This cohort study describes the long-term outcomes of PE in 150 children and adolescents followed-up at a single-center institution.
Patients were screened for abnormal functional outcomes using a local protocol (symptoms, lung and cardiac function, and exercise testing).
Visual Abstract
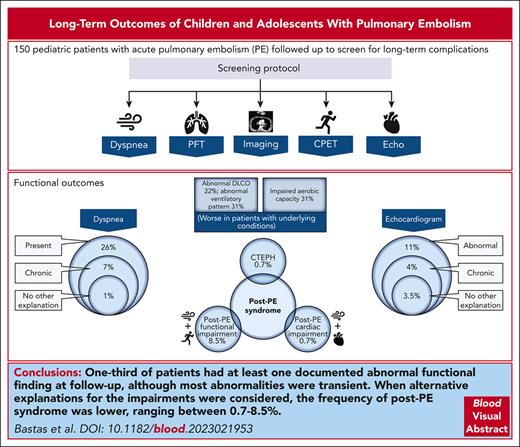
Knowledge regarding the long-term consequences of pulmonary embolism (PE) in children is limited. This cohort study describes the long-term outcomes of PE in children who were followed-up at a single-center institution using a local protocol that included clinical evaluation, chest imaging, echocardiography, pulmonary function tests, and cardiopulmonary exercise tests at follow-up, starting 3 to 6 months after acute PE. Children objectively diagnosed with PE at age 0 to 18 years, who had ≥6 months of follow-up were included. Study outcomes consisted of PE resolution, PE recurrence, death, and functional outcomes (dyspnea, impaired pulmonary or cardiac function, impaired aerobic capacity, and post-PE syndrome). The frequency of outcomes was compared between patients with/without underlying conditions. In total, 150 patients were included; median age at PE was 16 years (25th-75th percentile, 14-17 years); 61% had underlying conditions. PE did not resolve in 29%, recurrence happened in 9%, and death in 5%. One-third of patients had at least 1 documented abnormal functional finding at follow-up (ventilatory impairments, 31%; impaired aerobic capacity, 31%; dyspnea, 26%; and abnormal diffusing capacity of the lungs to carbon monoxide, 22%). Most abnormalities were transient. When alternative explanations for the impairments were considered, the frequency of post-PE syndrome was lower, ranging between 0.7% and 8.5%. Patients with underlying conditions had significantly higher recurrence, more pulmonary function and ventilatory impairments, and poorer exercise capacity. Exercise intolerance was, in turn, most frequently because of deconditioning than to respiratory or cardiac limitation, highlighting the importance of physical activity promotion in children with PE.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 652.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning Objectives
Upon completion of this activity, participants will:
Describe clinical characteristics, pulmonary embolism (PE) resolution, PE recurrence, and death among 150 children with PE, based on a single-center study
Determine functional outcomes (dyspnea, impaired pulmonary or cardiac function, impaired aerobic capacity, and post-PE syndrome) among 150 children with PE, based on a single-center study
Identify clinical implications of long-term outcomes of PE in children, based on a single-center study
Release date: February 15, 2024; Expiration date: February 15, 2025
Introduction
Thrombotic events are increasingly being recognized among hospitalized children. Data from the United States showed that the frequency of pediatric venous thrombosis in tertiary care institutions increased from 34 per 10 000 to 106 per 10 000 hospital admissions between 2001 and 2019.1,2 Pulmonary embolism (PE) accounted for ∼10% of these venous thrombotic events.1,2 Similarly, a 200% increase in pediatric PE was documented in pediatric tertiary institutions between 2001 and 2014.3
Approximately 50% of adult patients develop chronic complications after acute PE, or post-PE syndrome.4-8 Post-PE syndrome is defined by the diagnosis of chronic thromboembolic pulmonary disease (including chronic thromboembolic pulmonary hypertension [CTEPH]), post-PE cardiac impairment, or post-PE functional impairment after anticoagulation treatment for ≥3 months, in the absence of other comorbidities that can explain clinical findings.4,5,9-11 This multifactorial complication of PE typically manifests in adults with new or progressive dyspnea or exercise intolerance, and can lead to reduced health-related quality of life, loss of productivity, and increased health care cost.4,5,9,12,13
Despite the increasing diagnosis of PE in children, long-term consequences in the pediatric population are not characterized. The main goal of this study was to investigate the frequency of chronic sequelae of PE in the pediatric population, describing the results of a local protocol implemented at The Hospital for Sick Children (SickKids), Toronto, Canada, in 2004 to monitor for long-term complications of acute PE. The protocol includes clinical assessment and cardiac, pulmonary, and exercise testing, starting ∼3 to 6 months after PE. Furthermore, we sought to investigate whether the frequency of long-term complications was associated with the presence of underlying conditions at the time of PE diagnosis.
Methods
This cohort study enrolled children aged 0 to 18 years with objective diagnosis of acute PE who survived for at least 6 months after PE diagnosis and who were followed-up at SickKids between 2004 and 2022. Patients with tumor thrombus, septic PE, and in situ pulmonary artery thrombosis14 were excluded. Patients were identified in the Thrombosis Database at SickKids, enriched by the review of health records using specific the International Classification of Diseases, Ninth Revision and Tenth Revision codes, as per a published algorithm.15 Local ethics research board approval was obtained, and informed consent was waived. The following variables were collected:
Baseline clinical characteristics present at the time of acute PE, which included age, sex (male/female), height, weight, PE symptoms, underlying conditions that can impact cardiac or pulmonary function (none, autoimmune, cancer, infectious, cardiac, and other); and risk factors for PE including thrombophilia (major, minor, and none16), oral contraceptives, trauma/surgery, and anatomic variants (May-Thurner syndrome and Paget-Schroetter syndrome); concomitant objectively documented deep vein thrombosis (DVT), hospital-acquired PE (PE diagnosed during or up to 90 days after a hospital admission17), PE severity (massive, submassive, or low-risk, as per American Heart Association criteria18), management strategy (anticoagulation alone, thrombolysis/thrombectomy, or no anticoagulation), and treatment duration. Height and weight at the time of PE were used to estimate body mass index percentile according to the World Health Organization growth standards for children aged <2 years, and to the growth reference standards from the Centers for Disease Control and Prevention for children aged ≥2 years.
Outcomes of the study, which included previously reported outcomes (PE resolution, objectively documented thrombosis recurrence, and death), and functional outcomes (dyspnea, pulmonary function and ventilatory impairments, abnormal echocardiogram findings, impaired aerobic capacity, and post-PE syndrome). Outcomes were determined according to the results of the local PE follow-up protocol that included clinical evaluation, chest imaging (computerized tomography pulmonary angiography [CTPA], ventilation-perfusion [V/Q] scans), echocardiography, pulmonary function tests (PFT), and cardiopulmonary exercise test (CPET), starting at least 3 to 6 months after the diagnosis of acute PE. Outcomes were classified/defined as follows:
PE resolution was classified as complete vs residual obstruction (or nonresolution, including wall thickening),19 according to the results of CTPA, V/Q scans, or magnetic resonance angiography (MRA) at follow-up.
Thrombosis recurrence was defined as the objective diagnosis of a new thrombotic event, as per existing recommendations,20 in any location.
Death at follow-up, time to death in days, and cause of death were also collected.
Presence of dyspnea was defined as a “subjective experience of breathing discomfort”21 at rest or exertion during follow-up visits (yes/no). Dyspnea was considered chronic if documented at least twice, ≥4 weeks apart.22,23 In nonverbal patients, increased work of breathing was documented instead.
Pulmonary function and ventilatory impairments were determined from analysis of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, total lung capacity (TLC), and diffusing capacity for carbon monoxide (DLCO) obtained from PFTs meeting the European Respiratory Society (ERS) and the American Thoracic Society (ATS) acceptability and repeatability criteria.24 Poor quality tests were excluded; tests of questionable quality were reviewed by an expert pediatric respirologist (L.P.) to determine whether results were acceptable. PFTs were performed starting at age 5 years (spirometry only), depending on the patient’s ability to follow instructions. More complex testing (lung volumes and diffusion capacity) were only attempted if spirometry met acceptability and repeatability criteria.24 The Global Lung Function Initiative reference equations were used to determine sex-, height- and age-appropriate predicted values for each lung function parameter; observed and predicted values were used to estimate z scores.25-27 In keeping with the 2021 ERS/ATS technical standards, z scores of more than −1.65 were considered normal28; severity of pulmonary function impairment was classified as mild (z scores between −1.65 and −2.50), moderate (z score between −2.51 and −4.00), and severe (z scores of less than −4.10).28 Ventilatory impairments were classified according to the 2021 ERS/ATS technical standards as obstructive, restrictive, nonspecific pattern, mixed disorder, and dysanapsis (ie, mismatch between the lung size and airway caliber).28
Abnormal echocardiogram findings included right ventricle dysfunction or dilation, or pulmonary hypertension in follow-up imaging. Right ventricle dysfunction was measured qualitatively; pulmonary hypertension was derived from the tricuspid valve regurgitation velocity and intraventricular septum position. Changes in cardiac recovery were considered chronic if present for ≥3 months. In patients with documented cardiac abnormalities before PE, an experienced pediatric cardiologist (V.G.) evaluated whether the abnormalities worsened after PE.
Impaired aerobic capacity was assessed using CPET. Testing was performed according to the local protocol using a cycling ergometry step protocol29 and starting at age 7 years, depending on the maturity level of the child. Peak rate of oxygen consumption (VO2 peak; ie, highest VO2 recorded) was collected. Predicted VO2 peak was calculated using pediatric equations30 and used to estimate z scores. A z score of −1.96 or less was considered abnormal. Maximal heart rate and percentage of predicted maximal heart rate were collected. Maximal heart rate was calculated using the Tanaka method.31 Workload (in watts) and peripheral oxygen saturation (%) at rest and peak level were collected. Exercise oxygen desaturation was defined as a decrease of >4% in oxygen saturation from rest to peak.32 Of note, CPET data were included in the analysis only if peak data was achieved, defined by satisfying at least 2 of the following criteria: (1) ≥85% maximal heart rate at highest level, (2) respiratory quotient of >1.05, or (3) reaching the anaerobic threshold. Criteria to define reduced exercise capacity are shown in supplemental Table 1, available on the Blood website. When available, 6-minute walk tests for patients without CPET data were included.33 For this submaximal exercise test, we collected information on distance walked (in meters), maximal and peripheral oxygen saturation, as outlined earlier. Distance walked was considered abnormal if it was 2 standard deviations below pediatric normative values.34 The 6-minute walk test can be performed with children as young as 3 years of age.35
Post-PE syndrome was defined as follows: CTEPH was defined according to the European Society of Cardiology/ERS criteria by a mean pulmonary arterial pressure of >20 mmHg36 with pulmonary artery wedge pressure of ≤15 mmHg in right heart catheterization; mismatched perfusion defects in perfusion scans; and signs of CTEPH in CTPA, MRA, or conventional angiography.37,38
Chronic thromboembolic pulmonary disease without pulmonary hypertension at rest was defined by the presence of symptoms attributed to post-thrombotic changes in the pulmonary arteries.11
Post-PE cardiac impairment was defined by the presence of echocardiographic changes (as described earlier) and exertional dyspnea.5,9,10
Post-PE functional impairment was defined by the presence of dyspnea and exercise intolerance and/or impaired function with no alternative explanation.5,9,10
Statistical analysis
Descriptive statistics were used to report data distribution; continuous variables were described using measures of central tendency and dispersion, and categorical variables were summarized using percentages. Because underlying conditions can affect PE outcomes,9,39-41 baseline characteristics and outcome frequency were compared between patients with/without underlying conditions using Wilcoxon rank sum test, Pearson χ2 test, or Fisher exact test. The Kaplan-Meier estimator was used to determine cumulative incidence of recurrence. The association between dyspnea and abnormal echocardiogram findings, and between dyspnea and abnormal DLCO, adjusted for the presence or absence of underlying conditions, was analyzed using the Cochran-Mantel-Haenszel test. Missing data on outcomes were not imputed. Analyses were carried out using R Core Team (2022).
Results
In total, 165 patients sustained a PE in the study period; 15 were excluded (early death, n = 10; hospital transfer, n = 4; and lung transplantation after PE, n = 1), resulting in a final sample of 150 patients included in the analysis. Patients were followed-up for a median of 521 days (25th-75th percentile, 286-973 days) and had a median of 3 outpatient visits per patient (25th-75th percentile, 2-5 visits).
Median age at PE diagnosis was 16 years (25th-75th percentile, 14-17 years). Underlying conditions were present in 92 of 150 (61%) patients, including autoimmune diseases (33 of 150, 22%), cancer (20 of 150, 13%), infectious diseases (7 of 150, 5%), and cardiac disease (7 of 150, 5%). Twenty-five patients (17%) had ≥1 additional comorbidities.
Most patients had ≥1 risk factor for PE (Table 1), were diagnosed with low-risk PE (125 of 150, 83%), and were treated with anticoagulation alone (122 of 150, 81%) for a median of 6 months (25th-75th percentile, 3-12 months). Concomitant DVT was found in 83 of 150 (55%) patients; 33% of the DVTs were central venous catheter related. Table 1 compares baseline characteristics of patients with/without underlying conditions.
Patient baseline characteristic and PE characteristics (n = 150)
| Characteristic . | No underlying conditions, n = 58∗ . | Underlying conditions present, n = 92∗ . | P value† . |
|---|---|---|---|
| Age, y | 16 (15, 17) | 16 (14, 17) | .08 |
| Male sex | 21 (36%) | 52 (57%) | .02 |
| BMI percentile | 77 (54, 96) | 85 (56, 96) | .61 |
| Symptomatic PE | 52 (90%) | 69 (76%) | .04 |
| Thrombophilia | .006 | ||
| Major | 11 (20%) | 23 (32%) | |
| Minor | 12 (22%) | 3 (4%) | |
| None | 31 (57%) | 47 (64%) | |
| Oral contraceptive use | 29 (78%) | 12 (30%) | <.001 |
| Anatomic variations | 5 (9%) | 4 (4%) | .31 |
| Trauma/surgery | 11 (19%) | 13 (14%) | .43 |
| Concomitant DVT | 27 (52%) | 56 (65%) | .13 |
| Hospital-acquired PE | 17 (29%) | 58 (63%) | <.001 |
| Massive/submassive PE at presentation | 8 (14%) | 17 (19%) | .44 |
| Treatment | |||
| Anticoagulation + thrombolysis/thrombectomy | 11 (19%) | 12 (13%) | .15 |
| Anticoagulation | 47 (81%) | 75 (82%) | |
| None | 0 (0%) | 5 (5%) |
| Characteristic . | No underlying conditions, n = 58∗ . | Underlying conditions present, n = 92∗ . | P value† . |
|---|---|---|---|
| Age, y | 16 (15, 17) | 16 (14, 17) | .08 |
| Male sex | 21 (36%) | 52 (57%) | .02 |
| BMI percentile | 77 (54, 96) | 85 (56, 96) | .61 |
| Symptomatic PE | 52 (90%) | 69 (76%) | .04 |
| Thrombophilia | .006 | ||
| Major | 11 (20%) | 23 (32%) | |
| Minor | 12 (22%) | 3 (4%) | |
| None | 31 (57%) | 47 (64%) | |
| Oral contraceptive use | 29 (78%) | 12 (30%) | <.001 |
| Anatomic variations | 5 (9%) | 4 (4%) | .31 |
| Trauma/surgery | 11 (19%) | 13 (14%) | .43 |
| Concomitant DVT | 27 (52%) | 56 (65%) | .13 |
| Hospital-acquired PE | 17 (29%) | 58 (63%) | <.001 |
| Massive/submassive PE at presentation | 8 (14%) | 17 (19%) | .44 |
| Treatment | |||
| Anticoagulation + thrombolysis/thrombectomy | 11 (19%) | 12 (13%) | .15 |
| Anticoagulation | 47 (81%) | 75 (82%) | |
| None | 0 (0%) | 5 (5%) |
BMI, body mass index.
Median (25th-75th percentile); n (%).
Wilcoxon rank sum test; Pearson χ2 test; Fisher exact test.
PE resolution
Chest imaging was performed at follow-up in 136 of 150 patients (91% of the cohort, 132 CTPA, 3 V/Q scans, and 1 MRA) at a median of 104 days (25th-75th percentile, 91-177 days) after PE. Nonresolution was documented in 40 of 136 (29%) patients. There was no evidence of difference in resolution/nonresolution according to underlying conditions (Table 2).
Long-term outcomes of PE by health status (n = 150)
| Outcomes . | No underlying conditions, n = 58∗ . | Underlying conditions present, n = 92∗ . | P value† . |
|---|---|---|---|
| Nonresolution of PE at follow-up‡ | 14 (26%) | 26 (32%) | .47 |
| Recurrence | 1 (1.7%) | 12 (13%) | .016 |
| Death | 1 (1.7%) | 6 (6.5%) | .25 |
| Dyspnea or increased work of breathing | |||
| Chronic | 3 (5.2%) | 7 (7.6%) | .84 |
| Transient | 12 (21%) | 17 (18%) | |
| Abnormal echocardiogram findings§ | |||
| Chronic | 1 (1.9%) | 5 (5.8%) | .11 |
| Transient | 1 (1.9%) | 8 (9.3%) | |
| Chronic thromboembolic pulmonary hypertension | 0 (0%) | 1 (1.1%) | >.99 |
| Post-PE cardiac impairment§ | 0 (0%) | 1 (1.2%) | >.99 |
| Post-PE functional impairment‖ | 2 (7.1%) | 3 (9.7%) | >.99 |
| Outcomes . | No underlying conditions, n = 58∗ . | Underlying conditions present, n = 92∗ . | P value† . |
|---|---|---|---|
| Nonresolution of PE at follow-up‡ | 14 (26%) | 26 (32%) | .47 |
| Recurrence | 1 (1.7%) | 12 (13%) | .016 |
| Death | 1 (1.7%) | 6 (6.5%) | .25 |
| Dyspnea or increased work of breathing | |||
| Chronic | 3 (5.2%) | 7 (7.6%) | .84 |
| Transient | 12 (21%) | 17 (18%) | |
| Abnormal echocardiogram findings§ | |||
| Chronic | 1 (1.9%) | 5 (5.8%) | .11 |
| Transient | 1 (1.9%) | 8 (9.3%) | |
| Chronic thromboembolic pulmonary hypertension | 0 (0%) | 1 (1.1%) | >.99 |
| Post-PE cardiac impairment§ | 0 (0%) | 1 (1.2%) | >.99 |
| Post-PE functional impairment‖ | 2 (7.1%) | 3 (9.7%) | >.99 |
Median (25th-75th percentile); n (%).
Pearson χ2 test; Fisher exact test.
n = 54 and 82, respectively.
n = 54 and 86, respectively.
n = 28 and 31, respectively.
Thrombosis recurrence
This outcome was documented in 13 of 150 (9%) patients at a median of 87 days (25th-75th percentile, 28-206 days) after PE diagnosis. The event rate was 4.1 per 100 person-years; the cumulative incidence of recurrence at 2 and 4 years were 9% (95% confidence interval [CI], 5-15) and 11% (95% CI, 6-18), respectively. Most recurrences (12 of 13) were found in patients with underlying conditions (Table 2). Nine recurrent events were DVT and/or PE. In total, 8 of 13 (62%) patients recurred while on documented anticoagulation. All patients with recurrence had ≥1 persisting risk factors (supplemental Tables 2a/2b).
Death
Death occurred in 7 of 150 patients (4.7%) a median of 381 days from diagnosis (25th-75th percentile, 341-666 days). The cause of death in 6 of 7 patients was their underlying condition (4 cancer, 2 cardiac); in 1 patient the cause of death was unknown.
Functional outcomes
Presence of dyspnea
Either dyspnea or increased work of breathing were documented in 39 of 150 (26%) patients on follow-up (Table 2). Only 2 patients, who had severe underlying conditions, had increased work of breathing documented at rest; the remaining 37 of 150 (25%) patients had dyspnea at exertion only. In total, 10 of 150 (7%) patients had chronic symptoms and only 2 of 10 patients with dyspnea (2 of 150, 1%) had no alternative explanation for their dyspnea (supplemental Table 3). Overall, dyspnea was first documented a median of 168 days from PE diagnosis (25th-75th percentile, 69-329 days). There was no significant difference in the frequency of dyspnea between those with/without underlying conditions (Table 2).
Pulmonary function and ventilatory impairments
PFTs were performed in 126 of 150 (84%) patients; 118 patients (79% of the cohort) had technically acceptable tests. Included tests were performed a median of 232 days (25th-75th percentile, 109-632 days) from PE diagnosis. Patients with underlying conditions had significantly lower z scores for FEV1, FVC, total lung capacity, and DLCO; and higher frequency of FEV1, FVC, and DLCO impairments (Table 3; supplemental Table 4). Normal or obstructive ventilatory patterns were more frequent among patients without underlying conditions; restrictive, mixed, or nonspecific patterns were more common among children with underlying conditions.
Results of pulmonary function tests at follow-up by health status (n = 118)
| Characteristic . | No underlying conditions, n = 50∗ . | Underlying conditions present, n = 68∗ . | P value† . |
|---|---|---|---|
| DLCO, z score | −0.31 (−1.02, 0.28) | −1.05 (−2.28, −0.25) | .002 |
| DLCO, impairment severity‡ | |||
| None | 40 (95%) | 33 (65%) | .001 |
| Mild | 2 (4.8%) | 9 (18%) | |
| Moderate | 0 (0%) | 8 (16%) | |
| Severe | 0 (0%) | 1 (2.0%) | |
| Ventilatory impairment | |||
| Normal | 39 (78%) | 43 (63%) | .049 |
| Restrictive | 3 (6.0%) | 12 (18%) | |
| Airway dysanapsis | 4 (8.0%) | 3 (4.4%) | |
| Non-specific pattern | 1 (2.0%) | 6 (8.8%) | |
| Obstruction | 3 (6.0%) | 1 (1.5%) | |
| Possible mixed | 0 (0%) | 3 (4.4%) |
| Characteristic . | No underlying conditions, n = 50∗ . | Underlying conditions present, n = 68∗ . | P value† . |
|---|---|---|---|
| DLCO, z score | −0.31 (−1.02, 0.28) | −1.05 (−2.28, −0.25) | .002 |
| DLCO, impairment severity‡ | |||
| None | 40 (95%) | 33 (65%) | .001 |
| Mild | 2 (4.8%) | 9 (18%) | |
| Moderate | 0 (0%) | 8 (16%) | |
| Severe | 0 (0%) | 1 (2.0%) | |
| Ventilatory impairment | |||
| Normal | 39 (78%) | 43 (63%) | .049 |
| Restrictive | 3 (6.0%) | 12 (18%) | |
| Airway dysanapsis | 4 (8.0%) | 3 (4.4%) | |
| Non-specific pattern | 1 (2.0%) | 6 (8.8%) | |
| Obstruction | 3 (6.0%) | 1 (1.5%) | |
| Possible mixed | 0 (0%) | 3 (4.4%) |
Data shown for n = 118 patients with acceptable tests as per ERS/ATS criteria.24
Median (25th-75th percentile); n (%).
Wilcoxon rank sum test; Fisher exact test.
n = 42 and 51, respectively.
Abnormal echocardiogram findings
A total of 723 echocardiograms were completed in 141 of 150 patients (94% of the cohort). Median time to last echocardiogram was 167 days (25th-75th percentile, 100-588 days) after acute PE. Abnormal findings were seen in 15 patients (15 of 141, 11%; Table 2), including right ventricle dilation (n = 6), right ventricle dysfunction (n = 4), and both (n = 5); four of these 15 patients also had documented pulmonary hypertension. Echocardiogram abnormalities were chronic in 6 of 141 patients (4%); 4 patients had known echocardiogram abnormalities before PE diagnosis, but only 1 had evidence of worsening right ventricle function after the PE. Including this patient, a total of 5 children (5 of 141, 3.5%; 3 of 86 with, and 2 of 54 without, underlying conditions) had either no alternative explanation for their echocardiogram findings or evidence of worsening after PE (supplemental Table 5).
Impaired aerobic capacity
CPET was performed in 62 of 150 patients (41%); 4 patients did not reach peak levels because of deconditioning, 3 because of fatigue, and 1 because the patient stopped the test; the 54 remaining patients were included in the analysis. CPET was performed a median of 258 days (25th-75th percentile, 119-621 days) after PE.
Abnormal aerobic capacity (VO2 peak z score of −1.96 or less) was seen in 17 of 54 (31%) patients, 12 of whom had underlying conditions (71%) (Table 4). Reduced exercise capacity was attributed to deconditioning or to leg fatigue in 14 of 17 (82%) patients, and to respiratory limitation in 2 patients. Of note, 8 of these patients had DVT, but none had postthrombotic syndrome. One patient with a normal aerobic capacity desaturated (peripheral oxygen saturation [SPO2] difference of 6%). Patients with underlying conditions had significantly poorer exercise capacity (lower VO2 peak z score and higher frequency of abnormal VO2 peak z score; Table 4).
Results of cardiopulmonary exercise tests at follow-up by health status (n = 54)
| Characteristic . | No underlying conditions, n = 27∗ . | Underlying conditions present, n = 27∗ . | P value† . |
|---|---|---|---|
| Age (y) at the time of exercise test | 16.8 (15.6, 17.5) | 16.8 (15.5, 17.6) | .79 |
| VO2 peak, z score | −0.73 (−1.53, −0.16) | −1.68 (−2.80, −0.80) | .042 |
| Abnormal VO2 peak z score‡ | 5 (19%) | 12 (44%) | .040 |
| Percent peak heart rate (bpm) | 93 (91, 97) | 92 (86, 97) | .16 |
| Peak workload (watts) | 145 (130, 193) | 144 (123, 166) | .26 |
| Peak SPO2 | 99 (99, 100) | 99 (99, 100) | .95 |
| Exercise desaturation | 1 (3.7%) | 0 (0%) | >.99 |
| Characteristic . | No underlying conditions, n = 27∗ . | Underlying conditions present, n = 27∗ . | P value† . |
|---|---|---|---|
| Age (y) at the time of exercise test | 16.8 (15.6, 17.5) | 16.8 (15.5, 17.6) | .79 |
| VO2 peak, z score | −0.73 (−1.53, −0.16) | −1.68 (−2.80, −0.80) | .042 |
| Abnormal VO2 peak z score‡ | 5 (19%) | 12 (44%) | .040 |
| Percent peak heart rate (bpm) | 93 (91, 97) | 92 (86, 97) | .16 |
| Peak workload (watts) | 145 (130, 193) | 144 (123, 166) | .26 |
| Peak SPO2 | 99 (99, 100) | 99 (99, 100) | .95 |
| Exercise desaturation | 1 (3.7%) | 0 (0%) | >.99 |
bpm, beats per minute.
Median (25th-75th percentile); n (%).
Wilcoxon rank sum test; Fisher exact test; Pearson χ2 test.
Abnormal defined by a z score of −1.96 or less.
Six patients without CPET had 6-minute walk test data available. One patient walked a distance that was 2–standard deviations below pediatric normative values.34 Two patients desaturated during the test, 1 with CTEPH (SPO2 difference of 9%); the second patient (SPO2 difference of 10%) had a history of resolved pulmonary hypertension and resolved chronic changes on CTPA; the latter patient also had phrenic nerve damage and orthopnea from a surgery for thoracic outlet syndrome, likely affecting the results.
Post-PE syndrome
CTEPH
CTEPH was observed in 1 of 150 patients (0.7%). The patient had autoimmune hemolytic anemia at the time of PE diagnosis and was later confirmed to have antiphospholipid antibody syndrome. The patient was fully anticoagulated throughout their follow-up at our institution. Pulmonary hypertension, which was not present at the time of PE, was documented in an echocardiogram performed 60 days later. Cardiac catheterization (first procedure performed 3 months after PE) demonstrated high pulmonary artery pressures of ∼50 mmHg, with normal wedge pressures [6-9 mmHg]); CTPA noted tapering of vessels in all but 1 lung lobes, interlobal artery and segmental branch narrowing and wall thickening, and signs of hypoperfusion in both lungs. Dyspnea persisted at last follow-up, 3.3 years after PE diagnosis. The patient had abnormal DLCO and a restrictive ventilatory pattern.
Post-PE cardiac impairment
Only 1 patient fulfilled these criteria. The patient had chronic dyspnea and preexisting impaired right ventricular function that worsened after PE (1 of 141, 0.7%). The patient died because of progression of severe restrictive cardiomyopathy 316 days after PE diagnosis. Of note, the 2 patients with transient echocardiogram findings mentioned earlier in "Abnormal echocardiogram findings” had no dyspnea at follow-up.
Post-PE functional impairment
Nine of 59 tested patients (15%) had both abnormal exercise test results and dyspnea at follow-up. Excluding the patient with CTEPH, only 5 of 59 (8.5%) patients had no alternative explanation for the findings. Dyspnea resolved in all but 1 patient who had chronic pulmonary hypertension and chronic thrombotic changes in CTPA that fully resolved after ∼6 months, as mentioned earlier. Although <50% of the patients with dyspnea underwent exercise testing, 7 of 10 patients with chronic dyspnea were tested. The remaining 3 patients had severe underlying conditions, 2 of whom had severe cognitive impairments, and exercise testing was not feasible.
Cochran-Mantel-Haenszel test showed no association between abnormal echocardiogram findings and dyspnea, (M2, 0.49; df, 2; P = .78) or between abnormal DLCO and dyspnea (M2, 0.84; df, 2; P = .66, adjusted for the presence or absence of underlying conditions; supplemental Figure 1).
Discussion
In this study, we characterized the long-term outcomes in 150 children with PE, and compared the outcomes of those with and without existing underlying conditions, including outcomes previously reported in the pediatric literature (PE resolution, thrombosis recurrence, and death)39,40,42-45 as well as functional outcomes comprising the full spectrum of post-PE syndrome.
In terms of previously reported outcomes, nonresolution of PE was documented in ∼30% of patients, a frequency that is slightly higher than the ∼25% frequency of nonresolution documented in 4 different pediatric studies that included a total of 111 children with PE and follow-up imaging.40,44-46 The 9% frequency of thrombosis recurrence found in our study is similar to the 9% to 14% frequency documented in previous pediatric studies,40,45,47 although other researchers have reported lower (0%)44 and higher recurrence frequencies (19%).42 Lastly, the frequency of death (5%) was lower than that shown in other pediatric cohorts (17%-21%),45,47,48 which can be explained by the exclusion of patients with early death and therefore no long-term follow-up in this cohort, as well as the exclusion of in situ pulmonary artery thrombosis and tumor thrombus. Previously, 1 study reported that only inflammatory conditions were associated with recurrence/nonresolution (composite outcome).40 Another study reported that PE severity was associated with cardiac conditions, and that both variables were associated with PE-related mortality/progression/recurrence, although only having a cardiac condition was an independent predictor of the composite outcome in multivariable analysis.39 In our study, underlying conditions were associated with recurrence but not with mortality.
We observed dyspnea, transient or chronic, and generally exertional dyspnea, in 26% of children in our cohort. This frequency is similar to the 22% to 55% frequency of exertional dyspnea in adults after PE.6,7,49-51 Whereas in 1 study researchers reported that all adults with exertional dyspnea had underlying conditions, largely cardiopulmonary comorbidities, that explained the finding,7 others found no evidence that comorbidities predict this symptom.6,8 In line with the latter studies, we found that the presence of underlying conditions was not associated with a higher frequency of dyspnea or increased work of breathing. Furthermore, only 2 patients had no alternative explanations for their chronic dyspnea; whereas asthma, anxiety, and other diseases were thought to explain these findings in the remaining patients.
Pulmonary function parameters were normal in the majority of patients with no underlying conditions in our cohort, with a frequency of abnormal findings among these patients ranging between 2% and 14%, which is similar to the ∼8% frequency reported in adults with PE without major comorbidities.52 Abnormal ventilatory patterns were seen in 22% of children with no underlying conditions, with airway dysanapsis, obstruction, and restriction being more common. This is in line to the frequency of patterns reported in the general population. According to data from 14 populational-based cohorts involving nearly 50 000 children and young adults, the frequency of obstructive ventilatory patterns in this age group is 3% to 11%, and the frequency of restrictive ventilatory pattern is 2% to 8%.53 The prevalence of airway dysanapsis among children without asthma has been estimated at ∼7% to 15%.54
Overall, we observed that most of the pulmonary function parameter z scores were lower, and ventilatory impairments were more frequent among those with underlying conditions. Although it is likely that impairments in lung function were driven by the underlying conditions, the extent to which PE further affected these patients remains unknown. In addition, although impaired DLCO has been associated with the presence of dyspnea in adults with PE,8 we found no association between dyspnea and abnormal DLCO.
We found abnormal echocardiogram findings with no alternative explanation in 4% and 3% of patients with and without underlying conditions, respectively. Abnormal right ventricle function or systolic pressure were reported in ∼4% to 6% of adults with no major comorbidities, 1 year after PE.52 In keeping with studies that found no evidence that echocardiogram parameters at follow-up predict dyspnea in adults,6,8 we found no association between the frequency of abnormal echocardiogram findings on follow-up and dyspnea in our cohort, after adjusting for the presence or absence of underlying conditions.
Reduced exercise capacity was observed in approximately one-third of patients who were tested in our cohort. In a prospective study of adults with PE without major comorbidities, ∼50% of the patients were found to have reduced exercise capacity 1 year after PE diagnosis, which was associated with worse dyspnea, lower health-related quality of life, and shorter walking distance.52 Similarly, a cohort study that included adults with PE and persistent dyspnea and/or functional limitations found that 42% of patients had reduced exercise capacity at a median of 6.4 months from PE diagnosis.55 Reduced exercise capacity in patients with PE has been attributed to both cardiopulmonary limitations56 and to deconditioning.38,52,57,58 Deconditioning, in turn, is thought to be the result of reduced physical activity participation after the PE, exacerbated by a vicious cycle of dyspnea, fatigue, emotional impact, and inactivity.52,58 For this reason, a gradual resumption of pre-PE physical activity participation is encouraged in adult patients as early as diagnosis,4 counselling patients to slowly resume physical activity after hospital discharge.4 In our study, deconditioning or leg fatigue were present in 82% of children with reduced exercise capacity, and among children with reduced exercise capacity, 71% had underlying conditions, suggesting an overlap between deconditioning and underlying conditions in the pediatric population. Of note, patients with more severe illnesses precluding exercise testing did not undergo CPET. The presence of deconditioning in children with PE highlights the importance of early promotion of physical activity to help prevent functional decline. In a scoping review of physical activity in patients with venous thromboembolism, low to moderate noncontact physical activity after the first 4 weeks of anticoagulation was found to be safe.59 Furthermore, the Canadian 24-hour movement guidelines for children aged 5 to 17 years recommend 60 minutes of moderate to vigorous activity per day at minimum.60 Thus, encouraging patients to meet these guidelines whenever possible will be helpful to mitigate deconditioning after PE. Prevention of deconditioning may require a multidisciplinary approach in children with underlying conditions.
We observed an association between underlying conditions and PFTs and exercise testing results. There are multiple mechanisms by which underlying conditions can affect the pulmonary and cardiovascular function. For instance, the activated inflammatory state seen in systemic lupus erythematosus, antiphospholipid syndrome, inflammatory bowel disease, and nephrotic syndrome can lead to endothelial cell injury and dysfunction; there is also immune-mediated damage to pulmonary vessels that can result in vascular remodeling.61-66 Cancer therapy can affect the cardiopulmonary system via oxidative damage to lung tissue, endothelial damage, inflammatory response in vessel walls, and collagen deposition into the lungs.67
Post-PE syndrome, as defined in adults, was found in 7 patients, including 1 with CTEPH (0.7%), 1 with post-PE cardiac impairment (0.7%), and 5 with post-PE functional impairment (8.5%), of whom only 1 did not resolve on follow-up (1.7%). Although only 20 of 39 (51%) patients with dyspnea underwent exercise testing, all patients with chronic dyspnea who were able to exercise were tested, and therefore persistent post-PE functional impairments were likely captured in all patients. The low frequency of post-PE syndrome in children is in stark contrast to that reported in adults, in whom it has been documented in ∼50% after acute PE.4-8 Perhaps this difference in frequency of post-PE syndrome could be explained by the normal decline in function of the lung and cardiovascular system with aging. Lung decline is characterized by a reduced response to stress, damage accumulation, and loss of adult stem cells, which interferes with regenerative capacity.68,69 Aging, considered the most important risk factor for the development of cardiovascular conditions, leads to endothelial and myocardial cell dysfunction and impaired angiogenesis.70-72 Aging has been associated with persistent dyspnea,7 cardiopulmonary problems,73 and impaired functional capacity6 after acute PE in adults. As our understanding of the impact of PE in children evolves, and in view of the increasing life expectancy and advancing survival frontier,74,75 educating children who sustained a PE on lifestyle strategies to reduce or prevent cardiovascular risk factors as they age is of critical importance.
There are limitations to this study. First, CPET was not performed in 59% of patients because of underlying conditions restricting participation, movement limitations, young age, and cancelled appointments during the COVID-19 pandemic. Second, dyspnea was not assessed using a scale, as typically done in adult patients. Although there are some scales developed for children aged ≥8 years, these are not widely accepted. Third, the study included data over nearly 20 years, during which reference values changed. To overcome this limitation, only raw data was extracted, and predictive values for PFTs and CPET data were calculated using the same updated equations for all patients. The equations and reference values used in our study were derived from large pediatric samples, including 31 840 healthy children (27 419 children aged 0-15 years, and 4421 aged 15-20 years) from 13 different countries that participated in the Global Lung Function Initiative,25-27 and 282 healthy Canadian children aged 12-17 years not involved in high-performance competitive sports who participated in a study that estimated reference values for CPET.30 Fourth, the limited number of persistent functional outcomes precluded us from exploring potential predictors, and therefore the role of variables such as PE severity, body mass index, age, or sex could not be investigated in the present study. Lastly, because of the advanced imaging/functional testing used in this study, patients with underlying conditions may benefit from referral to specialized centers to monitor for potential worsening in function. Our study also has strengths, including the high frequency of patient follow-up and protocol completion, homogeneous protocol implementation given its single-center nature, and centralized multidisciplinary involvement in patient follow-up.
In conclusion, abnormal functional findings were seen in up to 20% to 30% of children diagnosed with thromboembolic PE, most commonly ventilatory impairments, impaired aerobic capacity, exertional dyspnea, and DLCO impairment. Post-PE syndrome was less frequent, ranging between 1% and 8.5%. Persistent post-PE findings were only seen in 2% of patients (1 CTEPH, 1 post-PE cardiac impairment, and 1 post-PE functional impairment). Underlying conditions were associated with a higher frequency of recurrence, ventilatory and pulmonary functional impairments, and reduced exercise capacity, which was, in turn, attributed to deconditioning or to leg fatigue in most patients. Strategies to promote physical activity should be incorporated in the clinical follow-up of children with PE.
Acknowledgments
The authors thank the Thrombosis team, the Pulmonary Function Laboratory, the Labatt Family Heart Centre, and the Pulmonary Exercise Laboratory at The Hospital for Sick Children for supporting the implementation of the PE follow-up protocol.
Authorship
Contribution: D.B. collected, entered, and analyzed data, and wrote the manuscript; M.L.A. designed the study, and collected, entered, and analyzed data, and wrote the manuscript; D.W. collected data and critically reviewed the manuscript; L.P. and S.T. reviewed data, and critically reviewed the manuscript; V.G. reviewed data, and critically reviewed the manuscript; G.W. collected and entered data, and critically reviewed the manuscript; J.V. organized patient follow-up, and critically reviewed the manuscript; J.E.S. performed cardiopulmonary exercise tests, and critically reviewed the manuscript; L.R.B., R.F.B., N.A., and S.W. critically reviewed the manuscript; and S.W. and L.R.B. drafted the local protocol.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. Laura Avila, Division of Pediatric Hematology/Oncology, The Hospital for Sick Children, 555 University Ave, Toronto, ON M5G 1X8, Canada; email: laura.avila@sickkids.ca.
References
Author notes
∗S.W. and M.L.A. are joint senior authors.
Data are available on request from the corresponding author, M. Laura Avila (laura.avila@sickkids.ca).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal