Visual Abstract
Thrombocytopenia is a common hematologic abnormality in pregnancy, encountered in ∼10% of pregnancies. There are many possible causes, ranging from benign conditions that do not require intervention to life-threatening disorders necessitating urgent recognition and treatment. Although thrombocytopenia may be an inherited condition or predate pregnancy, most commonly it is a new diagnosis. Identifying the responsible mechanism and predicting its course is made challenging by the tremendous overlap of clinical features and laboratory data between normal pregnancy and the many potential causes of thrombocytopenia. Multidisciplinary collaboration between hematology, obstetrics, and anesthesia and shared decision-making with the involved patient is encouraged to enhance diagnostic clarity and develop an optimized treatment regimen, with careful consideration of management of labor and delivery and the potential fetal impact of maternal thrombocytopenia and any proposed therapeutic intervention. In this review, we outline a diagnostic approach to pregnant patients with thrombocytopenia, highlighting the subtle differences in presentation, physical examination, clinical course, and laboratory abnormalities that can be applied to focus the differential. Four clinical scenarios are presented to highlight the pathophysiology and treatment of the most common causes of thrombocytopenia in pregnancy: gestational thrombocytopenia, preeclampsia, and immune thrombocytopenia.
Introduction
Thrombocytopenia, defined as a platelet count <150 × 109/L, is common in pregnancy, affecting 7% to 11% of all pregnancies, with most occurrences in the third trimester.1-4 The differential diagnosis is broad, ranging from benign processes in which observation alone is appropriate to conditions that can threaten both mother and fetus and require timely intervention. Accurately identifying the cause is the critical element of management but can be challenging given tremendous overlap in the symptoms and laboratory data between normal pregnancy physiology and pathologic thrombocytopenias, particularly at onset. Confidence in the diagnosis often comes only with time or depending on response to initial therapies, because few processes are definitively identified with a singular confirmatory test.
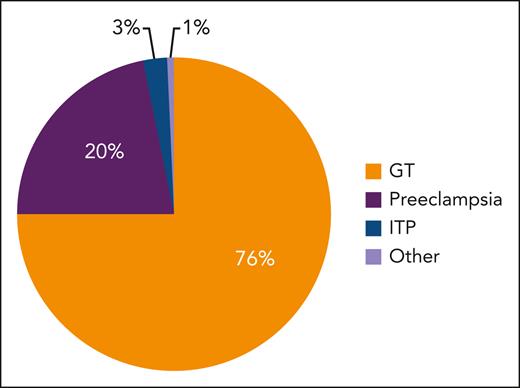
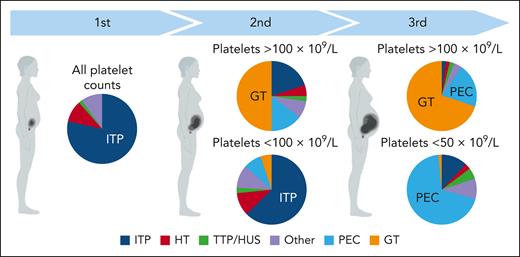
Figure 1 displays the relative proportion of causes of thrombocytopenia in pregnancy, with Figure 2 depicting the most likely cause based on trimester of onset and severity, which are the most important features to direct an appropriate differential. Table 1 highlights the salient features of presentation and management of commonly encountered or sinister thrombocytopenic processes in pregnancy. Among the many potential causes, this review focuses on the 3 most common: gestational thrombocytopenia (GT), preeclampsia, and immune thrombocytopenia (ITP).
Relative proportion of causes of thrombocytopenia in pregnancy. Figure modified from Fogerty.5
Relative proportion of causes of thrombocytopenia in pregnancy. Figure modified from Fogerty.5
Causes of thrombocytopenia by trimester and severity. HT, hereditary thrombocytopenia; PEC, preeclampsia; TTP/HUS, thrombotic thrombocytopenic purpura/hemolytic uremic syndrome. The figure was modified from Cines and Levine.6
Causes of thrombocytopenia by trimester and severity. HT, hereditary thrombocytopenia; PEC, preeclampsia; TTP/HUS, thrombotic thrombocytopenic purpura/hemolytic uremic syndrome. The figure was modified from Cines and Levine.6
Characteristic features and management of specific thrombocytopenias in pregnancy
| . | Presenting stage of pregnancy . | Degree of thrombocytopenia . | Associated clinical features . | Primary treatment . | |
|---|---|---|---|---|---|
| GT | Late | Mild | Normal physical examination and laboratory profile | Observation | |
| ITP | Any trimester or PP | Mild to severe | Bleeding when thrombocytopenia is significant Consider acute or chronic infections as potential triggers, or syndrome with other autoimmune diseases | First-line therapy Corticosteroids and IVIG Second-line therapies Azathioprine Cyclosporine Rituximab Splenectomy (rarely performed) TPO mimetics (off-label use) | |
| TMAs | PEC/HELLP | Must be 20+ wk Typically third trimester Possible early PP | Mild/moderate | Transaminitis Normal to severe Renal injury Rare/mild Coagulopathy Rare/mild Neurologic Headache common Seizure/CVA rare | Fetal delivery In addition to delivery Aggressive HTN control Aspirin Magnesium ∗Betamethasone as needed for fetal lung maturity |
| AFLDP | Late in pregnancy or immediately PP | Mild/moderate | Transaminitis Severe (RUQ pain, nausea, and vomiting are common) Renal injury Mild/moderate Coagulopathy Often severe Neurologic Encephalopathy | Fetal delivery Unlikely to fully resolve liver injury Liver transplantation may be required Assess for fatty acid oxidation enzyme defects | |
| TTP | Any trimester or PP | Severe | Transaminitis Rare Renal injury Mild or absent Coagulopathy Rare Neurologic Transient focal defects possible, progressive without treatment | Plasma exchange for acquired TTP Fresh frozen plasma simple transfusion for congenital TTP | |
| cm-HUS | Typically PP Evenly distributed in trimesters when ante partum | Moderate | Transaminitis Rare/mild Renal injury Severe Coagulopathy Rare/mild Neurologic Rare | Eculizumab Renal replacement therapy may be required | |
| CAPS | Any trimester or PP | Mild to severe | Transaminitis Rare/mild Renal injury Rare to severe Coagulopathy Common Neurologic Increased risk for CVA | Therapeutic anticoagulation | |
| . | Presenting stage of pregnancy . | Degree of thrombocytopenia . | Associated clinical features . | Primary treatment . | |
|---|---|---|---|---|---|
| GT | Late | Mild | Normal physical examination and laboratory profile | Observation | |
| ITP | Any trimester or PP | Mild to severe | Bleeding when thrombocytopenia is significant Consider acute or chronic infections as potential triggers, or syndrome with other autoimmune diseases | First-line therapy Corticosteroids and IVIG Second-line therapies Azathioprine Cyclosporine Rituximab Splenectomy (rarely performed) TPO mimetics (off-label use) | |
| TMAs | PEC/HELLP | Must be 20+ wk Typically third trimester Possible early PP | Mild/moderate | Transaminitis Normal to severe Renal injury Rare/mild Coagulopathy Rare/mild Neurologic Headache common Seizure/CVA rare | Fetal delivery In addition to delivery Aggressive HTN control Aspirin Magnesium ∗Betamethasone as needed for fetal lung maturity |
| AFLDP | Late in pregnancy or immediately PP | Mild/moderate | Transaminitis Severe (RUQ pain, nausea, and vomiting are common) Renal injury Mild/moderate Coagulopathy Often severe Neurologic Encephalopathy | Fetal delivery Unlikely to fully resolve liver injury Liver transplantation may be required Assess for fatty acid oxidation enzyme defects | |
| TTP | Any trimester or PP | Severe | Transaminitis Rare Renal injury Mild or absent Coagulopathy Rare Neurologic Transient focal defects possible, progressive without treatment | Plasma exchange for acquired TTP Fresh frozen plasma simple transfusion for congenital TTP | |
| cm-HUS | Typically PP Evenly distributed in trimesters when ante partum | Moderate | Transaminitis Rare/mild Renal injury Severe Coagulopathy Rare/mild Neurologic Rare | Eculizumab Renal replacement therapy may be required | |
| CAPS | Any trimester or PP | Mild to severe | Transaminitis Rare/mild Renal injury Rare to severe Coagulopathy Common Neurologic Increased risk for CVA | Therapeutic anticoagulation | |
AFLDP, acute fatty liver disease of pregnancy; CAPS, catastrophic antiphospholipid antibody syndrome; cm-HUS, complement-mediated hemolytic uremic syndrome; CVA, cerebrovascular accident; HTN, hypertension; PEC/HELLP, preeclampsia/hemolysis, elevated liver enzymes and low platelets; PP, postpartum; RUQ, right upper quadrant.
General pathophysiologic aspects of thrombocytopenia during pregnancy
GT
GT accounts for 70% to 75% of thrombocytopenia encountered in pregnancy, occurring in ∼10% of all pregnancies,1,2,4 thus complicating >9 million pregnancies worldwide each year.7,8 Despite its frequency, the precise mechanism of thrombocytopenia is not known. As such, GT remains a diagnosis of exclusion that can only be confirmed after a postpartum complete blood count (CBC) reveals recovery to a normal platelet count. The salient features of GT are listed in Table 2.
Features of GT
| History of thrombocytopenia in prior pregnancies that resolved after delivery |
| Later onset of thrombocytopenia (usually late second to early third trimesters) |
| Overall mild thrombocytopenia (platelet count expected to remain above >70 × 109/L) |
| Absence of fetal thrombocytopenia or bleeding9 |
| Spontaneous and rapid recovery to normal platelet count postpartum |
| Recurrence in future pregnancies with similar course and platelet nadirs10,11 |
| History of thrombocytopenia in prior pregnancies that resolved after delivery |
| Later onset of thrombocytopenia (usually late second to early third trimesters) |
| Overall mild thrombocytopenia (platelet count expected to remain above >70 × 109/L) |
| Absence of fetal thrombocytopenia or bleeding9 |
| Spontaneous and rapid recovery to normal platelet count postpartum |
| Recurrence in future pregnancies with similar course and platelet nadirs10,11 |
Numerous hypotheses for the mechanism of GT have been proposed, including hemodilution, increased von Willebrand factor (VWF), insufficient thrombopoietin (TPO) response, or reduced ADAMTS13 activity levels, but none provide complete explanation. Although there is a physiologic increase in VWF production in all pregnancies,12-14 this quantitative change alone is not likely responsible for GT, which occurs in only 10% of pregnancies. ADAMTS13 levels decrease progressively in pregnancy, starting at about week 12 to 16 through the early postnatal period,15 but without correlation to platelet count or identified association with GT. Women with GT had a higher TPO level (187 pg/mL) than that of pregnant women with normal platelet counts (65 pg/mL), nonpregnant women with ITP (88 pg/mL), and nonpregnant controls (37 pg/mL), but this was far less than the TPO level of pregnant patients with ITP (1283 pg/mL), even when the respective platelet counts overlapped.16 Although the sensitivity/specificity of this test have not yet been evaluated, TPO levels may help distinguish GT from ITP but do not explain the mechanism of GT.
An investigation into potential mechanisms of GT suggests a unique maternal physiology. In a case control study of >3500 pregnancies17 in which 12% of cases were diagnosed with GT, platelet counts in women with GT declined 31.8%, which was significantly greater than that of controls with an 18.3% decrease. There was also no difference in maternal weight, weight gain, or hemoglobin (Hb) decline between women with GT and controls, arguing against dilution as the cause of GT. In addition, although mean platelet volumes (MPVs) rose in both groups throughout gestation, most prominently in the third trimester, women with GT had a statistically greater increase in MPV during pregnancy and at each trimester. An elevated MPV suggests increased platelet turnover.18,19
Onset of thrombocytopenia later in pregnancy, significant increase in MPV compared with normal pregnancies, and rapid recovery post partum suggest that women susceptible to GT may unmask an enhanced platelet turnover in the presence of the uniquely high shear blood flow of the third trimester utero-placental circuit. That GT will recur in future pregnancies10,11 suggests that the responsible mechanism may be genetically driven. Prior publications have demonstrated that some genetic VWF polymorphisms can result in enhanced platelet adhesion only under conditions of high shear flow.14,20,21
Although observation alone is appropriate for women with GT, its management is a frequently encountered clinical predicament, particularly when the GT diagnosis is uncertain. There are no randomized data defining a safe platelet threshold for epidural anesthesia, which can lead to variability between institutions in accepted platelet targets and may drive unnecessary “treatment” or denial of epidural anesthesia when an arbitrary target is strictly enforced. Several retrospective studies have shown no complications associated with regional anesthesia to parturients with platelets <100 × 109/L.7,22-24 The overall risk of epidural hematoma with epidural anesthesia is very low, estimated at 1/150 000 in the general population,25 and reported as 0.2 to 3.7 per 100 000 in obstetric cases.26 The interdisciplinary consensus statement of the Society of Obstetric Anesthesia and Perinatology concluded via a modified Delphi process that the risk of spinal epidural hematoma is likely to be very low with platelets >70 × 109/L in women with GT, ITP, or hypertensive disorders of pregnancy in the absence of additional risk factors.27 We encourage regular dialogue between multidisciplinary providers to develop guidelines that incorporate these data and revisit any protocols that may have been established by custom or uncertainty.
ITP
ITP is defined as a platelet count <100 × 109/L in the absence of other explanations or cytopenias.28 ITP is diagnosed in <0.1% of all pregnancies4 and occurs at any time during pregnancy. It is the most common cause of thrombocytopenia in the first and early second trimesters.29 Although many women experience thrombocytopenia (even ITP) before pregnancy, for 30% this is the first diagnosis of ITP.30 Few resolve fully after delivery.31
ITP is due to antiplatelet antibodies and lymphocytes that increase platelet destruction as well as inhibit the production of platelets from megakaryocytes.32,33 Little is known about differences in ITP “secondary” to pregnancy compared with that in nonpregnant patients, but it has been speculated that the normal shift from Th2 cytokines (affecting cell–mediated immunity) toward Th1 cytokines (favoring humoral immunity) late in gestation might be abnormal and initiate autoimmune processes.34 In general, 80% of nonpregnant patients with ITP have an antiplatelet antibody targeting glycoproteins IIb/IIIa, Ib/IX, and Ia/IIa35 (all of which can also cross the placenta). Antibody-coated platelets then bind to Fcγ receptors on macrophages, usually in the spleen, resulting in their increased clearance. The expected compensatory rise in platelet production from bone marrow megakaryocytes is often blunted by the very same antibodies or lymphocytes.36-38
In accordance with current guidelines,39,40 during the first 8 months of pregnancy, we advocate treatment when the platelet count falls below 20 × 109/L or if the patient experiences bleeding. We increase the intensity of therapy approaching delivery as needed for a target platelet count >70 × 109/L to allow for epidural anesthesia. In those women eschewing epidural anesthesia, we target a platelet count >50 × 109/L, recognizing the increased hemostatic demand of labor and delivery.40,41
Treatment of the pregnant patients with ITP differs from that of the nonpregnant patient only in the exclusion of medications regarded as toxic to the fetus or insufficiently studied for safety. The general treatment approach is either to decrease the rate of platelet destruction, increase the rate of platelet production, or a combination of both.39,40 First-line therapy in pregnancy is corticosteroids and IV immunoglobulin (IVIG). In a retrospective study of 137 pregnancies, there was no significant difference in maternal platelet count response with treatment of IVIG or corticosteroids (39% vs 38%).42 Although there was suggestion of reduced efficacy of corticosteroids and IVIG in pregnant patients with ITP compared with nonpregnant patients with ITP,43 this has not been confirmed by others44,45 and is not our experience. The choice of corticosteroids is limited to prednisone, which appears only in small amounts in cord blood compared with dexamethasone, which reaches higher fetal concentrations due to less efficient placental metabolism.46 The onset of prednisone effect is slightly slower than with dexamethasone but is manifest in most patients within 2 to 3 days.47
Prednisone can usually be deferred until about week 35 or 36 in preparation for delivery to minimize exposure and associated maternal side effects, which can include aggravated gestational diabetes, hypertension, mood lability, or insomnia.48 Once started, prednisone is continued until spontaneous delivery, thereafter tapered if the platelet count is >20 × 109/L. Some consider a brief trial of prednisone 40 mg daily for 5 days at about week 30 to 32 to assess response, which may guide management for labor and delivery, because steroid response is often consistent in an individual patient. This practice is usually reserved for more significant thrombocytopenia, because failed response to prednisone started at week 35 or 36 still provides time to add IVIG in most patients.
IVIG has many therapeutic effects that mitigate thrombocytopenia. Total doses of 0.4 to 2 g/kg can be administered in divided doses over 1 to 5 days.49,50 In the nonemergency patient, we prefer an IVIG dose of 0.4 g/kg every 1 to 5 days, which can be infused thereafter as needed (usually every 2 to 3 weeks) to maintain adequate platelet counts; higher IVIG doses often cause headache, necessitating concern over central nervous system hemorrhage and potential need for imaging. Most patients do not require significant treatment during the first 8 months of pregnancy, but intermittent pulses of corticosteroids or IVIG are often effective if needed.
Treatment is more challenging in the refractory patient with platelet counts <20 × 109/L. Second-line medications include azathioprine, cyclosporine, and rituximab. Each has a response rate of 40% to 50%, but onset of response is usually several weeks39 and thus unlikely to be effective for delivery management if started after trials of corticosteroids and IVIG. Splenectomy and anti-RhD immunoglobulin are rarely used. Platelet transfusion is restricted to management of acute bleeding because it is only transiently beneficial and thus not appropriate management for epidural anesthesia when a sustained platelet response from the time of placement until 2 hours after catheter removal is indicated.
The great success of recombinant human TPO (rhTPO) and the TPO receptor agonists (TPO-RA) in ITP51-53 may carry over to the pregnant patient, but data remain scant. Only 1, rhTPO, available in China, does not cross the placenta. In the only prospective study of TPO agents in pregnant patients,54 31 women with platelets <30 × 109/L near delivery who had no response to corticosteroids and IVIG were treated daily for 14 days with rhTPO; 23 (75%) raised their platelet count >30 × 109/L, and 10 developed platelet counts >100 × 109/L. There were no bleeding events, and adverse events were minimal. There were no observed effects on the neonates.
All TPO-RAs (romiplostim, eltrombopag, and avatrombopag) cross the placenta. Despite evidence of fetal effects in preclinical animal toxicology studies only at levels much higher than those clinically used,55 these agents are not approved for pregnant patients, and we do not advocate their routine use. A retrospective analysis of the use of TPO-RAs in the third trimester during 17 pregnancies (8 women managed with eltrombopag and 7 women with romiplostim) showed that baseline platelet counts of 10 × 109/L rose to a median of 94 × 109/L with no bleeding at delivery. No serious adverse events or clinically symptomatic thromboembolic events were reported, but 2 patients reported mild headache.56 Median TPO-RA exposure was limited to 4.4 weeks. Six of the neonates (43%) with available platelets counts had thrombocytopenia, and 1 had a platelet count of 558 × 109/L. There were no fetal losses or birth defects attributable to TPO-RA; 29% were delivered preterm (34-38 weeks).
Our approach has been to restrict the use of TPO-RAs only for patients who have an inadequate response to corticosteroids and IVIG, for whom there is no time for other options (rituximab, cyclosporine, and azathioprine) to act, and only in the last trimester. If used, we prefer romiplostim, given its potentially fewer off target effects, for example, no iron chelation as is seen with eltrombopag.
Preeclampsia
Preeclampsia is diagnosed in about 3% to 4% of all pregnancies and is defined as maternal hypertension (blood pressure, 140/90 mm Hg) with proteinuria and/or end-organ dysfunction after 20 weeks.57 It may also occur in the early postpartum window. Preeclampsia is a thrombotic microangiopathy (TMA) and thus characterized by schistocytes present on the peripheral blood film. The pathogenesis includes both abnormal placentation and angiogenic imbalance,58 with excess secretion of the placentally derived antiangiogenic factor, soluble fms-like tyrosine kinase-1 (sFlt-1). sFlt-1 sequesters proangiogenic molecules vascular endothelial growth factor and placental growth factor. Increased sFlt-1/placental growth factor ratios are seen in preeclampsia, both at diagnosis and weeks before the onset of clinical symptoms,58-60 suggesting causality. Aspirin has consistently been shown to prevent or delay onset of preeclampsia.61,62 A Cochrane analysis of >36 000 women showed an 18% reduction.
A platelet count <150 × 109/L occurs in ∼20% of patients with preeclampsia,63 diagnosed as “severe” if the platelet count is <100 × 109/L. Microangiopathic endothelial injury and activation yield platelet and fibrin thrombi throughout the microvasculature, leading to accelerated platelet consumption. Serious maternal complications can include cerebral hemorrhage, hepatic rupture, renal failure, pulmonary edema, myocardial infarction, acute respiratory distress syndrome, seizure, and death.
Identifying the correct TMA is critical because management differs but can be difficult given overlap in clinical presentation and laboratory abnormality. As presented in Table 1, subtle differences in symptoms, timing of onset, severity of thrombocytopenia, and the most prominent end organ involved help discriminate between the possible causes. Thrombotic thrombocytopenic purpura (TTP) is the only TMA with a confirmatory laboratory test (ADAMTS13 <10%), but this test may not be readily available in some hospitals. Thus, the presumed diagnosis of a particular TMA may depend upon response to treatment.
The mainstay of treatment of the pregnancy-specific TMAs, preeclampsia, HELLP (hemolysis, elevated liver enzymes and low platelets), and acute fatty liver disease of pregnancy is fetal delivery. The appropriate timing of delivery is best directed by the obstetric team who factor the mother’s clinical status, gestational week, and fetal maturity. TTP, complement-mediated hemolytic uremic syndrome, and catastrophic antiphospholipid antibody syndrome in pregnancy are treated the same as in nonpregnant patients and do not mandate fetal delivery.
General approach to thrombocytopenia in pregnancy
Our diagnostic approach is presented in Table 3. The initial determination is always whether urgent therapy is indicated for clinically significant bleeding, thrombosis, concern for end-organ damage or imminent delivery. The next branch point is to distinguish isolated thrombocytopenia from syndromes involving other cytopenias or end-organ damage, with particular attention to peripheral smear review to assess for TMA, which always requires active management. Onset and severity of thrombocytopenia (Figure 2) further focus the differential, guiding the appropriate supporting laboratory data and physical examinations.
Diagnostic approach to thrombocytopenia in pregnancy
| 1. History | Bleeding symptoms Infectious symptoms Prior platelet counts: pregnant and nonpregnant Family history of thrombocytopenia Autoimmune disorders Medications Headache | ||
| 2. Physical examination | Bleeding: bruising, petechiae, purpura, oral mucosal blood blisters, conjunctival hemorrhages Blood pressure Lymphadenopathy, hepatomegaly, splenomegaly Abdominal tenderness Lower extremity edema Rashes or synovitis | ||
| 3. Timing | Categorize by onset and severity of thrombocytopenia (Figure 2) | ||
| 4. Peripheral smear examination | Platelet clumping (Pseudothrombocytopenia) | Normal morphology of all 3 cell lines  | Schistocytes (TMA) |
| Laboratory assessment | None indicated | Investigate potential contributors Infection Screen for HIV, HBV, HCV, H pylori Thorough assessment of any current infectious symptoms Liver injury LFTs Coagulation profile Autoimmune processes APLA panel ANA  | Hemolytic panel LDH Reticulocyte count Haptoglobin LFTs (bilirubin) Urinalysis Renal function ADAMTS13 activity Coagulation profile  |
| Management | Standard obstetric care | Trend platelet count Reserve treatment for clinically significant processes/bleeding or as needed to prepare for delivery | Initiate therapy for the most likely TMA Revisit the diagnosis and escalate or redirect therapies pending clinical course |
| 1. History | Bleeding symptoms Infectious symptoms Prior platelet counts: pregnant and nonpregnant Family history of thrombocytopenia Autoimmune disorders Medications Headache | ||
| 2. Physical examination | Bleeding: bruising, petechiae, purpura, oral mucosal blood blisters, conjunctival hemorrhages Blood pressure Lymphadenopathy, hepatomegaly, splenomegaly Abdominal tenderness Lower extremity edema Rashes or synovitis | ||
| 3. Timing | Categorize by onset and severity of thrombocytopenia (Figure 2) | ||
| 4. Peripheral smear examination | Platelet clumping (Pseudothrombocytopenia) | Normal morphology of all 3 cell lines  | Schistocytes (TMA) |
| Laboratory assessment | None indicated | Investigate potential contributors Infection Screen for HIV, HBV, HCV, H pylori Thorough assessment of any current infectious symptoms Liver injury LFTs Coagulation profile Autoimmune processes APLA panel ANA  | Hemolytic panel LDH Reticulocyte count Haptoglobin LFTs (bilirubin) Urinalysis Renal function ADAMTS13 activity Coagulation profile  |
| Management | Standard obstetric care | Trend platelet count Reserve treatment for clinically significant processes/bleeding or as needed to prepare for delivery | Initiate therapy for the most likely TMA Revisit the diagnosis and escalate or redirect therapies pending clinical course |
ANA, antinuclear antibody; APLA, antiphospholipid antibody antibodies; LDH, lactate dehydrogenase.
A thorough history is important, particularly relevant family history, remote CBCs for baseline platelet count, and available data from prior pregnancies. The presence of other prior or concurrent autoimmune conditions, fevers, infectious symptoms, new drug, or toxin exposures should be reviewed.
Cases
Patient 1
A 27-year-old female (G3P2002) at 34 weeks is referred for a platelet count of 108 × 109/L. She feels well. She is unsure of platelet nadirs in her prior pregnancies but recalls mention of thrombocytopenia. She received neuraxial anesthesia and delivered vaginally without complications. On physical examination, she is well appearing, afebrile, and normotensive. There is no leg edema, rash, purpura, petechiae, or joint inflammation. Other laboratory tests include a white blood cell (WBC) count of 7.6 μL and an Hb level of 11.4 g/dL; negative HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV) tests; and normal liver function tests (LFTs), basic metabolic panel, prothrombin time (PT), and partial thromboplastin time (PTT).
Discussion
As depicted in Figure 2, the most likely diagnosis is GT based on presentation in the third trimester and platelets >100 × 109/L. This suspicion is strengthened by reports of similar trends in prior pregnancies. Smear review confirms no schistocytes, and she is normotensive, arguing against TMAs and preeclampsia. ITP is the leading alternative diagnosis, but with no confirmatory test for either GT or ITP, the diagnosis will be chiefly informed by platelet trend over time. We plan to repeat the CBC in 2 weeks.
At 36 weeks, the platelet count is 96 × 109/L. She remains asymptomatic and normotensive without bleeding, bruising, or headache. In discussion with anesthesia and obstetrics, a threshold platelet count of 70 × 109/L is agreed to for delivery. We follow weekly CBCs, and she presents in labor at 39 weeks with a platelet count of 78 × 109/L. She delivers vaginally with epidural anesthesia with no bleeding complications. The neonate has a normal platelet count. Her platelet count starts to rebound on postpartum day 1, and she is discharged. At her 6-week postpartum visit, her platelet count is 187 × 109/L. A diagnosis of GT is now confirmed. We expect a similar pattern of thrombocytopenia in future pregnancies. CBC monitoring between pregnancies is not indicated, barring any new clinical developments, and standard CBC monitoring will be appropriate in future pregnancies.
Patient 2
A 29-year-old female (G1P0) at 16 weeks is referred for a platelet count of 58 × 109/L. She denies any bleeding or bruising. She had a viral respiratory process 4 weeks ago, now fully resolved. Her only medication is a prenatal vitamin. She has no known history of thrombocytopenia and a prior CBC from college was normal (platelets, 216 × 109/L). There is no family history of thrombocytopenia or bleeding disorders. She is well appearing, afebrile, and normotensive. There is no palpable hepatosplenomegaly or lymphadenopathy, leg edema, rash, purpura, petechiae or bruising. Other laboratory results included: WBC 4.6 × 109/L, Hb 12.8 g/dL; negative HIV, HBV, and HCV tests; and normal LFTs, basic metabolic panel, PT, and PTT.
Discussion
At 16 weeks with a platelet count <100 × 109/L, her most likely diagnosis is ITP. Her recent viral illness is of note because infections can decrease platelet counts. She has no prior history of autoimmune conditions and tests negative for antinuclear antibodies, antiphospholipid antibodies, and Helicobacter pylori antibody. Peripheral blood smear shows only thrombocytopenia, large platelets, and no schistocytes. Her prior documented normal platelet count argues against inherited thrombocytopenia.
Many pregnant patients with ITP, such as this one, do not require treatment and tolerate platelet counts as low as 15 × 109/L to 20 × 109/L during pregnancy with minimal bleeding. We discuss with her the potential use of steroids or IVIG if her platelet count falls below 20 × 109/L or to manage bleeding and the likely need for therapy closer to delivery if thrombocytopenia persists. We monitor her CBC every 4 weeks during the first 2 trimesters and increase the frequency to every 1 to 2 weeks in the third trimester or more frequently for any clinically significant bleeding or a substantial decline in platelet count.
Her platelet counts were 55 × 109/L to 69 × 109/L between weeks 20 and 30 and are now 48 × 109/L at week 33. She has no bleeding or hypertension. No schistocytes are evident on serial review of her blood smears.
She desires epidural anesthesia, and multidisciplinary discussion sets a threshold platelet count of 70 × 109/L. In preparation, she starts prednisone 60 mg daily at week 36. Her platelet count is 103 × 109/L after 7 days of treatment, and prednisone is continued. At 39 weeks, she presents in spontaneous labor, epidural is placed uneventfully, and she delivers vaginally without complications. Her neonate has a normal platelet count. Corticosteroids are tapered off over 10 days post partum. Her platelets decrease to ∼80 × 109/L and persist in this range for the next 12 months. She continues without any bleeding complications or need for active therapy. She is diagnosed with chronic ITP.
Patient 3
A 28-year-old female (G1P0) at 8 weeks has a platelet count of 11 × 109/L, an MPV of 14.5 fL, and otherwise normal CBC. She has a known history of ITP, previously managed with eltrombopag, which she discontinued about 3 months before pregnancy. She is alert, afebrile, and normotensive. She has scattered bruising to all 4 limbs and streaks of petechiae on her left shoulder, on which she carries her backpack, but no oral or nasal bleeding and no headaches. She had light vaginal bleeding for 5 days around week 6, now resolved.
Discussion
This case presents a management but not a diagnostic challenge. ITP is the most likely cause of severe isolated thrombocytopenia in the first trimester, and an ITP diagnosis predates her pregnancy. Although she has no clinically significant bleeding, we generally prefer a platelet count >20 × 109/L if possible to provide a greater margin of safety. We anticipate difficulty achieving a satisfactory platelet count for delivery and recommend early referral to anesthesia.
We prefer avoiding corticosteroids in the first trimester given reports, albeit few, suggesting increased risk of fetal cleft palate and urogenital congenital anomalies.64-66 After a single dose of IVIG 0.4 g/kg, her platelet count is 31 × 109/L. We continue IVIG 0.4 g/kg every 2 weeks between weeks 8 and 30, and her platelets remain between 15 × 109/L and 20 × 109/L with no significant adverse events. Starting week 30, however, her platelets remain <15 × 109/L despite adding prednisone and escalating IVIG dosing. We discuss with her the risks of delivery with a platelet count <20 × 109/L and the possible risks and benefits of TPO-RA, emphasizing the limited data on safety and efficacy in pregnancy. In a shared decision with the patient, obstetrics, anesthesia, and hematology, we start romiplostim 5 μg/kg at week 34. Her platelet count at week 35 is 48 × 109/L, and we increase romiplostim to 6 μg/kg weekly. At 36 weeks, she presents with premature rupture of membranes and a platelet count of 71 × 109/L. She receives epidural anesthesia without complications. Care is taken to avoid fetal instrumentation during delivery, given the risk of fetal thrombocytopenia, which carries a reported incidence of 8.9% to 14.7% in neonates born to mothers with ITP, with fetal intracranial hemorrhage occurring in 1.5% of cases.67 A healthy male baby with a platelet count of 62 × 109/L is delivered vaginally.
Patient 4
A 36-year-old female (G2P0010) at 33 weeks is referred for a platelet count of 113 × 109/L. Her WBC count is 12.3 × 109/L and Hb is 9.8 g/dL. Prior platelet counts in this pregnancy were normal. She reports no bleeding or bruising but has pedal edema, which worsens by the end of the day. She had 2 episodes of dull headache in the last week, which resolved after drinking water. She is afebrile without infectious symptoms. She is taking prenatal vitamins, iron supplements, and stool softener. On physical examination, she is well appearing. Her heart rate and blood pressure are 98 beats per minute and 136/82 mm Hg, respectively. She has trace nonpitting pedal edema bilaterally, without calf tenderness or palpable cord. Her abdomen is gravid without tenderness or palpable hepatosplenomegaly. There are no rashes, bruising, petechiae, or purpura. Other laboratory tests include normal aspartate transferase, alanine transaminase, PT, and PTT; alkaline phosphatase 121 U/L; creatinine 1.21 mg/dL; and negative HIV, HBV, and HCV tests.
Discussion
Although GT is the most common diagnosis for thrombocytopenia initially presenting in the third trimester with a platelet count >100 × 109/L, this patient also has anemia, mild hypertension, headaches, and a relative increase in creatinine. Her peripheral smear demonstrates schistocytes, suggesting a TMA. We send a hemolytic panel, including ADAMTS13 level. Her lactate dehydrogenase is elevated at 647 U/L, total bilirubin is 3.2 mg/dL (direct bilirubin 0.7 mg/dL), reticulocyte count is 5.9%, and haptoglobin is <10 mg/dL. Of the TMAs, preeclampsia is the prime suspect. TTP is less likely given that thrombocytopenia is mild, so, we do not recommend plasma exchange. Normal LFTs make HELLP and acute fatty liver disease of pregnancy unlikely.
In coordination with obstetrics, close follow-up is planned. Her repeat blood pressure 2 days later is 146/92 mm Hg, and urinalysis shows proteinuria. She is diagnosed with preeclampsia, admitted to obstetrics, and started on aspirin, hydralazine for blood pressure management, and magnesium therapy per protocol. Her neurologic examination remains nonfocal, but creatinine increases to 1.84 mg/dL, repeat platelet count is 83 × 109/L, and hypertension persists despite hydralazine. With a platelet count <100 × 109/L, she is classified as having preeclampsia with severe features. Now at 34 weeks, obstetrics prepares for delivery. She responds to induction of labor, receives an epidural with no bleeding complications, and delivers vaginally.
Her platelets continue to decline steadily, however, down to 44 × 109/L at 48 hours post partum. Her creatinine continues to rise, now 2.23 mg/dL. The previously sent ADAMTS13 activity is 49%.
Placental delivery is the cornerstone of management for preeclampsia. Although hypertension and laboratory abnormalities may not resolve immediately after delivery, progression of abnormalities is unusual. We now favor an alternative TMA diagnosis to preeclampsia. TTP is ruled out with an ADAMTS13 of 49%. In addition to progressive thrombocytopenia, her renal function is the predominant laboratory abnormality, making complement-mediated hemolytic uremic syndrome the most likely diagnosis. We treat her with eculizumab with excellent hematologic response. She does not require renal replacement therapy, and her creatinine and blood pressure normalize over the ensuing weeks. We recommend testing for mutations in complement genes. Eculizumab is stopped per international working group guidelines.70
Summary and conclusions
Thrombocytopenia is a common complication of pregnancy. Specific etiologies range from the most common and benign, such as GT, to those that are life threatening. A better understanding of the specific pathophysiology of GT is needed, so that a laboratory test might confirm this diagnosis early. The severity of thrombocytopenia, trimester of onset, and whether it is isolated vs part of a more complex presentation will guide appropriate next steps in data collection and management. TMAs merit special consideration and should be specifically sought by examining the peripheral smear for schistocytes.
The distinction between severe ITP and GT is usually readily apparent but is less certain in patients with platelet counts between 60 × 109/L and 90 × 109/L. In very small case studies, TPO level16 or the detection of antiplatelet antibodies in platelet eluate71 may help distinguish between these 2 disorders, but ultimately, the trend over time will be the prime distinguishing feature. Corticosteroids and IVIG are first-line therapies when ITP requires treatment in pregnancy. Further studies are needed to assess the safety of TPO-RA and potentially other newer treatments for ITP in pregnant patients before being considered for routine clinical use.
Platelet nadirs in GT and preeclampsia rarely drop to levels posing significant risk of spontaneous bleeding. Even more severe thrombocytopenia in ITP is generally well tolerated. Despite this low risk, many clinicians will assess thrombocytopenia as prohibitive for epidural anesthesia. Future goals should focus on developing clinical consensus that might lower minimum acceptable platelet counts for epidural anesthesia and promoting research that will better inform such recommendations.
Diagnosis and treatment of pregnant patients with thrombocytopenia should be collaborative between hematology, obstetrics, and anesthesia and revisited often with updated data. Treatment plans and therapeutic goals should be developed via multidisciplinary recommendation and shared decision-making with the patient.
Authorship
Contribution: D.J.K. and A.E.F. were involved in the conception/development of the manuscript and reviewed, revised, and approved the final version of the manuscript.
Conflict-of-interest disclosure: D.J.K. reports research for Alnylam, Biocryst, Novartis, Rigel Pharmaceuticals, Sanofi (Principia), Takeda (Bioverativ), and UCB; consulting fees from AIRx, Alexion (Syntimmune), Alnylam, Alpine ImmuneSciences, Amgen, Argenx, BioCryst, Bristol Myers Squibb, Caremark, Cellularity, Cellphire Therapeutics, Chugai, CRICO, Daiichi Sankyo, Dianthus Therapeutics, Electra Therapeutics, Fujifilm Diosynth Biotechnologies, Hemopure, Hengrui, Immunovant, Incyte, Inmagene Bio, Kezar Life Sciences, Kyowa-Kirin, Merck Sharp & Dohme, Momenta, Novartis, Nuvig Therapeutics, Pfizer, Platelet BioGenesis, Platelet Disorder Support Association, Principia Biopharma (Sanofi), Protagonist Therapeutics, Rigel Pharmaceuticals, Sanofi (Bioverativ), Sanofi (Genzyme), Sanofi, Sobi (Dova Pharmaceuticals), Takeda, UCB, UpToDate, and Zafgen; and stock ownership in Rubius Therapeutics. A.E.F. reports consulting fees from Amgen.
Correspondence: Annemarie E. Fogerty, Hematology Division, Massachusetts General Hospital, Harvard Medical School, Bartlett Hall 154, 55 Fruit St, Boston, MA 02114-2603; email: afogerty@mgb.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal