a lymphocytic predominance (35%). Subsequently, the diagnosis of HCT-OP was established. The patient was started on prednisone of 40 mg (0.5 mg/kg) daily and had symptomatic and radiographic improvement. His prednisone was tapered at a rate of 5 mg every 2 weeks, guided by monthly clinic visits and PFTs (Figure 4). He was weaned off prednisone and maintained respiratory stability. Two months later, his cough and pleuritic chest pain recurred. Radiographic features and an unrevealing infectious workup favored the diagnosis of relapsed HCT-OP. Prednisone (40 mg/d) was restarted, with clinical and radiological improvement. After a discussion with his transplant physician, ibrutinib (140 mg/d) was initiated as a corticosteroid-sparing agent. The patient was placed on infectious prophylaxis, including acyclovir, posaconazole, and sulfamethoxazole/trimethoprim. Prednisone was tapered at an average rate of 5 mg every 2 weeks, and he was successfully weaned off prednisone, without recurrence of HCT-OP.
Graph of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and DLCO (percentage) in relation to immunosuppressive therapy (prednisone and ibrutinib [mg]) and HCT-OP relapse for case 3.
Graph of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and DLCO (percentage) in relation to immunosuppressive therapy (prednisone and ibrutinib [mg]) and HCT-OP relapse for case 3.
Discussion of case 3
Unlike COP, where spontaneous resolution can be observed, immunosuppression is often needed in HCT-OP.1,12,53,56,57 Systemic corticosteroids remain the primary treatment at the time of diagnosis. Typically, we start prednisone at 1 mg/kg per day. For a patient who is not hypoxemic, prednisone at 0.5 mg/kg per day may be sufficient. However, for a critically ill patient who requires mechanical ventilatory support or is severely hypoxemic and needing intensive care unit (ICU) admission, we recommend methylprednisolone pulse at 1 to 2 mg/kg per day, with tapering at the physician’s discretion, depending on clinical stability, until the patient can be tapered to prednisone of 1 mg/kg per day.
Response to corticosteroids should be apparent after 1 to 2 weeks of initiation. Once clinical improvement is observed, we try to taper the prednisone dose by 10 mg every week, until a dose of 50 to 60 mg/d is achieved. A repeat CT scan of the chest is performed to ensure radiographic improvement and to monitor for possible opportunistic infections related to immunosuppression. Thereafter, we typically will taper at rate of 5 mg every 2 weeks. Corticosteroid-sparing agents can be introduced at this stage, if necessary, particularly if there is evidence of a flare of HCT-OP during the corticosteroid taper. Figure 5 summarizes a general approach of corticosteroid initiation, tapering, and the approach to corticosteroid refractory HCT-OP.
Approach to treatment for HCT-OP. BMT, bone marrow transplant; ICID, immunocompromised infectious disease.
Approach to treatment for HCT-OP. BMT, bone marrow transplant; ICID, immunocompromised infectious disease.
Throughout the treatment phase, complications, such as an exacerbation of extrapulmonary cGVHD, infection, and complications of chronic corticosteroid use, should be routinely monitored. Appropriate infectious prophylaxis is paramount, as opportunistic infections are the most commonly observed complication. Therefore, in the early phase of HCT-OP, we typically monitor patients monthly, using PFT, oxygen saturation measurements, and exercise capacity. During the taper, we maintain a low threshold to perform follow-up imaging to guide adjustment of the corticosteroid dose. Once a prednisone dose of 20 mg/d is achieved, we favor a slower taper, as we have observed an increased risk of HCT-OP relapse below this dose. Conversely, if significant corticosteroid-induced adverse effects are observed, the taper may occur more rapidly or corticosteroid-sparing agents may be introduced earlier in the treatment course.
We typically do not use corticosteroid-sparing agents at the first occurrence of HCT-OP. We reserve their use if the disease relapses, as illustrated in this case. However, if the clinical situation warrants a more rapid corticosteroid taper, attributable to corticosteroid intolerance (eg, uncontrolled diabetes, osteoporosis, vertebral fractures, or myopathy), a corticosteroid-sparing agent can be considered at the index episode of HCT-OP. At present, there are no evidence-based standards for the use of medications as corticosteroid-sparing agents in HCT-OP. Because there are no consensus guidelines or research on the optimal treatment approach, there is wide variability in clinical practice. A prospective, open-label trial by Yanik et al indicated that the simultaneous administration of corticosteroids and etanercept led to improvement in forced vital capacity (FVC) by >10% in 33% of patients with restrictive lung disease. Additionally, the 5-year estimated survival was 44%, with etanercept being well tolerated and not associated with an increased risk of infections.58 Data on other agents used in HCT-OP are limited to case reports and series (Table 4). When there is a lack of specific HCT-OP data, we have provided references for medications used in OP occurring in the non-HCT population. As corticosteroid-sparing agents can have complex drug-drug interactions, a substantial impact on immunosuppression profile, and variable efficacy on nonpulmonary cGVHD, agent selection should be made in collaboration with a transplant physician, a pulmonologist, and an infectious disease specialist.
Corticosteroid-sparing agents used in HCT-OP and non–HCT-OP with related conditions
| Agent class . | Medications used in HCT-OP∗ . | Other medications used in non–HCT-OP . | Non–HCT-OP conditions . |
|---|---|---|---|
| Antibiotic | Erythromycin16 Macrolide15 | Erythromycin59 Clarithromycin60 Azithromycin61 | COP |
| Antiproliferative | MMF62 | Azathioprine63 MMF63 Cyclophosphamide63,64 Methotrexate65 | COP, IgG4-related disease, CTD |
| Bruton tyrosine kinase inhibitor | Ibrutinib†,66 | ||
| Calcineurin inhibitor | Cyclosporine20,62,67 | Cyclosporine63 Tacrolimus68 | COP, CTD |
| Cytokine-directed therapy‡ | Etanercept§,58 Tocilizumab69,70 | Etanercept71 Infliximab72 Rituximab73,74 Tocilizumab75 IVIG76 | RA, immune check point inhibitor, COP, Sjogren disease |
| JAK1/2 inhibitor | Ruxolitinib†,46,66 | ||
| Other | ECP46,77 LABA/inhaled corticosteroid15 | Inhaled corticosteroid78 | Hodgkin lymphoma |
| Agent class . | Medications used in HCT-OP∗ . | Other medications used in non–HCT-OP . | Non–HCT-OP conditions . |
|---|---|---|---|
| Antibiotic | Erythromycin16 Macrolide15 | Erythromycin59 Clarithromycin60 Azithromycin61 | COP |
| Antiproliferative | MMF62 | Azathioprine63 MMF63 Cyclophosphamide63,64 Methotrexate65 | COP, IgG4-related disease, CTD |
| Bruton tyrosine kinase inhibitor | Ibrutinib†,66 | ||
| Calcineurin inhibitor | Cyclosporine20,62,67 | Cyclosporine63 Tacrolimus68 | COP, CTD |
| Cytokine-directed therapy‡ | Etanercept§,58 Tocilizumab69,70 | Etanercept71 Infliximab72 Rituximab73,74 Tocilizumab75 IVIG76 | RA, immune check point inhibitor, COP, Sjogren disease |
| JAK1/2 inhibitor | Ruxolitinib†,46,66 | ||
| Other | ECP46,77 LABA/inhaled corticosteroid15 | Inhaled corticosteroid78 | Hodgkin lymphoma |
CTD, connective tissue disease; ECP, extracorporeal photopheresis; IgG4, immunoglobulin G4; IVIG, intravenous immunoglobulin; JAK, Janus kinase; LABA, long-acting β agonist; MMF, mycophenolate mofetil; RA, rheumatoid arthritis.
These agents (except etanercept) were historically used, as documented in case reports and limited case series.
Agents approved by the US Food and Drug Administration for corticosteroid-refractory GVHD.
Consider checking serum cytokine level (sTNFRI [soluble tumor necrosis factor receptor type I], sTNFRII, interleukin 6 [IL-6], IL-8, and transforming growth factor-β).
Agent studied in a prospective clinical trial.
Case 4: complication during treatment of HCT-OP and concomitant BOS
A 57-year-old woman with relapsed Hodgkin lymphoma received a nonmyeloablative allogeneic PBSCT. Two years later, she developed cGVHD of the eyes and liver. Her immunosuppressant regimen included ruxolitinib, 10 mg/d, extracorporeal photopheresis, and prednisone, 10 mg/d. She was referred for subacute onset of dyspnea and found to have bilateral, predominantly basilar ground-glass opacities (Figure 6B). PFTs showed a decline in DLCO (Figure 6A, time 0). Infectious workup, including BAL, was unrevealing. The patient was started on 1 mg/kg of prednisone for HCT-OP, with subjective improvement in respiratory status. Four weeks later, she was hospitalized in the ICU with Klebsiella bacteremia and CMV viremia. She was treated with antimicrobial therapy and discharged home. Her prednisone dose was tapered from 40 to 15 mg/d within 2 weeks under close monitoring. During the corticosteroid taper, radiographic worsening was observed, indicating a flare of her HCT-OP (Figure 6C). However, escalation of immunosuppression was not pursued given her clinical stability and recent ICU admission. Shortly thereafter, she started to complain of worsening dyspnea and cough. There were no interval radiographic changes (Figure 6D), but her PFTs showed a decline in the forced expiratory volume in 1 second (FEV1) and the FEV1/FVC ratio to 0.76, concerning for worsening obstructive lung disease. PFTs did not meet the NIH criteria for BOS as the FEV1/FVC ratio remained >0.7. A chest CT scan with postprocessing lung density–based parametric response mapping (PRM) showed unchanged HCT-OP findings, but revealed significant air trapping (ie, functional low-density area of 32%) (Figure 7), consistent with the diagnosis of BOS. Following the use of fluticasone propionate/salmeterol and montelukast, the patient noted improvement in dyspnea, and had an improved PFT trend (Figure 6A).
Representative of PFT trend in relation to clinical course and axial images of noncontrast CT scan of the chest for case 4. (A) PFT trend (FVC, forced expiratory volume in 1 second [FEV1], and DLCO) during clinical course. (B) Axial CT image, showing basilar predominant ground-glass opacities with peripheral and peribronchovascular distribution, representing HCT-OP. (C) CT scan, showing worsened subpleural ground-glass opacities while immunosuppression was rapidly tapered during reactivation of CMV viremia. Escalation of prednisone was not pursued given clinical stability and ICU admission for CMV viremia and Klebsiella bacteremia. (D) CT image, showing similar subpleural ground-glass opacities, while FEV1 continued to decline and patient experienced worsening dyspnea on exertion.
Representative of PFT trend in relation to clinical course and axial images of noncontrast CT scan of the chest for case 4. (A) PFT trend (FVC, forced expiratory volume in 1 second [FEV1], and DLCO) during clinical course. (B) Axial CT image, showing basilar predominant ground-glass opacities with peripheral and peribronchovascular distribution, representing HCT-OP. (C) CT scan, showing worsened subpleural ground-glass opacities while immunosuppression was rapidly tapered during reactivation of CMV viremia. Escalation of prednisone was not pursued given clinical stability and ICU admission for CMV viremia and Klebsiella bacteremia. (D) CT image, showing similar subpleural ground-glass opacities, while FEV1 continued to decline and patient experienced worsening dyspnea on exertion.
Representative of parametric response mapping analysis for case 4. Dual-phase chest CT scanned at both end inspiration (total lung capacity) and end expiration (residual volume) and analyzed using parametric response mapping, showing 32% functional low-density area representing air trapping (yellow). This was taken at time point 3 on PFT trend (Figure 6A).
Representative of parametric response mapping analysis for case 4. Dual-phase chest CT scanned at both end inspiration (total lung capacity) and end expiration (residual volume) and analyzed using parametric response mapping, showing 32% functional low-density area representing air trapping (yellow). This was taken at time point 3 on PFT trend (Figure 6A).
Discussion of case 4
This case underscores the complexity in management of HCT-OP and clinical issues that may arise during treatment, including the following: (i) a relapse of HCT-OP during corticosteroid taper; (ii) a concern for concomitant infection; and (iii) the occurrence of BOS. The complexity of HCT patients warrants a determination of risk and benefit with the introduction of more intensive immunosuppression, including infection and relapse of malignancy. It is essential to involve the transplant team on initiation of immunosuppression to ensure appropriate infectious prophylaxis and that drug interactions have been thoroughly considered. As illustrated in this case, although increasing corticosteroids initially improved HCT-OP, it also heightened the patient’s risk for infections, resulting in adverse outcomes and clinical setbacks. Rapid corticosteroid tapering may appear counterintuitive given ongoing HCT-OP, but the patient ultimately attained a meaningful recovery with a modified treatment regimen guided by a multidisciplinary discussion.
A restrictive ventilatory pattern and a reduced DLCO are the hallmark PFT findings for HCT-OP. This contrasts with BOS, which is characterized by a reduction in FEV1 and an obstructive spirometric pattern. These 2 distinct patterns are helpful in phenotyping disease and guiding workup and management. However, apart from BOS and HCT-OP, HCT patients are susceptible to other pulmonary complications, such as drug-induced pneumonitis and truncal sclerosis. These disease processes are not mutually exclusive. The sum of these disease entities could contribute to an isolated decline of one PFT metric or a combined decline in FEV1, FVC, and DLCO, which poses challenges in accurate phenotyping. Radiologic features of BOS, such as small airway thickening and air trapping by expiratory CT, may not be apparent with concomitant HCT-OP and rely on the experience of the interpreter. Acknowledging these limitations, studies have shown the value of dynamic chest CT scans analyzed using PRM, which can assess the change of lung attenuation through voxel-by-voxel comparison between inspiration and expiration. This permits qualitative and quantitative analysis of obstructive and restrictive diseases of the lung parenchyma. Algorithms have been developed to quantify respiratory changes into normal lung, air trapping, and parenchymal consolidation. We have shown that quantitative CT imaging through PRM not only improves the sensitivity and the specificity for the diagnosis BOS but also aids in differentiating late pulmonary complications after HCT.79,80Table 1 summarizes the PFT patterns and PRM findings in different noninfectious pulmonary complications after HCT.
Conclusion
Although there has been recent improvement in understanding and management for BOS, a comparable advance in HCT-OP is hindered by the absence of uniform definition, diagnostic criteria, and its generalization with the presentation and clinical course of non–HCT-OP. This lack of clarity results in a limited understanding of its epidemiology, including a variability in reported incidence and prevalence, and hinders a clear understanding of its risk factors. The strategies in the approach and treatment for HCT-OP we have outlined are derived from a single-center experience and, thus, may not be broadly applicable given variations in patient profiles and transplant practices. This highlights the need for a large multicenter study to ascertain the feasibility and efficacy of our proposed methods. Nevertheless, our case series stands as an initial effort to provide a structured care model for HCT-OP. The distinctive features of HCT-OP and complexity of multiorgan involvement in patients with GVHD necessitate a multidisciplinary collaboration among pulmonary, chest radiology, transplant, and infectious disease clinicians that allows for appropriate disease detection, careful selection of immune suppressive agents, monitoring, and evaluation of other HCT- or treatment-related comorbidities. Further research is needed in each of these domains to improve outcomes for this highly morbid disease.
Acknowledgments
The authors thank Guang-Shing Cheng and Ajay Sheshadri for their careful read of the manuscript and H. Henry Guo for providing radiology images.
J.L.H. is funded by National Institutes of Health, National Heart, Lung, and Blood Institute grant R01HL157414-01.
Authorship
Contribution: Y.K.L. was responsible for conducting the literature review, case preparation, and drafting the initial manuscript; and H.S. and J.L.H. provided critical input and revision and finalized the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joe L. Hsu, Division of Pulmonary, Allergy and Critical Care Medicine, Stanford University School of Medicine, 1701 Page Mill Rd, Room 146, Palo Alto, CA 94305-5236; email: joehsu@stanford.edu.



![Graph of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and DLCO (percentage) in relation to immunosuppressive therapy (prednisone and ibrutinib [mg]) and HCT-OP relapse for case 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/10/10.1182_blood.2023023249/2/m_blood_bld-2023-023249-c-gr4.jpeg?Expires=1765883499&Signature=1i8ksfTSdkfCsACdQDHl6Ocyt4FaCVZd94~dS3Su270p5eEQdieET74j~AaVr0FbSjoCpKrYkVAUVA-hCMQmWX9l71rxW1-7x6Y~Zhb1oEREFNS-CVp2EBuo8zDC3tZ~XEpPsuybR7~-pKMTnywcTZ-r2rT0nc-NO8WFlG4TujXIUBT-TSXKIrox184mnZ9D3PVhZArCYLyt907ulabMe9y0q5s00kyrO8rhOkJ4tU7Ff4aFueXIdq0~t5RAdfvtdt7IZyqFu1TfIH~2ROS7aA7gFCI-~jAvqXwfVWkJRcHO1B61QHC9y7M59nWgY5noyJzmADKvo9AsHRHsXJNUlg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Representative of PFT trend in relation to clinical course and axial images of noncontrast CT scan of the chest for case 4. (A) PFT trend (FVC, forced expiratory volume in 1 second [FEV1], and DLCO) during clinical course. (B) Axial CT image, showing basilar predominant ground-glass opacities with peripheral and peribronchovascular distribution, representing HCT-OP. (C) CT scan, showing worsened subpleural ground-glass opacities while immunosuppression was rapidly tapered during reactivation of CMV viremia. Escalation of prednisone was not pursued given clinical stability and ICU admission for CMV viremia and Klebsiella bacteremia. (D) CT image, showing similar subpleural ground-glass opacities, while FEV1 continued to decline and patient experienced worsening dyspnea on exertion.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/10/10.1182_blood.2023023249/2/m_blood_bld-2023-023249-c-gr6.jpeg?Expires=1765883499&Signature=JsuilKkWG4CJWkBpDzDicLEcgGpukhsmtoPfE7a6h4D1niFBKXwYofAGW31JV8RJLk4qrNa8qBtPeEAphLH~miuSsqHlSjE7ATLcpUC1DLxFX5Cc2srTD9yCjOtqBnRScv7vkSuDjtJzqDGu1zLL9SJEP-9N5iJxgwk8an5nSOFZ1LR3zSvycC81RmNR6CwBncIeAAA0onTL9TqWF21uLQ1hIUj4HeaoPQfWOmpq3iFu-dTXDGH4qF3eKQQRnFH-bPh06Yd1tHTWzN4zdgbH4KNVr1xV4T9qHIC0KZ9~Hs3ro-7jKDrcs5vApn3A6iKbckik05Ln9vJ~uC9vpMbMSA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments
Reply to: How I diagnose and treat organizing pneumonia in hematopoietic cell transplant recipients
Pulmonary complications after HCT persist in up to 30–60% of HCT recipients despite improvements in supportive care and remain fatal. Among non-infectious complications, idiopathic pneumonia syndrome (IPS), diffuse alveolar haemorrhage (DAH), peri-engraftment respiratory distress syndrome, nonspecific interstitial pneumonia (NSIP), lymphoid interstitial pneumonia (LIP), and OP2 can occur. These conditions can inherently exhibit clinical features like both infectious and other non-infectious conditions, often requiring a lung biopsy for definitive diagnosis3, so doing to avoid improper therapy.
We believe it is important to emphasize that, particularly in atypical presentation cases where the clinical, radiological findings do not allow for a highly confident diagnosis of OP, lung biopsy should be included in the diagnostic workup. In fact, in these specific cases, prolonged corticosteroid therapy or immunosuppression therapy may be more harmful for the patient than undergoing a lung biopsy.
Transbronchial lung cryobiopsy (TBLC) has recently been recognized as a valid and less invasive alternative to SLB for diagnosing interstitial lung diseases (ILDs). TBLC allows for the collection of larger and higher-quality lung tissue samples without the crush artifacts typically associated with conventional transbronchial lung biopsy performed with flexible forceps4.
Moreover, recent randomized clinical trials have demonstrated an acceptable safety profile for TBLC when performed even in intensive care unit settings, both in terms of haemorrhage and pneumothorax, particularly when conducted by experienced pulmonologists5.
References
1. Cherian SV, Patel D, Machnicki S, et al. Algorithmic Approach to the Diagnosis of Organizing Pneumonia: A Correlation of Clinical, Radiologic, and Pathologic Features. Chest. 2022 Jul;162(1):156-178. doi: 10.1016/j.chest.2021.12.659.
2. Bondeelle L, Bergeron A. Managing pulmonary complications in allogeneic hematopoietic stem cell transplantation. Expert Rev Respir Med. 2019;13(1):105-119. doi:10.1080/17476348.2019.1557049
3. Patel SS, Ahn KW, Khanal M, et al. Noninfectious Pulmonary Toxicity after Allogeneic Hematopoietic Cell Transplantation. Transplantation and Cellular Therapy, Official Publication of the American Society for Transplantation and Cellular Therapy. 2022;28(6):310-320. doi:10.1016/j.jtct.2022.03.015
4. Ravaglia C, Poletti V. Transbronchial lung cryobiopsy for the diagnosis of interstitial lung diseases. Curr Opin Pulm Med. 2022;28(1):9-16. doi:10.1097/MCP.0000000000000848
5. Loor K, Culebras M, Sansano I, et al. Lung allograft transbronchial cryobiopsy for critical ventilated patients: a randomised trial. European Respiratory Journal. 2023;61(1). doi:10.1183/13993003.02354-2021