Bispecific antibodies directed against CD20 and CD3 have recently captivated the attention of lymphoma oncologists, challenging chimeric antigen receptor (CAR) T-cell therapy in terms of enthusiasm, yet also raising questions about predictive biomarkers and underlying mechanisms of resistance. In this issue of Blood, Schuster et al show that antigen escape, predominantly related to acquired mutations in MS4A1 (the gene encoding CD20), is a major cause of B-cell lymphoma progression after treatment with the CD20xCD3 bispecific antibody mosunetuzumab.1 Using tumor samples from the phase 1/2 GO29781 trial, which enrolled patients with a variety of relapsed/refractory B-cell lymphomas,2 the authors found loss of CD20 expression in 34% of biopsies collected at the time of disease progression after mosunetuzumab treatment.
Importantly, Schuster et al also observed no responses to mosunetuzumab in lymphomas with a low (<10%) baseline expression of CD20. A similar phenomenon has been reported for glofitamab,3 suggesting that a minimum antigen density is required for the activity of bispecific antibodies. Pending confirmatory data from trials of epcoritamab and odronextamab, the cutoff requirement for CD20 expression may distinguish bispecific antibodies from CAR T-cell therapy, which retain efficacy even with minimal target expression. Nevertheless, the frequent loss of CAR-binding CD19 epitopes observed in post-CAR T-cell relapses supports antigen escape as a common evasion mechanism in targeted immunotherapy. Clinicians increasingly need to play the game of catching up to sequential antigen loss when choosing among the available antibodies or antibody-drug conjugates targeting CD19, CD20, CD79b, or CD30, with the promise of more targets, like ROR1, CD22, CD25, and CD38. Repeated biopsies at every relapse are now necessary, and treatment on the mere basis of radiographic progression is inadequate when using targeted agents. Consistent, quantitative assessment of CD20 expression in clinical practice remains a challenge, with variable technical approaches (flow cytometry, single or duplex immunohistochemistry), tumor content, and specimen sources.
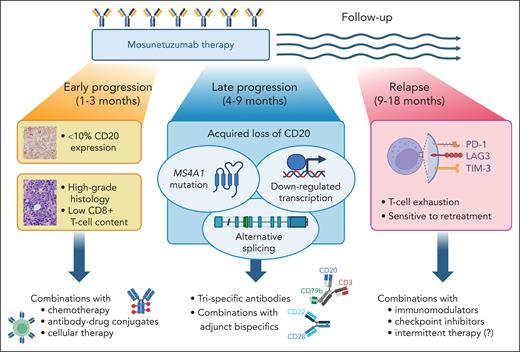
Apart from the baseline lack of CD20 expression, the GO29781 analysis reveals 3 types of progression events on mosunetuzumab, each with distinct timing, mechanism, and therapeutic implications (see figure). First, approximately half of patients experience immediate progression within weeks of starting treatment despite strong CD20 expression. Primary refractoriness to mosunetuzumab is more common in aggressive B-cell histologies.2 It hypothetically relates to a hostile microenvironment, which can be either devoid of cytotoxic T cells ("immune exclusion") or dominated by immunosuppressive elements (strong checkpoint molecule expression, overabundance of regulatory T cells and myeloid suppressors) with the "inflamed" phenotype.4 Unlike the CAR T cells, bispecific antibodies may be unable to draw enough cytotoxic T cells into the tumor, or the trafficking gets outpaced by an extremely high tumor proliferation rate. Low intratumoral CD8+ T-cell density, which correlates with MYC activation and TP53 dysfunction, is a histologic feature associated with worse response rates to CD20xCD3 bispecifics, both in the relapsed/refractory and first-line settings.3,5
Diverse mechanisms and clinical features of tumor progression events on mosunetuzumab therapy, and potential ways to overcome them. Figure created with BioRender.com.
Diverse mechanisms and clinical features of tumor progression events on mosunetuzumab therapy, and potential ways to overcome them. Figure created with BioRender.com.
Second, approximately one-third of progression events occur 3 to 9 months from the first dose—either while still on therapy (sometimes following an initial response) or soon after completion. It is within this group that the acquired CD20 loss and MS4A1 mutations concentrate, affecting both aggressive and indolent B-cell histologies. According to Schuster et al, somatic MS4A1 mutations emerge relatively often on progression after mosunetuzumab in relapsed/refractory lymphomas. This contrasts with an exceedingly low rate of mutation-associated CD20 loss in diffuse large B-cell lymphoma at diagnosis (1%) or at first relapse after rituximab-based immunochemotherapy (7%).6 Similarly to the first relapse setting,6 most MS4A1 mutations detected before mosunetuzumab administration were in the transmembrane domain. In contrast, variants emerging at progression were more often in the extracellular domain or splicing sites, and they abrogated T-cell activation by mosunetuzumab in vitro.
Because of the consistent timing of progression and already lower pretreatment CD20 expression in this group, it is plausible that CD20-negative relapses arise from already existing subclones with an MS4A1 mutation. The mutated clone gains an advantage during an "evolutionary bottleneck" of anti-CD20 therapy. Further support for this hypothesis comes from a recent case series in which a reemergence of a CD20+ subclone was observed after some time off mosunetuzumab.7MS4A1 mutations are not the only mechanism of CD20 loss after mosunetuzumab. Ang et al have described an alternative (and potentially reversible) splicing mechanism causing a shift toward an untranslated mRNA isoform.8 Using RNA sequencing, Schuster et al additionally describe transcriptional downregulation of MS4A1 in 16% of CD20-negative relapses, which remains to be mechanistically explained.
The third and most curious group of progression events after mosunetuzumab occurs much later, 6 to 24 months after completing therapy, and following attainment of complete response. These relapses are associated with retained CD20 expression on the tumor and remain highly sensitive to retreatment with mosunetuzumab.2 Duell et al have demonstrated increased markers of CD8+ T-cell exhaustion in cases of CD20+ (but not CD20-negative) relapse after mosunetuzumab, with the accumulation of potentially hyperexhausted effector T cells.7 It remains to be investigated whether similar patterns emerge during open-ended therapy with epcoritamab or odronextamab, or during first-line treatment among patients without prior exposure to immunosuppressive or lymphodepleting chemotherapy.5
The findings from the study by Schuster et al highlight important points for clinical practice and directions for further research. Evaluating CD20 expression both before therapy and at progression has value when weighing the potential benefits of CD20- vs CD19-directed therapy or considering retreatment with a bispecific antibody. Overcoming resistance to mosunetuzumab cannot be accomplished with a single strategy. Antigen escape can be likely mitigated by targeting 2 separate tumor markers simultaneously, by either using trispecific antibodies (eg, JNJ-8543, which binds CD79b, CD20, and CD3)9 or combining a CD20xCD3 drug with an adjunct bispecific (eg, odronextamab plus CD22xCD28).10 The latter approach offers an additional advantage of improving the cytotoxic T-cell function by delivering the costimulatory “signal 2.” Other modalities to achieve this enhancement may include combining bispecific antibodies with immunomodulatory agents, like lenalidomide, or with checkpoint inhibitors, or potentially instituting periodic breaks in therapy to promote T-cell recovery. The biggest challenge may lie in preventing primary refractoriness in high-grade tumors characterized by immune exclusion. Here, combinations with chemotherapy or antibody-drug conjugates might offer recourse.
Conflict-of-interest disclosure: A.J.O. consulted for Schrödinger, Genmab, Beigene, and Bristol-Myers Squibb and received research funding from Genentech/Roche, the Leukemia & Lymphoma Society, the Institute for Follicular Lymphoma Innovation, Genmab, Artiva Biotherapeutics, Precision BioSciences, and Kymera Therapeutics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal