Key Points
Platelet-derived TGF-β1 modulates MDSC function and profiling via TGF-β/Smad pathway in ITP.
Beyond an indicator of treatment response, platelet TGF-β paves the way for immune homeostasis in immune-mediated thrombocytopenia.
Visual Abstract
Platelet α-granules are rich in transforming growth factor β1 (TGF-β1), which is associated with myeloid-derived suppressor cell (MDSC) biology. Responders to thrombopoietin receptor agonists (TPO-RAs) revealed a parallel increase in the number of both platelets and MDSCs. Here, anti-CD61 immune-sensitized splenocytes were transferred into severe combined immunodeficient mice to establish an active murine model of immune thrombocytopenia (ITP). Subsequently, we demonstrated that TPO-RAs augmented the inhibitory activities of MDSCs by arresting plasma cells differentiation, reducing Fas ligand expression on cytotoxic T cells, and rebalancing T-cell subsets. Mechanistically, transcriptome analysis confirmed the participation of TGF-β/Smad pathways in TPO-RA–corrected MDSCs, which was offset by Smad2/3 knockdown. In platelet TGF-β1–deficient mice, TPO-RA-induced amplification and enhanced suppressive capacity of MDSCs was waived. Furthermore, our retrospective data revealed that patients with ITP achieving complete platelet response showed superior long-term outcomes compared with those who only reach partial response. In conclusion, we demonstrate that platelet TGF-β1 induces the expansion and functional reprogramming of MDSCs via the TGF-β/Smad pathway. These data indicate that platelet recovery not only serves as an end point of treatment response but also paves the way for immune homeostasis in immune-mediated thrombocytopenia.
Introduction
Platelets play dual physiological roles as cellular mediators of thrombosis and inflammation.1 They originate from megakaryocytes that express numerous immune sensors and cross-present antigen-MHC class I complex to CD8+ T cells.1,2 These immune properties were spread during the formation of proplatelets and constitute major granular contents including platelet factor 4 (PF4), CD40L, interleukin-1 (IL-1), and transforming growth factor β1 (TGF-β1).1,3 Platelets contain 40 to 100 times higher TGF-β1 than other cells, which has been linked to the pathophysiology of pulmonary arterial hypertension and cervical carcinoma.4-6
TGF-β is a crucial enforcer of immune homeostasis, and perturbations in TGF-β signaling underly inflammatory and autoimmune diseases.7 As a member of the TGF-β superfamily, TGF-β1 is the dominant isoform in immune cells and exerts pleiotropic immunosuppressive effects, including inhibition of T-cell activation, B-cell survival, proliferation, immunoglobulin (Ig) synthesis, and promotion of peripheral regulatory T-cell (Treg) and T helper cell 17 (Th17) differentiation.8,9 Null mutations in the Tgfb1 gene can cause systemic inflammation and untimely death in mice.10,11 Mechanistically, TGF-β1 engages TGF-β receptor II (TGF-βRII) and then recruits and phosphorylates TGF-βRI. The receptor complex then initiates the canonical Smad2- and Smad3-dependent pathways and/or noncanonical pathways to regulate target gene expression in immune cells.11-13
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous group of immature myeloid cells with potent immunosuppressive functions, including the inhibition of T-cell proliferation/activation and promotion of Tregs recruitment/amplification.14-17 TGF-β1 can expand the MDSC population and enhance their immunosuppressive functions.18,19 In turn, MDSCs functions through induced nitric oxide synthase (iNOS), arginase-1(Arg-1), reactive oxygen species, and a variety of secreted cytokines, including TGF-β1.20-23 Given that platelets are rich in TGF-β1, the impact of platelet-derived TGF-β1 on the cell fate of MDSCs requires further exploration.
Impaired MDSCs have been described in multiple autoimmune diseases.17 Immune thrombocytopenia (ITP) is an acquired bleeding disorder characterized by increased platelet destruction and insufficient platelet production due to self-tolerance breakdown.24 Our previous studies showed that MDSCs were impaired in number and function in patients with ITP, and dexamethasone corrected MDSC dysfunction via Ets1, whereas decitabine enhanced the oxidative metabolism of MDSCs via liver kinase B1. Additionally, impaired aerobic metabolism in MDSCs contributed to the pathophysiology of glucocorticoid resistance in ITP.25-27 Dramatically decreased production and serum levels of TGF-β1 has been observed in fatal thrombocytopenic syndromes as well as active ITP,28-30 whereas elevated TGF-β1 was associated with treatment response of ITP.31,32 However, whether platelet recovery can restore immune homeostasis through TGF-β1 is still unclear.
Thrombopoietin receptor agonists (TPO-RAs), the first choice among second-line ITP treatments, stimulate megakaryopoiesis and platelet production.24,33-35 Previous studies implicated that changes in the platelet mass are significant in modulating Tregs, irrespective of the treatment used in ITP, no matter steroids, IVIg, rituximab, or TPO-RAs.36 Moreover, improved Tregs activity, restored regulatory B-cell compartment, and reduced phagocytic capacity of macrophages were reported in patients with ITP responding to thrombopoietic treatment.31,37-39 The above alterations in the immune cells are seen with increased platelet mass,40 although the detailed mechanisms remain to be elucidated. Our previous study showed a significant elevation in plasma TGF-β1 levels in TPO-RA responders.39,41 In addition, TGFB1 was identified as an upstream regulator of eltrombopag-induced genes.42 Thus, we speculated that platelet-derived TGF-β1 could reprogram MDSCs during ITP convalescence.
The objective of this study was to elucidate the underlying molecular mechanisms of the functional reprogramming of MDSCs by platelet-derived TGF-β1. Here, active-ITP murine models and MDSC cell therapy were used to evaluate the regulation of elevated TGF-β1 on MDSCs induced by thrombopoiesis. Additionally, platelet-specific TGF-β1–deficient mice were used to validate the effect of platelet-derived TGF-β1 on MDSCs.
Materials and methods
Patients and controls
For in vitro MDSC studies, 72 patients with ITP and 34 age- and sex-matched healthy controls (supplemental Table 1, available on the Blood website) were enrolled at the Department of Hematology, Qilu Hospital, Shandong University, Jinan, China, between September 2019 and February 2024. For in vivo studies, an additional 8 patients with ITP receiving TPO-RA and 8 sex-matched healthy controls (supplemental Table 3) were enrolled.
The study included 3 retrospective cohorts to evaluate the long-term outcome of patients with an initial response to eltrombopag, dexamethasone, or rituximab. Information of patients are detailed in supplemental Methods and supplemental Tables 4-6.
This study was approved by the medical ethical committee of the Qilu Hospital, Shandong University. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Mice
Anti-CD61 immune-sensitized splenocytes were transferred into severe combined immunodeficient mice to establish an active-ITP murine model.43 A total of 2 × 106 MDSCs were sorted and intraperitoneally injected into the active-ITP mice after pharmacological interventions to build the MDSC therapy model. MDSC-specific Smad2 or Smad3 was knocked down in the active-ITP mice. Adeno-associated virus (AAV)–DJ-SMAD2-GFP -miR30-shRNA, AAV-DJ-SMAD3-GFP-miR30-short hairpin RNA (shRNA) with CD11b as specific promoter, and control AAV were injected into severe combined immunodeficient mice through the tail vein 4 weeks ahead. The passive-ITP murine model was established as previously reported.27 C57BL/6J, Pf4cre-Tgfb1fl/fl, or Pf4cre+Tgfb1fl/fl mice were injected intraperitoneally with a rat anti-mouse CD41a monoclonal antibody. The strains and backgrounds of mice and procedures are detailed in the supplemental Methods.
Animal experiments were conducted under the approval of the Animal Care and Use Committee of Qilu Hospital of Shandong University.
Medication
Romiplostim and SB431542 (TGF-β1 inhibitor) were applied in this study. To determine the role of platelet TGF-β1 in the active model, ITP mice were transfused with 100 μL of 108 platelets from Pf4cre-Tgfb1fl/fl or Pf4cre+Tgfb1fl/fl mice weekly for 4 weeks. Dose and route of administration, as well as preparation of platelets, are provided in supplemental Methods.
Isolation and culturing of peripheral blood mononuclear cells and MDSCs
Peripheral blood mononuclear cells from patients with ITP and healthy controls were isolated and cultured with recombinant human IL-6 and granulocyte-macrophage colony stimulating factor to induce MDSCs.
Transwell coculture assays
Homogeneous platelets (0, 20 × 109/L, or 200 × 109/L) from healthy controls were seeded in the upper chamber, whereas MDSCs from patients with ITP were seeded in the lower chamber, together or alone. Platelets from patients with ITP (supplemental Table 2) treated with TPO-RA or not were also collected and cultured alone.
Suppression capacity of MDSCs
Carboxyfluorescein diacetate succinimidyl ester–labeled effector T cells (Teffs) (CD4+CD25–) were cocultured with MDSCs. Samples were acquired for flow cytometry after 5 days of coculture. Inhibition of MDSCs toward cytotoxic T cell (CTL)–induced platelet apoptosis is described in the supplemental Methods.
Transcriptome sequencing
Total RNA was extracted from the MDSCs of active-ITP mice treated by romiplostim or not. Transcriptome sequencing was completed by Beijing Nuohezhiyuan Technology Service Co, Ltd. Detailed descriptions of RNA quantification, sequencing, and data analysis are described in the supplemental Methods.
Cytokine analysis
Cell-culture supernatant of MDSCs and platelets and serum samples of ITP mice were tested for TGF-β1 concentrations via enzyme-linked immunosorbent assay (ELISA) kits. Besides, serum total Ig levels were detected by ELISA kits.
Flow cytometry
We detected the cell cycle stages of T and B cells, the phenotypic markers of MDSCs, Tregs, Th1, Th2, Th17, CTLs, and B cells, as well as functional molecules of MDSCs by flow cytometry. Antibodies are listed in supplemental Table 7.
Immunofluorescence analysis
Femurs were harvested from active-ITP mice, and paraffin sections (6 μm) were incubated with primary anti-Ly-6G/Ly-6C and anti-Smad2/anti-phospho Smad2/anti-Smad3/anti-phospho Smad3 antibodies, followed by incubation with secondary antibodies.
siRNA transfection
SMAD2 small interfering RNA (siRNA), SMAD3 siRNA, negative control (NC) siRNA, and FAM siRNA were incubated with cells for a total of 48 hours and functionally evaluated by real-time quantitative polymerase chain reaction (qPCR).
AAV transfection
We established AAVs targeted CD11b and encoded the murine SMAD2, SMAD3, or NC shRNA (supplemental Figure 1). AAV transfection efficiency was detected by flow cytometry.
Procedures of all the above are detailed in supplemental Methods.
Statistical analysis
Normally distributed continuous data were expressed as the mean ± standard deviation, differences between 2 groups were compared by paired or unpaired t test, comparisons between groups were made by 1-way or 2-way analysis of variance or mixed-effects analysis, and the relationships between variables were assessed by the Pearson correlation test. Nonnormally distributed continuous data were expressed as the median and extreme values, and the differences between 2 groups were compared by the Mann-Whitney test. Categorical data were analyzed using the Pearson χ2 test. SPSS 25.0 (IBM SPSS, Armonk, NY) and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA) were used for statistical analyses. P < .05 was considered statistically significant.
This study was approved by the Medical Ethical Committee of the Qilu Hospital, Shandong University. The study was conducted in accordance with the principles of the Declaration of Helsinki. Animal experiments were conducted under the approval of the Animal Care and Use Committee of Qilu Hospital of Shandong University.
Results
Platelets promoted the expansion and immunosuppressive function of MDSCs via TGF-β1
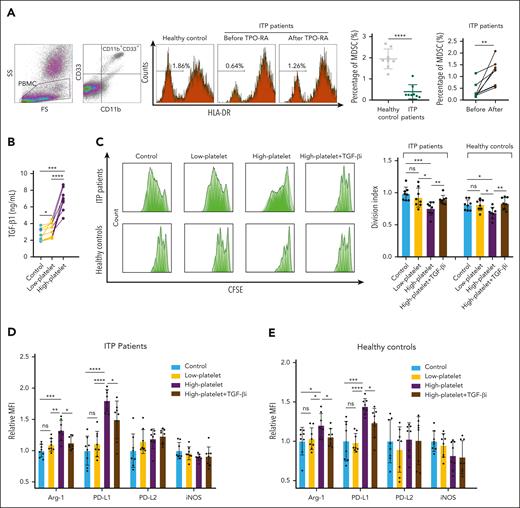
The proportion of circulating MDSCs was significantly lower in patients with ITP patients than in healthy controls (P < .0001). After TPO-RA treatment, there was a significant increase in the percentage of circulating MDSCs in all 8 patients with ITP (P < .01; Figure 1A). In the supernatant of transwell cocultures, TGF-β1 levels in the high-platelet group (platelets at 200 × 109/L) were significantly higher than those in the low-platelet group (platelets at 20 × 109/L) and MDSCs-alone group (P < .001; Figure 1B). Platelet alone cultures were also analyzed as shown in supplemental Figure 2A-D. Besides, the division index of Teffs was significantly lower when cocultured with high-platelet–modulated MDSCs (P < .001; Figure 1C), indicating that the immunosuppressive function of MDSCs was enhanced by platelets at high concentration but not at low concentration. Additionally, T-cell proliferation modulated by platelets alone was shown in supplemental Figure 2E-I. Significantly higher levels of Arg-1 and programmed cell death ligand (PD-L) 1 were found in MDSCs of the high-platelet group than those in the low-platelet and control groups (P < .01; Figure 1D-E) The levels of PD-L2 and iNOS were relatively stable between the groups. Furthermore, the platelet-induced enhanced suppressive function of MDSCs was offset by a TGF-β1 inhibitor (TGF-βi; P < .01; Figure 1C), indicating that platelets induced the functional reprogramming of MDSCs via TGF-β1. A similar phenomenon was observed in MDSCs from healthy controls (Figure 1C). Additionally, the T-cell inhibition capacity of MDSCs was significantly lower in nonresponders than responders to TPO-RA (P < .05; supplemental Figure 3A). However, upon modulation by platelets at high concentration, MDSCs could be corrected despite treatment response (supplemental Figure 3B-C).
Platelet-associated TGF-β1 stimulated the function of MDSCs in vitro. (A) Representative flow-cytometric plots of MDSCs (CD11b+ CD33+ HLA-DRlow cells) in peripheral blood mononuclear cells (PBMCs) from healthy controls and patients with ITP before and after TPO-RA treatment. (B) TGF-β1 level in control and low-platelet and high-platelet cocultured groups. (C) MDSC-mediated suppression of CD4+ Teffs proliferation was measured according to the division index. (D-E) Relative mean fluorescence intensity (MFI) of Arg-1, PD-L1, PD-L2, and iNOS expressed by MDSCs cocultured with low-platelet, high-platelet, high-platelet plus TGF-βi, or none. Relative MFI is the fold of average MFI in controls. (D) MDSC from patients with ITP. (E) MDSC from healthy controls.
Platelet-associated TGF-β1 stimulated the function of MDSCs in vitro. (A) Representative flow-cytometric plots of MDSCs (CD11b+ CD33+ HLA-DRlow cells) in peripheral blood mononuclear cells (PBMCs) from healthy controls and patients with ITP before and after TPO-RA treatment. (B) TGF-β1 level in control and low-platelet and high-platelet cocultured groups. (C) MDSC-mediated suppression of CD4+ Teffs proliferation was measured according to the division index. (D-E) Relative mean fluorescence intensity (MFI) of Arg-1, PD-L1, PD-L2, and iNOS expressed by MDSCs cocultured with low-platelet, high-platelet, high-platelet plus TGF-βi, or none. Relative MFI is the fold of average MFI in controls. (D) MDSC from patients with ITP. (E) MDSC from healthy controls.
Platelet-associated TGF-β1 augmented the population of MDSCs in the active-ITP murine model.
Romiplostim (Romi),44 a TPO-RA, was used to raise the platelet count in an active-ITP murine model (supplemental Figure 9A). The optimal concentration for platelet elevation in a passive-ITP murine model was determined to be 30 μg/kg (supplemental Figure 4). The platelet count in the Romi-treated group was significantly higher than that in the control group (P < .0001), which was counteracted by TGF-βi (P < .05; Figure 2A). Significantly expanded MDSC percentage and higher levels of Arg-1 and PD-L1 were found in Romi-treated mice than controls, which was offset by TGF-βi (P < .05; Figure 2B-C). The levels of PD-L2 and iNOS were relatively stable beween the groups.
Platelet-associated TGF-β1 stimulated the function and modulated the transcriptome profiles of MDSCs in active-ITP mice. (A) Platelet counts among the control, Romi, and Romi + TGF-βi groups differed significantly on day 28. (B) Percentage of MDSCs in bone marrow nucleated cells evaluated by FACS. (C) Relative MFI of Arg-1, PD-L1, PD-L2, and iNOS expressed by MDSC from active-ITP mice treated with TGF-βi, Romi, Romi plus TGF-βi, or none. Relative MFI is the fold of average MFI in controls. (D) Serum TGF-β1 level detected by ELISA. Pearson correlation analysis of (E) platelet counts with serum TGF-β1 level and (F) serum TGF-β1 level with MDSC percentage. (G) Gene ontology (GO) enrichment score [−log10(P value)] analysis of DEGs identified between Romi-treated and control mice. (H) KEGG enrichment analysis based on the expression of Romi-altered genes. DEGs, differentially expressed genes; FACS, fluorescence activated cell sorter; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Platelet-associated TGF-β1 stimulated the function and modulated the transcriptome profiles of MDSCs in active-ITP mice. (A) Platelet counts among the control, Romi, and Romi + TGF-βi groups differed significantly on day 28. (B) Percentage of MDSCs in bone marrow nucleated cells evaluated by FACS. (C) Relative MFI of Arg-1, PD-L1, PD-L2, and iNOS expressed by MDSC from active-ITP mice treated with TGF-βi, Romi, Romi plus TGF-βi, or none. Relative MFI is the fold of average MFI in controls. (D) Serum TGF-β1 level detected by ELISA. Pearson correlation analysis of (E) platelet counts with serum TGF-β1 level and (F) serum TGF-β1 level with MDSC percentage. (G) Gene ontology (GO) enrichment score [−log10(P value)] analysis of DEGs identified between Romi-treated and control mice. (H) KEGG enrichment analysis based on the expression of Romi-altered genes. DEGs, differentially expressed genes; FACS, fluorescence activated cell sorter; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Parallel increase in platelet and TGF-β1 levels modulated the transcriptome profiles of MDSCs in the active-ITP murine model
The ELISA results suggested that the serum TGF-β1 level in the Romi-treated group was significantly higher than that in the control group (P < .01; Figure 2D). Strong positive correlations between the TGF-β1 concentration and platelet count (P < .0001; R2 = 0.6476; Figure 2E) and the proportion of MDSCs and TGF-β1 concentration (P < .0001; R2 = 0.6188; Figure 2F) were observed.
Messenger RNA sequencing analysis was performed on MDSCs from control and Romi-treated mice. The quantitative analysis showed that 188 genes were upregulated and 96 downregulated in the Romi-treated group compared with those in the control group (supplemental Figure 5A). The 5 most differentially upregulated genes were Ace, Hic1, Rbm44, Ptgdr2, and Fzd7. The 5 most differentially downregulated genes were Efr3b, Slc15a2, Cdk5rap1, Mt1, and AC133103.1.
Gene ontology analysis showed that differentially expressed genes (DEGs) in the Romi-treated group were linked with positive regulation of cytokine production, myeloid leukocyte differentiation, cytokine receptor binding, and TGF-βR binding (Figure 2G). Kyoto Encyclopedia of Genes and Genomes analysis showed that these genes were associated with the Hippo, TGF-β, JAK-STAT, and pluripotency regulating signaling pathways (Figure 2H). Reactome analysis showed that these genes were mainly linked to bone morphogenetic protein signaling, rhodopsin-like receptors, G protein-coupled receptor ligand binding, fatty acid metabolism, and signaling by TGF-β family members (supplemental Figure 5B). These results confirmed the participation of the TGF-β signaling pathway in TPO-RA–corrected MDSCs.
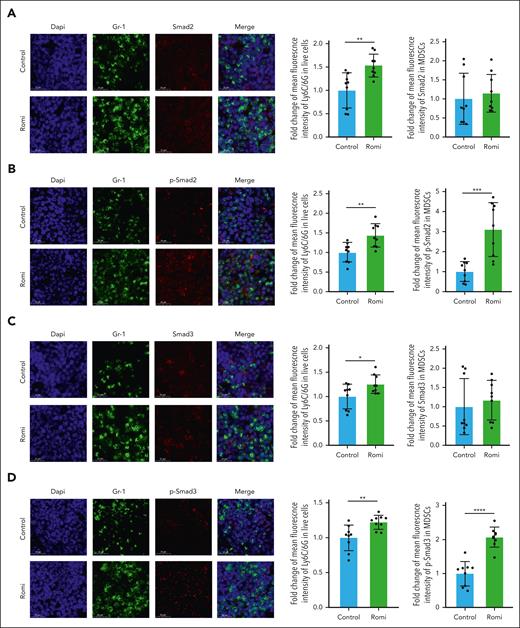
An immunofluorescence assay suggested that the mean fluorescence intensity of Gr-1 was higher in the Romi-treated group than in the control group (P < .05; Figure 3). Smad2 and Smad3 expressions of MDSCs were similar between the Romi-treated and control groups (Figure 3A,C), whereas the phosphorylation levels of Smad2 and Smad3 were significantly higher in the Romi-treated group than in the control group (P < .001; Figure 3B,D), indicating that parallel increase in platelets and TGF-β1 levels activated Smad signaling.
Platelet-associated TGF-β1 affected the TGF-β/Smad pathway in active-ITP mice. Immunofluorescence images of femurs from control and Romi-treated mice. (A) MFI of Ly6C/6G in live cells and Smad2 in Gr-1+ cells. (B) MFI of Ly6C/6G in live cells, and p-Smad2 in Gr-1+ cells. (C) MFI of Ly6C/6G in live cells and Smad3 in Gr-1+ cells. (D) MFI of Ly6C/6G in live cells and p-Smad3 in Gr-1+ cells.
Platelet-associated TGF-β1 affected the TGF-β/Smad pathway in active-ITP mice. Immunofluorescence images of femurs from control and Romi-treated mice. (A) MFI of Ly6C/6G in live cells and Smad2 in Gr-1+ cells. (B) MFI of Ly6C/6G in live cells, and p-Smad2 in Gr-1+ cells. (C) MFI of Ly6C/6G in live cells and Smad3 in Gr-1+ cells. (D) MFI of Ly6C/6G in live cells and p-Smad3 in Gr-1+ cells.
Platelet-associated TGF-β1 enhanced the efficacy of MDSC cell therapy in the active-ITP murine model
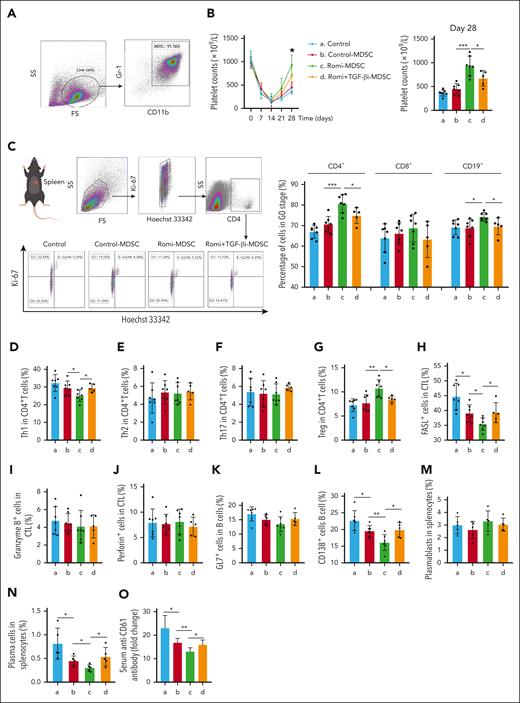
MDSCs from bone marrow of active-ITP mice were transferred for cell therapy (Figure 4A; supplemental Figure 9B). The platelet count in the Romi-MDSC treatment group was higher than that in the control-MDSC treatment group (P < .001) and Romi + TGF-βi–MDSC treatment group (P < .05; Figure 4B), indicating that TGF-βi could block the effect of elevated platelet levels on MDSC function via the TGF-β pathway.
Platelet-associated TGF-β1 enhanced immunosuppressive functions of MDSCs in active-ITP mice. (A) The dot plots of MDSC enriched populations for cell therapy. (B) Platelet counts among the control, control-MDSC, Romi-MDSC, and Romi + TGF-βi–MDSC groups differed significantly on day 28. Immune cells from all the groups were isolated and detected. (C) Representative flow-cytometric plots of cell cycle for CD4+ cells (G0 stage: Hoechst 33342– Ki-67–; G1 stage: Hoechst 33342– Ki-67+; S-G2/M stage: Hoechst 33342+ Ki-67+). Percentage of Th1 (D), Th2 (E), Th17 (F), and Tregs (G) in CD4+ cells. Percentage of FasL+ (H), granzyme B+ (I), and perforin+ CTLs (J). Percentage of GL-7+ B cells (K), CD138+ B cells (L), plasmablasts (M), and plasma cells (N). (O) The level of serum anti-CD61.
Platelet-associated TGF-β1 enhanced immunosuppressive functions of MDSCs in active-ITP mice. (A) The dot plots of MDSC enriched populations for cell therapy. (B) Platelet counts among the control, control-MDSC, Romi-MDSC, and Romi + TGF-βi–MDSC groups differed significantly on day 28. Immune cells from all the groups were isolated and detected. (C) Representative flow-cytometric plots of cell cycle for CD4+ cells (G0 stage: Hoechst 33342– Ki-67–; G1 stage: Hoechst 33342– Ki-67+; S-G2/M stage: Hoechst 33342+ Ki-67+). Percentage of Th1 (D), Th2 (E), Th17 (F), and Tregs (G) in CD4+ cells. Percentage of FasL+ (H), granzyme B+ (I), and perforin+ CTLs (J). Percentage of GL-7+ B cells (K), CD138+ B cells (L), plasmablasts (M), and plasma cells (N). (O) The level of serum anti-CD61.
Platelet-associated TGF-β1 enhanced MDSC-mediated suppression of T- and B-cell proliferation in the active-ITP murine model
The proportion of spleen CD4+ T and CD19+ B cells in the G0 phase was higher in the Romi-MDSC treatment group than in both the control-MDSC and Romi + TGF-βi–MDSC treatment groups (P < .001; P < .05; Figure 4C). However, no significant differences were observed in the proportion of CD8+ T cells in the G0 phase among the different treatment groups.
Platelet-associated TGF-β1 enhanced MDSC-mediated modulation of CD4+ T-cell subsets
The proportion of Th1 cells in the Romi-MDSC treatment group was significantly lower than that in both the control-MDSC and Romi + TGF-βi–MDSC treatment groups (P < .05; Figure 4D). However, no significant differences were observed in the percentages of Th2 and Th17 cells among the treatment groups (Figure 4E-F). The proportion of Tregs in the Romi-MDSC treatment group was higher than that in both the control-MDSC and Romi + TGF-βi–MDSC treatment groups (P < .01; P < .05; Figure 4G).
Platelet-associated TGF-β1 enhanced MDSC-mediated suppression of CTLs
The proportion of FasL+ CTLs was lower in the Romi-MDSC treatment group than in both the control-MDSC and Romi + TGF-βi–MDSC treatment groups (P < .05; Figure 4H). However, no significant difference was observed in the percentage of granzyme B+ or perforin-positive CTLs among the treatment groups (Figure 4I-J).
Platelet-associated TGF-β1 enhanced MDSC-mediated suppression of plasma cells and antibody production
The proportion of antibody-secreting cells and plasma cells in the Romi-MDSC treatment group was significantly lower than that in the control-MDSC or Romi + TGF-βi–MDSC treatment groups (P < .05; Figure 4L,N; supplemental Figure 6). However, no statistical difference was found in GL-7+ cells or plasmablasts between the groups (Figure 4K,M; supplemental Figure 6). The level of serum anti-platelet CD61-specific antibodies and Ab-sensitized platelets changed inversely with platelet counts (supplemental Figure 7). Besides, they were all significantly lower in the Romi-MDSC treatment group than in the control-MDSC (P < .05) or Romi + TGF-βi–MDSC (P < .05) treatment groups (Figure 4O).
Platelet-associated TGF-β1–induced functional reprogramming of MDSCs occurs through Smad2/Smad3 in the active-ITP murine model
Consistent with our previous results, Romi elevated both the platelet count and MDSC proportion in active-ITP mice (P < .01). Smad2 or Smad3 knockdown reversed this effect of TPO-RAs (P < .05; Figure 5A-B; supplemental Figure 9C). Furthermore, MDSCs were separated for cell therapy (supplemental Figure 9D). The platelet count in the Romi-NC–MDSC group was higher than that in the control-NC–MDSC (P < .001), Romi-Smad2––MDSC (P < .05) and Romi-Smad3––MDSC groups (P < .05; Figure 5C). The proportions of CD4+ T and CD19+ B cells in the G0 phase were higher in the Romi-NC–MDSC treatment group than in the control-NC–MDSC treatment groups (P < .01), which was reversed by Smad2 and Smad3 knockdown (P < .05; Figure 5D-E). Regarding Th-cell subsets, the percentage of Th1 cells was lower in the Romi-NC–MDSC treatment group than in the control-NC–MDSC treatment group (P < .01), whereas the proportion of Tregs in the Romi-NC–MDSC treatment group was higher than that in the control-NC–MDSC treatment group (P < .01); this effect was reversed by Smad2 and Smad3 knockdown (P < .05; Figure 5F-G). In assays against CTLs, the proportions of FasL+ cells were lower in the Romi-NC–MDSC treatment group than in the control-NC–MDSC treatment group (P < .01), which was reversed by Smad2 and Smad3 knockdown (P < .05; Figure 5H). In addition, the proportion of CD138+ B cells was lower in the Romi-NC–MDSC treatment group than in the control-NC–MDSC treatment group (P < .05), which was reversed by Smad2 and Smad3 knockdown (P < .05; Figure 5I).
Platelet-derived TGF-β1 induced functional reprogramming of MDSCs in active-ITP mice through Smad2/Smad3. (A) AAV-DJ-SMAD2-GFP-miR30-shRNA, AAV-DJ-SMAD3-GFP-miR30-shRNA with CD11b as the specific promoter, and control AAV were injected into SCID mice through tail vein. Four weeks later, the active model of ITP was established. Platelet counts were detected once a week. (B) Percentage of MDSCs in bone marrow nucleated cells. The proportion of GFP+ cells in MDSCs was 40% to 50%. (C) MDSCs of mice from Control-NC, Romi-NC, Romi-Smad2–, and Romi-Smad3– groups were individually transferred to the new active-ITP murine model. Platelet counts were detected once a week. (D) Percentage of CD4+ cells in G0 stage. (E) Percentage of CD19+ cells in G0 stage. Percentage of Th1 (F) and Tregs (G) in CD4+ cells. (H) Percentage of FasL+ CTLs. (I) Percentage of CD138+ B cells. SCID, severe combined immune deficiency.
Platelet-derived TGF-β1 induced functional reprogramming of MDSCs in active-ITP mice through Smad2/Smad3. (A) AAV-DJ-SMAD2-GFP-miR30-shRNA, AAV-DJ-SMAD3-GFP-miR30-shRNA with CD11b as the specific promoter, and control AAV were injected into SCID mice through tail vein. Four weeks later, the active model of ITP was established. Platelet counts were detected once a week. (B) Percentage of MDSCs in bone marrow nucleated cells. The proportion of GFP+ cells in MDSCs was 40% to 50%. (C) MDSCs of mice from Control-NC, Romi-NC, Romi-Smad2–, and Romi-Smad3– groups were individually transferred to the new active-ITP murine model. Platelet counts were detected once a week. (D) Percentage of CD4+ cells in G0 stage. (E) Percentage of CD19+ cells in G0 stage. Percentage of Th1 (F) and Tregs (G) in CD4+ cells. (H) Percentage of FasL+ CTLs. (I) Percentage of CD138+ B cells. SCID, severe combined immune deficiency.
Platelet-derived TGF-β1 induced expansion and functional reprogramming of MDSCs in ITP mice
Because Romi did not directly affect MDSCs (supplemental Figure 8), we hypothesized that Romi could induce the expansion and functional reprogramming of MDSCs through platelet–derived TGF-β1 activity. To verify this hypothesis, a passive-ITP murine model was established (supplemental Figure 9E) along with 5 treatment groups, including control, Romi, and Romi + TGF-βi groups in Pf4cre−Tgfb1fl/fl mice, as well as control and Romi groups in Pf4cre+Tgfb1fl/fl mice; the platelet count in the Romi group was significantly higher than that in the control group (P < .05; Figure 6A). The percentage of MDSCs in the Romi group was higher than that in the control group (P < .01; Figure 6B). TGF-βi and platelet–derived TGF-β1 deficiency counteracted this effect (P < .01; P < .05), with no statistical difference between the groups. The division index of Teffs was significantly lower when cocultured with MDSCs from Romi-treated mice than that in the other groups (P < .01; Figure 6C), indicating that the immunosuppressive function of MDSCs were enhanced in these mice. Furthermore, TGF-βi and platelet-derived TGF-β1 deficiency both offset this effect (P < .05), with no statistical difference between the groups. In addition, the control group in Pf4cre+Tgfb1fl/fl mice showed similar proportion and function of MDSCs, compared with the control group in Pf4cre−Tgfb1fl/fl mice (P > .05; Figure 6B-C). These results suggested that platelet TGF-β1 induced the expansion and functional reprogramming of MDSCs in passive-ITP mice.
Platelet-derived TGF-β1 induced functional reprogramming of MDSCs in the ITP murine model. (A) Passive-ITP mice in group a, b, and c presented Pf4cre–Tgfb1fl/fl. Group a was injected with sterile water as a control. Group b was treated with TPO-RA (romiplostim, 30 μg/kg, once every 3 days). Group c was treated with TPO-RA and TGF-βi (SB431542, 10 mg/kg, once every 3 days). Mice in groups d and e were Pf4cre+Tgfb1fl/fl and treated with TPO-RA or not. Platelet counts detected every other day. Platelet counts among the groups differed significantly on day 6. (B) Percentage of MDSCs in bone marrow nucleated cells. (C) MDSC-mediated suppression of CD4+ Teff proliferation measured according to the division index. Platelet counts (D) and percentage of MDSCs (E) of active-ITP mice after platelet transfusion. CFSE, carboxyfluorescein diacetate succinimidyl ester.
Platelet-derived TGF-β1 induced functional reprogramming of MDSCs in the ITP murine model. (A) Passive-ITP mice in group a, b, and c presented Pf4cre–Tgfb1fl/fl. Group a was injected with sterile water as a control. Group b was treated with TPO-RA (romiplostim, 30 μg/kg, once every 3 days). Group c was treated with TPO-RA and TGF-βi (SB431542, 10 mg/kg, once every 3 days). Mice in groups d and e were Pf4cre+Tgfb1fl/fl and treated with TPO-RA or not. Platelet counts detected every other day. Platelet counts among the groups differed significantly on day 6. (B) Percentage of MDSCs in bone marrow nucleated cells. (C) MDSC-mediated suppression of CD4+ Teff proliferation measured according to the division index. Platelet counts (D) and percentage of MDSCs (E) of active-ITP mice after platelet transfusion. CFSE, carboxyfluorescein diacetate succinimidyl ester.
Finally, active-ITP mice receiving Pf4cre−Tgfb1fl/fl platelet transfusion had significantly higher platelet counts and MDSC percentage than those receiving Pf4cre+Tgfb1fl/fl platelets or the control group (P < .05; Figure 6D-E), indicating that TGF-β1 knockout platelets had less rescuing effect than wild-type (WT) platelets.
Patients with ITP with complete platelet response to standard treatments presented better long-term treatment response
In ITP, platelet recovery fundamentally defines the depth of treatment response. The criteria for the treatment response were defined as follows: complete response (CR) as platelet count ≥100 × 109 /L and absence of bleeding; and partial response (PR) as platelet count ≥30 × 109 /L and at least a twofold increase of the baseline platelet count and absence of bleeding. In this study, initial response refers to the first response after taking/switching to the current medication. To explore the long-term outcomes of patients with ITP with different initial platelet responses, we reviewed the data of 3 retrospective cohorts from Qilu Hospital of Shandong University. In the eltrombopag treatment cohort, a Kaplan-Meier survival analysis revealed that the duration of response differed between patients with initial CR and PR (P = .022). The sustained response rate at 12 months was higher for patients with initial CR than for those with initial PR (80.6% vs 51.9%; P = .015; supplemental Figure 10A). We also observed differences in the duration of response between patients with initial CR and PR in both dexamethasone and rituximab treatment cohorts (P = .010; P = .016; supplemental Figure 10B-C). Moreover, in the dexamethasone treatment cohort, the sustained response rate at 6 months was higher for patients with initial CR than for those with initial PR (56.1% vs 30.4%; P = .048; supplemental Figure 10B). Similarly, in the rituximab treatment cohort, the sustained response rate at 18 months was higher in patients with initial CR than in those with initial PR (79.3% vs 46.2%; P = .032; supplemental Figure 10C). From our findings, we speculated that patients with ITP with early platelet response to a certain medication might benefit from platelet TGF-β–mediated immune regulation, which fosters long-term efficacy of the drug.
Discussion
Platelets have widely been recognized as versatile cells with multiple immune functions. To our knowledge, this is the first study to elucidate the enhancing effect of platelet TGF-β1 on MDSCs in vitro and in vivo. Notably, we showed that platelet recovery could induce the restoration of immune homeostasis via TGF-β1–Smad2/3 pathways in patients with ITP, indicating a common mechanism of ITP-specific therapies.
Owing to their abundant presence, platelets are central not only to hemostasis but also to tumor metastasis, inflammation, and immune modulation.45,46 Platelets participate in both innate and adaptive immune responses, because they can respond to various pathogens through their toll-like receptors, granule secretion, complement activation, immune complex-sensing through Fcγ receptor IIa, T-cell activation, and antigen presentation to dendritic cells.47,48 However, the effect of platelets on MDSCs was unclear until now. TGF-β1 is the most abundant platelet-derived immunosuppressive cytokine.49 Severe autoimmune diseases occur in TGF-β1–deficient mice.50 In humans, TGF-β1 deficiency has consistently been linked to polyautoimmunity and multiple autoimmune diseases, including systemic sclerosis, systemic lupus erythematosus, inflammatory bowel disease and encephalopathy, and ITP.30,51-53 The physiological and pathophysiological roles of platelet-released TGF-β1 have gradually gained attention and have been shown to participate in atherosclerosis, pulmonary arterial hypertension, aortic valve stenosis, and endometriosis.5,54-56 To our knowledge, studies assessing the role of platelet TGF-β1 in autoimmune diseases are lacking. Our data provided evidence that platelet TGF-β1 induced functional reprogramming of MDSCs in ITP.
MDSCs regulate immune responses under various pathological conditions, including cancer, chronic infection, sepsis, and autoimmunity, through the inhibition of CD4+ and CD8+ T-cell functions.57,58 MDSCs are a potential therapeutic target for autoimmune diseases, including rheumatoid arthritis, autoimmune diabetes, multiple sclerosis, systemic lupus erythematosus, and ITP.17 Multiple studies reported the significant role of MDSCs in refractory ITP.59 Besides, nonresponders to dexamethasone failed to retrieve MDSC activity,25 as was observed in nonresponders to TPO-RA in this study. Nevertheless, MDSCs could be corrected by increased platelet mass irrespective of response. Mechanistically, the immunosuppressive functions of MDSCs exert through the catabolism of L-arginine by arginase and iNOS, which leads to deprivation of the fundamental amino acid required for T-cell proliferation.57,60 In addition, MDSC-induced nitration of TCR/CD8 via hyperproduction of reactive oxygen species and peroxynitrite results in CD8+ T-cell tolerance.61 MDSCs also express immune checkpoint molecules such as PD-L and release inhibitory cytokines such as IL-10/TGF-β, which further contribute to T-cell suppression and expansion of Tregs.62 Hence, MDSCs potently downplay T-cell activity through multiple pathways involving but not limited to TGF-β1, which partly explains why suppression is amplified though platelet-modulated MDSCs compared with platelets alone, because platelets potentially enhance Treg responses but also biphasically regulate Th1/Th17 and effector T-cell activation via TGF-β1 or PF4.63-67
Clinical studies have reported that TPO-RAs, including eltrombopag and romiplostim, can increase platelet levels and reduce the risk of bleeding in patients with chronic ITP, with an overall response rate of 74% to 94%.68-70 Mechanistically, TPO-RA binds to and activates the TPO receptor on the megakaryocyte precursors, promoting cell proliferation and viability, thereby increasing platelet production via the JAK2-STAT5/3, PI3K/AKT, and extracellular regulated protein kinases downstream signaling pathways.35 Recently, several clinical studies have noted that 25% to 32% of the patients with ITP could achieve sustained treatment response after TPO-RA withdrawal, which could be explained by the immune modulation effect rather than the above mechanism.71,72
Several studies have reported alterations in the immune cells of patients with ITP before and after TPO-RA treatment, but the specific mechanisms of immune regulation remain unclear39,40,73 and may include (1) unknown direct effects of TPO-RAs on immune cells via c-Mpl–dependent or -independent mechanisms and/or (2) immune modulation after increased platelet mass.40 Based on a dose escalation study (0.1, 1, or 10 μg/kg) using naïve WT Bagg Albino cleared mice, Kapur et al adopted 10 μg/kg weekly of murine NPlate to treat ITP mice.73 In this study, the concentration of Romi (0, 10, 30, 50, and 100 μg/kg) was determined using passive-ITP mice (supplemental Figure 4) as 30 μg/kg every 3 days.39 Consistent with previous reports, the TPO-RAs synchronously increased the number of platelets and level of serum TGF-β1.41,42 The fact that megakaryocytes can transfer antigen-MHC class I molecules to proplatelets suggests that platelets acquire immune properties from their parent cells and further act as immune mediators in the periphery, such as inducing CD8+ T-cell suppression.3,74 This provides a plausible explanation for the underlying mechanism of T-cell modulation elicited by thrombopoietic agents. The decline in MDSC levels in the convalescent phase of severe COVID-19 was associated with a reduction in TGF-β and increase in inflammatory cytokine levels in the plasma samples of patients.75 In this study, we found that platelets could enhance the expansion and immunosuppressive function of MDSCs, and TGF-β/Smad pathway blockade could counteract this modulatory function, indicating the role of platelet-derived TGF-β1 in immune homeostasis. Previous report confirmed the efficacy of WT platelet transfusion in ITP mice.76 Our study further suggested that the rescuing effect of TGF-β1 knockout platelets was impaired, indicating the indispensable role of TGF-β1 in the immune modulatory functions of platelets. This also provides strong evidence that TPO-RAs exert immunomodulatory roles through platelet-derived TGF-β1 as well as a plausible mechanistic explanation for alterations in other immune cells in patients with ITP.31,37-39 Moreover, TGF-β1 has also been shown to inhibit megakaryopoiesis.77,78 These findings suggest that TGF-β1 not only mitigates immune-mediated platelet destruction but also inhibits megakaryopoiesis after the initial induction of platelet production, which helps to restore platelet homeostasis in patients responding to TPO-RAs.
There is growing evidence that TPO-RAs can trigger a sustained response in some patients after tapering and discontinuation. Owing to their expensive price and thrombotic risk, further exploration of an optimal TPO-RA tapering scheme is required against ITP. However, there is insufficient evidence to standardize the suspension of TPO-RAs. Montaño suggested that tapering and discontinuation be performed after 6 consecutive months with platelet counts ≥50 × 109/L, whereas Roy suggested when patient symptoms subside at the European Hematology Association conference. The major goal in ITP management is to maintain a safe platelet level, rather than a normal platelet count, and patients with a platelet count >30 × 109/L generally require no treatment unless they are under specific bleeding risks.24,33,34,79 Our retrospective findings revealed that patients achieving a complete platelet response showed superior long-term outcomes compared with those who only reached a PR. We also demonstrated that platelet recovery contributed to immune homeostasis at least partially through platelet-derived TGF-β1, which provides strong theoretical support for the long-term response of patients after tapering and discontinuation of TPO-RAs.
In conclusion, platelet-derived TGF-β1 enhanced the proliferation and immunosuppressive function of MDSCs via the TGF-β1–Smad2/3 pathway. In addition, TPO-RAs can regulate immune homeostasis both indirectly and directly, which explains the long-term treatment response of patients with ITP after TPO-RA withdrawal. Our results indicated that platelet recovery not only serves as an end point of treatment response but also paves the way for immune homeostasis in immune-mediated thrombocytopenia, indicating a common mechanism of ITP-specific therapies.
Acknowledgments
The authors thank Zhangyin Ming (Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China) for generously providing the Pf4cre−Tgfb1fl/fl and Pf4cre+Tgfb1fl/fl mice. They thank the Translational Medicine Core Facility of Shandong University for consultation and instrument availability that supported this work. They thank Editage for English language editing.
This work was supported by grants from the National Natural Science Foundation of China (82322003 [Y.H.], 82270131 [Y.H.], and 82370131 [M.H.]); the National Key R&D Program of China (2023YFC2507800 [J.P.]); Natural Science Foundation of Distinguished Young Scholars of Shandong Province (ZR2021JQ28 [Y.H.]); Key R&D Program of Shandong Province, China (2021LCZX05 [M.H.]); the Young Taishan Scholar Foundation of Shandong Province (tsqn201909175 [Y.H.]); the Natural Science Foundation of Shandong Province (ZR2023QH511 [J.P.]); and the Outstanding Young and Middle-aged Scholar of Shandong University and funded by the ECCM Program of Clinical Research Center of Shandong University (2021SDUCRCB009 [M.H.]).
Authorship
Contribution: Y.H. and L.W. conceived the study and designed the experiments; L.W., H.W., X.N., L.S., and W.W. performed the experiments and analyzed the data; Y.L., Y.X., J.X., and R.W. assisted with animal experiments; M.Z., S.H., and P.Z. assisted with in vitro experiments; L.W. and Y.H. wrote the manuscript; M.H. and J.P. edited the paper; Y.H. and M.H. funded the research; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yu Hou, Department of Hematology, Qilu Hospital of Shandong University, Shandong University, 107 Wenhuaxi Rd, Jinan, Shandong, 250012, China; email: houyu2009@sina.com.
References
Author notes
Data are available on request from the corresponding author, Yu Hou (houyu2009@sina.com).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Platelet-associated TGF-β1 stimulated the function and modulated the transcriptome profiles of MDSCs in active-ITP mice. (A) Platelet counts among the control, Romi, and Romi + TGF-βi groups differed significantly on day 28. (B) Percentage of MDSCs in bone marrow nucleated cells evaluated by FACS. (C) Relative MFI of Arg-1, PD-L1, PD-L2, and iNOS expressed by MDSC from active-ITP mice treated with TGF-βi, Romi, Romi plus TGF-βi, or none. Relative MFI is the fold of average MFI in controls. (D) Serum TGF-β1 level detected by ELISA. Pearson correlation analysis of (E) platelet counts with serum TGF-β1 level and (F) serum TGF-β1 level with MDSC percentage. (G) Gene ontology (GO) enrichment score [−log10(P value)] analysis of DEGs identified between Romi-treated and control mice. (H) KEGG enrichment analysis based on the expression of Romi-altered genes. DEGs, differentially expressed genes; FACS, fluorescence activated cell sorter; KEGG, Kyoto Encyclopedia of Genes and Genomes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/1/10.1182_blood.2023022738/3/m_blood_bld-2023-022738-gr2.jpeg?Expires=1767698362&Signature=cM2T4tKOImY837laLcKOxlB28dxHFLj4r2m4XMevlpSe6kj1D4cI2zC72HLDpzEmdgJHSOqFwf-OwZXKDE9-K6XH1mxV664FYtL8UqTBIFk5dKXharzDvq0ciZumQyItHKVCAZ8AIbbEEU4tkXxpgn8lP9gZWdbv993KkUtuHzZKuXB~LWhiaYbIpdmIBFOFSE9Q~ew0KeV6tFyaLQpCqLLI2~oFaQwRrg4HTWo2NWfKCGqO4d5WukCA0-TUjmAxMKwkl39y-zMI0wm-Gpi37Fb3lMwFTTTqrfXXvAJQZelgDcciFcrBjpjfjrEmewLul82kUG-5uC3ECGfg8NgJog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal