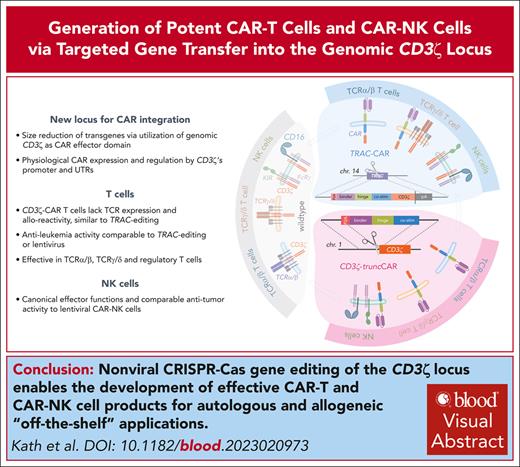
Key Points
Integration of ζ-deficient CARs into the CD3ζ gene allows generation of functional TCR-ablated CAR-T cells for allogeneic off-the-shelf use.
CD3ζ-editing platform allows CAR redirection of NK cells without affecting their canonical functions.
Visual Abstract
Chimeric antigen receptor (CAR)-redirected immune cells hold significant therapeutic potential for oncology, autoimmune diseases, transplant medicine, and infections. All approved CAR-T therapies rely on personalized manufacturing using undirected viral gene transfer, which results in nonphysiological regulation of CAR-signaling and limits their accessibility due to logistical challenges, high costs and biosafety requirements. Random gene transfer modalities pose a risk of malignant transformation by insertional mutagenesis. Here, we propose a novel approach utilizing CRISPR-Cas gene editing to redirect T cells and natural killer (NK) cells with CARs. By transferring shorter, truncated CAR-transgenes lacking a main activation domain into the human CD3ζ (CD247) gene, functional CAR fusion-genes are generated that exploit the endogenous CD3ζ gene as the CAR’s activation domain. Repurposing this T/NK-cell lineage gene facilitated physiological regulation of CAR expression and redirection of various immune cell types, including conventional T cells, TCRγ/δ T cells, regulatory T cells, and NK cells. In T cells, CD3ζ in-frame fusion eliminated TCR surface expression, reducing the risk of graft-versus-host disease in allogeneic off-the-shelf settings. CD3ζ-CD19-CAR-T cells exhibited comparable leukemia control to TCRα chain constant (TRAC)-replaced and lentivirus-transduced CAR-T cells in vivo. Tuning of CD3ζ-CAR-expression levels significantly improved the in vivo efficacy. Notably, CD3ζ gene editing enabled redirection of NK cells without impairing their canonical functions. Thus, CD3ζ gene editing is a promising platform for the development of allogeneic off-the-shelf cell therapies using redirected killer lymphocytes.
Introduction
The adoptive transfer of immune cells is a powerful tool to combat chronic diseases, such as cancer. Guiding lymphocytes to specifically bind and respond to antigens can be used to redirect the antitumor efficacy of cytotoxic T cells1 and natural killer (NK) cells2 as well as promote tissue-specific immunosuppression through regulatory T cells (Treg).3,4 To overcome the limitations associated with low frequencies of certain antigen-specific T cells in patients, gene transfer of chimeric antigen receptors (CAR) can be used to install the desired antigen-specificity to large numbers of cells needed for adoptive cell transfer and treatment success in severe disease. Autologous CAR-T cells are an approved treatment for B-cell malignancies, such as acute B-lymphoblastic leukemia,1,5 B-cell lymphoma6,7 and multiple myeloma.8
The TCR/CD3-complex is the endogenous antigen-receptor in T cells. It consists of a TCRα and a corresponding TCRβ chain which engage antigenic peptides presented by MHC molecules, as well as the accessory proteins CD3γ, CD3δ, CD3ε and CD3ζ which transduce the TCR signal downstream. While all CD3 proteins are required for TCR/CD3 assembly, biosynthesis of CD3ζ is the rate-limiting step in TCR/CD3 complex formation.9 Further, the intracellular domain of CD3ζ is sufficient to drive TCR-like activation in chimeric receptors.10,11 Therefore, all clinically approved (second-generation) CARs use the intracellular domain of CD3ζ as their primary TCR-activation-like effector domain. CARs further comprise an extracellular antigen-binding domain, a hinge domain, a transmembrane domain and an additional intracellular costimulatory domain, such as CD28 or 4-1BB. CARs without a main activation domain do not induce cytotoxicity, but have been proposed to boost T-cell function by providing costimulation.12
Most clinical CAR-T-cell products are generated by transduction with viral vectors which randomly integrate their cargo into the genome and drive CAR expression through strong promoters, such as EF1α.5-8,13-16 Positional effects and epigenetic silencing of transgenic expression cassettes have been linked to inconsistent CAR expression levels.17,18 While previous trials with virally transduced T cells were safe in most patients,19 gene transfer with (semi)random integration poses the risk of malignant transformation as highlighted by cases of clonal expansion after disruption of tumor suppressor genes TET220 or CBL21 by CAR provirus as well as by the development of CAR+ T-cell lymphoma after treatment with products generated via PiggyBac transposase technology22,23 and lentiviral (LV) vectors.24
Targeted gene transfer using gene editing can improve the consistency of redirected T-cell products by predictable antigen receptor expression.17,25,26 To this end, a programmable nuclease, such as CRISPR-Cas, is introduced into the T cells alongside a DNA repair template to exploit homology-directed DNA repair (HDR) for site-specific integration of the CAR-transgene. Multiple genomic sites have been proposed to redirect T cells with CARs, including the protein-coding genes TCRα chain constant (TRAC),17,27-29PDCD1 (encoding PD-1)28,30 and GAPDH31 as well as genomic safe harbor (GSH) loci, such as the (intragenic) human AAV-integration site (hAAVS1)30 or the extragenic GSH 6 (eGSH6) locus.18TRAC has emerged as the gold-standard for gene-edited CAR-T cells. One reason is the improved cell functionality associated with the temporary downregulation of the CAR after target engagement.17 This mirrors the natural regulation of the human TCR and protects from overt differentiation and T-cell exhaustion.17 An additional advantage is that the integration of CAR-transgenes into TRAC disrupts the TCR/CD3-complex. This creates CAR+ TCR− T cells which lack TCR-mediated alloreactivity, thereby offering a route towards safer application of CAR-T cells in allogeneic settings.32
In this study, we demonstrate virus-free CAR redirection via in-frame integration of truncated, CD3ζ-deficient CAR-transgenes (truncCARs) into an early exon of the CD3ζ-gene. Our knock-in strategy produces fusion genes composed of the exogenous truncCAR-transgene (encoding an antigen binder, a hinge, a transmembrane as well as a costimulatory domain but no main activation domain) and the endogenous CD3ζ-gene. This reduces the required transgene size and exploits the CD3ζ promoter for physiological CAR-regulation. CD3ζ-gene editing can also be used for redirection of regulatory T cells, TCRγ/δ T cells and most notably primary human NK cells which cannot be redirected by TRAC-targeting.
Material and methods
Cell culture
The study was performed in accordance with the declaration of Helsinki (Charité ethics committee approval EA4/091/19). Peripheral blood mononuclear cells (PBMC) were obtained from healthy donors via density gradient centrifugation from peripheral blood. T cells were enriched by magnetic cell separation (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany) using CD3-microbeads and cultured in T-cell medium, a 1:1 mixture of RPMI (Gibco, Thermo Fisher Scientific, Waltham, MA) and Click’s (Fujifilm Irvine Scientific, Santa Ana, CA) media supplemented with 10% fetal calf serum, interleukin-7 (IL-7) (10 ng/mL, Sartorius CellGenix, Freiburg, Germany) and IL-15 (5 ng/mL, Sartorius CellGenix). NK cells were enriched from the CD3-negative fraction using an NK isolation kit (Miltenyi) and cultured in NK MACS Medium (Miltenyi) supplemented with 10% fetal calf serum, IL-2 (500 IU/mL) and IL-15 (5 ng/mL).
Genetic engineering
Targeted virus-free CAR-integration was performed as recently described.33,34 In short, human T or NK cells were transfected with precomplexed CRISPR-Cas9 ribonucleoproteins and double-stranded DNA (dsDNA) (DNA/sgRNA sequences; supplemental Table 1, available on the Blood website). The dsDNA served as template for HDR and consisted of the (CAR/truncCAR) transgene flanked by 400 bp homology arms. Cells were resuspended in 20 μl P3 Electroporation Buffer (Lonza, Cologne, Germany) and electroporated with 1 μg of HDR-template and 1.38 μL of ribonucleoproteins consisting of synthetic modified single guide RNA (sgRNA, 100 μM, Integrated DNA Technologies [IDT], Coralville, IA), 15-50 kDa poly(l-glutamic acid)35 (100 μg/μL, Sigma-Aldrich, St Louis, MO) and recombinant SpCas9 protein (61 μM, IDT) in a 0.96:1:0.8 volume ratio using the 4D-Nucleofector (Lonza). T cells activated for 48 hours on αCD3/CD28-coated tissue culture plates were electroporated at a density of 5 × 104 cells/μL of buffer using program EH-115. Primary human NK cells were expanded in NK medium using NK activation/expansion beads (Miltenyi) for 6-7 days and electroporated using program DA-100. The NK-92 cell line was electroporated at 2.5 × 104 cells/μL with the program CA-137. 10 minutes postelectroporation, T cells were transferred into medium supplemented with 0.5 μM HDR-Enhancer v2 (IDT). For LV controls, activated T cells were transduced 1 day post–T-cell isolation while being kept on αCD3/CD28 coated tissue culture well plates for another day. After editing, cells were expanded in G-Rex 6-well plates (Wilson Wolf, St. Paul, MN).
Off-target analysis with CAST-Seq
Flow cytometry
Assessment of CAR+ rate, cytotoxicity, intracellular cytokine production, exhaustion, phenotype and CAR-regulation was performed on a Cytoflex LX device (Beckman Coulter) using the panels stated in supplemental Table 2 and as previously described.33 Activation-induced cell death of HER2-CAR-T cells was assessed after stimulation with plate-bound anti-Fc antibody (10 μg/mL, Jackson ImmunoResearch, West Grove, PA) via staining for annexin V Alexa Fluor 647 (Biolegend, San Diego, CA) and 7AAD (Biolegend). NK-cell degranulation was assessed after 4 hours of coculture with target cells in the presence of monensin A (1 μM, Golgistop, Becton Dickinson, Franklin Lakes, NJ) and BV785-conjugated anti-CD107a antibody (Biolegend). NK-cell-mediated antibody-dependent cellular cytotoxicity (ADCC) was assessed after 16 hours of coculture with CD20+ bGal− Jeko-1 cells in the presence of anti-CD20 or anti-bGal antibody (Invivogen, San Diego, CA).
Live cell imaging
In vitro tumor control of HER2-CAR-T cells was assessed via live cell imaging of GFP-expressing cancer cells on an Incucyte device (Sartorius).
Animal experiments
In brief, immunodeficient mice were infused with 0.5 × 106 Nalm-6 cells (expressing luciferase) via tail vein injection. Four days later, 0.5 × 106 or 1 × 106 TCR-deficient CD19-CAR-T cells were infused intravenously. CAR-T cells were generated either via targeted integration of a CAR or a truncCAR into the TRAC or CD3ζ-gene, respectively, or by LV gene transfer and consecutive TRAC-knockout (KO). Tumor burden was assessed as previously reported38 using bioluminescence imaging. The staff carrying out the mice experiments were blinded for the T-cell conditions. Mice were sacrificed according to study protocol either at ethical end points (models 1 + 3) or 5 weeks after tumor inoculation (model 2) according to the respective study protocols. For more detailed study protocols refer to supplementary Methods.
Data analysis, statistics and presentation
Flow cytometry data was analysed with FlowJo Software (BD). Prism 9 (GraphPad) was used to create graphs and perform statistics. Illustrations were created on BioRender.com.
The study with material from human participants was performed in accordance with the declaration of Helsinki (Charité ethics committee approval EA4/091/19). The in vivo CAR-T-cell potency studies were performed in accordance with the German animal welfare act and the EU-directive 2010/63. Animal studies 1 and 3 were approved by local authorities (Landesamt für Gesundheit und Soziales, Berlin, Germany) under the permission A0010/19. Model 2 was approved by the Lower Saxony Office for Consumer Protection and Food Safety-LAVES (permit number 16/2222).
Results
Integration of truncated CD3ζ-deficient (trunc)CARs in CD3ζ enables redirection of T cells
We performed targeted delivery of a 1419-bp-sized CD19-specific truncCAR (CD19-IgG1-CD28) into CD3ζ (exon 2, beginning of intracellular domain) and TRAC (exon 1) using CRISPR-Cas9 (Figure 1A). As additional control, we integrated a full-length 2015-bp-sized CAR (CD19-IgG1-CD28-CD3ζ) into TRAC as recently described.33 Transgene expression in primary human T cells was confirmed by flow cytometry (Figure 1B). Like TRAC-editing, CAR-integration into the CD3ζ-gene disrupted TCR/CD3 surface expression in the majority of cells. In a VITAL-assay,39 which monitors relative antigen-specific cytotoxicity, TRAC-edited truncCAR-T cells did not elicit any antigen-specific cytotoxicity as expected due to the lack of a main activation domain (Figure 1C). In contrast, CD3ζ-edited truncCAR-T cells effectively lysed CD19+ cells similar to TRAC-edited T cells transfected with the full-length CAR (Figure 1C), confirming the generation of functionally active truncCAR-CD3ζ fusion protein after insertion of CAR moieties into the endogenous CD3ζ-gene.
Integration of a truncated CD19-specific CAR into CD3ζ, but not TRAC, conveys cytotoxicity in conventional T cells toward CD19+ leukemia cells. (A) full-length second-generation CAR protein (left) and virus-free knock-in strategies to integrate a full-length CAR into TRAC or a truncated CAR (truncCAR) into TRAC or CD3ζ. (B) Flow cytometry dot plots after knock-in. Transgene integration into TRAC or CD3ζ disrupts expression of the TCR/CD3 complex. (C) Relative cytotoxicity in coculture with (CD19+) Nalm-6 target cells and CD19 KO Nalm-6 control cells (VITAL assay). Calculation of relative cytotoxicity according to formula stated in methods section. (n = 2 biological replicates each in 2 technical replicates; ordinary one-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance). (D-G) Functional testing of CD3ζ truncCAR, T cells in comparison to TRAC CAR and LV CAR-T cells. (D) Mean fluorescence intensity (MFI) determined by flow cytometry as a measure of cellular CAR expression and normalized to each donor’s mean CAR MFI in the TRAC condition. (n = 7 biological replicates each in 2-5 technical replicates; mixed-effects analysis with Geisser-Greenhouse correction + Holm-Šídák multiple comparison test with individual variances computed for each comparison). (E) Relative cytotoxicity towards CD19+ cells assessed in a 6-hour VITAL assay. (mock-E′: mock-electroporated controls without ribonucleoproteins/HDR templates) (n = 4 biological replicates each in 1-3 technical replicates; two-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance (F) Changes in CAR-expression levels (MFI normalized to start) after target cell encounter. (TRAC and LV in 4 biological replicates; CD3ζ in 2 biological replicates). (G) Acute lymphoblastic leukemia xenograft mouse model using luciferase-labeled Nalm-6 (CD19+) tumor cells. 4 days post Nalm-6 administration, 1 × 106 cryopreserved, 14-day expanded TCR-deleted CAR+ T cells were injected systemically. Tumor burden was assessed via bioluminescence imaging. (n = 5-6; 2-way ANOVA with Geisser-Greenhouse correction of log-transformed bioluminescence imaging data followed by Holm-Šídák multiple comparison test, with individual variances computed for each comparison). Asterisks in this and all further figures represent different P values calculated in the respective statistical tests (not significant [ns], P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001).
Integration of a truncated CD19-specific CAR into CD3ζ, but not TRAC, conveys cytotoxicity in conventional T cells toward CD19+ leukemia cells. (A) full-length second-generation CAR protein (left) and virus-free knock-in strategies to integrate a full-length CAR into TRAC or a truncated CAR (truncCAR) into TRAC or CD3ζ. (B) Flow cytometry dot plots after knock-in. Transgene integration into TRAC or CD3ζ disrupts expression of the TCR/CD3 complex. (C) Relative cytotoxicity in coculture with (CD19+) Nalm-6 target cells and CD19 KO Nalm-6 control cells (VITAL assay). Calculation of relative cytotoxicity according to formula stated in methods section. (n = 2 biological replicates each in 2 technical replicates; ordinary one-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance). (D-G) Functional testing of CD3ζ truncCAR, T cells in comparison to TRAC CAR and LV CAR-T cells. (D) Mean fluorescence intensity (MFI) determined by flow cytometry as a measure of cellular CAR expression and normalized to each donor’s mean CAR MFI in the TRAC condition. (n = 7 biological replicates each in 2-5 technical replicates; mixed-effects analysis with Geisser-Greenhouse correction + Holm-Šídák multiple comparison test with individual variances computed for each comparison). (E) Relative cytotoxicity towards CD19+ cells assessed in a 6-hour VITAL assay. (mock-E′: mock-electroporated controls without ribonucleoproteins/HDR templates) (n = 4 biological replicates each in 1-3 technical replicates; two-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance (F) Changes in CAR-expression levels (MFI normalized to start) after target cell encounter. (TRAC and LV in 4 biological replicates; CD3ζ in 2 biological replicates). (G) Acute lymphoblastic leukemia xenograft mouse model using luciferase-labeled Nalm-6 (CD19+) tumor cells. 4 days post Nalm-6 administration, 1 × 106 cryopreserved, 14-day expanded TCR-deleted CAR+ T cells were injected systemically. Tumor burden was assessed via bioluminescence imaging. (n = 5-6; 2-way ANOVA with Geisser-Greenhouse correction of log-transformed bioluminescence imaging data followed by Holm-Šídák multiple comparison test, with individual variances computed for each comparison). Asterisks in this and all further figures represent different P values calculated in the respective statistical tests (not significant [ns], P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001).
Off-target assessment of CD3ζ gene editing
To ensure high precision of CRISPR-Cas9-mediated CD3ζ-targeting, we performed off-target assessment with CAST-Seq36 which did not reveal any chromosomal translocations. The analysis revealed only the expected on-target aberrations including a very rare 15 Mb deletion between CD3ζ and a potential off-target site located on the same chromosome (supplemental Figure 1).
CD19-specific CD3ζ-truncCAR and TRAC-CAR-T cells have comparable CAR-regulation and antileukemia activity in vivo
We next compared CD19-CAR-expression levels and antileukemia potential of CD3ζ-truncCAR-T cells, TRAC-CAR-T cells and lentivirus-transduced (LV) TRAC-KO CAR-T cells in vitro. CAR-expression levels in CD3ζ-truncCAR-T cells were lower than in TRAC-integrated and LV counterparts (Figure 1D). Compared to TRAC-CAR-T cells, CD3ζ-truncCAR-T cells and LV CAR-T cells displayed significantly reduced dose-dependent killing in a 6-hour VITAL assay at some effector:target cell ratios (Figure 1E). Upon CD19+ Nalm-6 target cell engagement, CD3ζ-truncCAR and TRAC-CAR-T cells downregulated the CAR for 12-24 hours before returning to their relative baseline levels (Figure 1F). In contrast, LV CAR-T cells upregulated CAR-expression in response to stimulation and exceeded their baseline levels after 48 hours. Previous studies demonstrated that physiological control of CAR-expression in the TRAC locus enhances their antitumor performance in vivo.17 Therefore, we evaluated the antitumor efficacy of the differently engineered T cells (LV, TRAC, CD3ζ-truncCAR) in 2 independent, blinded xenograft models of acute lymphoblastic leukemia using immunodeficient mice. In both experiments, 0.5 × 106 luciferase-labeled CD19+ Nalm-6 tumor cells were administered systemically prior to the infusion of TCR-deficient CAR-T cells 4 days later. In mouse model 1 (Figure 1G; supplemental Figure 2a), mice received 14-day expanded cryopreserved CAR-T cells at a dose of 1 × 106 CAR+ cells. All 3 CAR-T treatments slowed tumor growth to a similar extent (control: L1CAM-CAR40). In vivo efficacy was also observed in mouse model 2 (supplemental Figure 2b). Here, fresh, 14-day expanded CAR-T cells were administered at a dose of 0.5 × 106 CAR+ cells. Five weeks after tumor inoculation, mice treated with TRAC- and CD3ζ-edited CAR-T cells had significantly lower leukemia burden than animals which received lentivirus-transduced CAR-T cells (supplemental Figure 2b).
Tightly-controlled HER2-CAR expression from CD3ζ avoids antigen-independent differentiation, but leads to low cytokine production
To test CD3ζ-editing for another CAR-target antigen, we generated HER2-specific CAR-T cells via integration of a truncCAR into CD3ζ.41-44 As controls, we integrated the full-length HER2-CAR into TRAC, or into the safe-harbor locus hAAVS1 driven by an exogenous LTR/EF1α-promoter. CD3ζ-edited HER2-truncCAR-T cells demonstrated the lowest CAR-expression level (supplemental Figure 3a). TRAC-edited T cells displayed unexpectedly high HER2-CAR-surface density, exceeding the LTR/EF1α-driven CAR expression from the hAAVS1 locus and CD19-CAR expression from TRAC. Phenotype analysis demonstrated antigen-independent differentiation in an expression level dependent manner (supplemental Figure 3b). TRAC-HER2-CAR-T cells expressed the highest levels of inhibitory receptors PD-1, Lag-3 and Tim-3 after 2 weeks expansion (supplemental Figure 3c). In contrast, CD3ζ-HER2-truncCAR-T cells displayed differentiation and exhaustion marker profiles mirroring the CAR– T-cell fraction which indicates reduced or absent tonic signaling. Further, CD3ζ-edited HER2-truncCAR-T cells showed less activation-induced cell death than TRAC- or AAVS1-edited CAR-T cells after CAR stimulation using plate-bound anti-CAR antibody (supplemental Figure 3d). CD3ζ-truncCAR-T cells showed similar cytotoxicity toward 3 different HER2+ tumor cell lines when compared to TRAC-HER2-CAR-T cells (supplemental Figure 3e). However, HER2-CD3ζ-truncCAR-T cells secreted less TNFα and IFNγ when cocultured with tumor cells (supplemental Figure 3f), indicating that lower HER2-CAR expression may reduce tonic signaling but potentially impairs other functions.
Increasing CAR expression from the CD3ζ locus improves cytokine production and antitumor efficacy
We hypothesized that the reduced effector functions of CD19- and HER2-specific CAR-T cells generated via CD3ζ-truncCAR integration is caused by the lower amounts of CAR molecules available for synapse formation. Optimization of the 2A-cleavage peptide by the addition of a GSG-linker has been shown to increase protein expression in multi-cistronic transgenes.45,46 In the CD3ζ-truncCAR condition, an optimized GSG-P2A (Figure 2A) increased CD19-CAR expression even above the TRAC-CAR condition (Figure 2B). This modification increased CAR-mediated cytotoxicity (Figure 2C) and intracellular cytokine production to levels similar to TRAC-CAR-T cells (Figure 2D; supplemental Figure 4).
Evaluation of an optimized CD3ζ truncCAR transgene and its impact on CAR-T cell function in vitro. (A) dsDNA templates for targeted delivery of a CAR or truncCAR respectively into TRAC (left) or CD3ζ (middle), as in Figure 1A, and for targeted delivery of a GSG-P2A-linker-modified truncCAR into CD3ζ (right). (B) Top: Mean fluorescence intensity (MFI) determined by flow cytometry at steady state (n = 4 biological replicates in 4-6 technical replicates in 2 independent experiments, data normalized to mean of TRAC for each donor; mixed-effects analysis with Geisser-Greenhouse correction followed by Holm-Šídák multiple comparison test, with individual variances computed for each comparison). Bottom: dynamics of CAR MFI after CAR-stimulation using CD19+ Nalm-6 tumor cells. (n = 3-4 biological replicates in 1-2 technical replicates). (C) Relative cytotoxicity assessed in a 6-hour VITAL assay (similar to Figure 1C, n = 4 biological replicates in 3 technical replicates; two-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance.). (D) Cytokine expression in CAR+ cells in response to control (CD19−) cell or target (CD19+) cell encounter (n = 3 biological replicates). (E-H) CAR-T-cell rechallenge in serial cocultures with Nalm-6 target cells. (E) Top: CAR MFI normalized to TRAC condition at steady state (n = 2 biological replicates in 4 technical replicates; statistics as in B). Bottom: dynamics of CAR MFI after target cell engagement (n = 2-4 biological replicates in 1-2 technical replicates). (F) 6-hour VITAL assay. (n = 3 biological replicates in 3-4 technical replicates; two-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance.). (G) Top: relative expansion of CAR+ T cells. Bottom: CAR+ frequency within T-cell products. (n = 4 biological replicates). (H) Cell surface expression of inhibitory receptors (LAG-3, PD-1, TIM-3; means of n = 4 biological replicates). (I) In vivo CAR-T-cell efficacy tested in Nalm-6 acute lymphoblastic leukemia xenograft mouse model (n = 5-6 mice/group; multiple log-rank tests).
Evaluation of an optimized CD3ζ truncCAR transgene and its impact on CAR-T cell function in vitro. (A) dsDNA templates for targeted delivery of a CAR or truncCAR respectively into TRAC (left) or CD3ζ (middle), as in Figure 1A, and for targeted delivery of a GSG-P2A-linker-modified truncCAR into CD3ζ (right). (B) Top: Mean fluorescence intensity (MFI) determined by flow cytometry at steady state (n = 4 biological replicates in 4-6 technical replicates in 2 independent experiments, data normalized to mean of TRAC for each donor; mixed-effects analysis with Geisser-Greenhouse correction followed by Holm-Šídák multiple comparison test, with individual variances computed for each comparison). Bottom: dynamics of CAR MFI after CAR-stimulation using CD19+ Nalm-6 tumor cells. (n = 3-4 biological replicates in 1-2 technical replicates). (C) Relative cytotoxicity assessed in a 6-hour VITAL assay (similar to Figure 1C, n = 4 biological replicates in 3 technical replicates; two-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance.). (D) Cytokine expression in CAR+ cells in response to control (CD19−) cell or target (CD19+) cell encounter (n = 3 biological replicates). (E-H) CAR-T-cell rechallenge in serial cocultures with Nalm-6 target cells. (E) Top: CAR MFI normalized to TRAC condition at steady state (n = 2 biological replicates in 4 technical replicates; statistics as in B). Bottom: dynamics of CAR MFI after target cell engagement (n = 2-4 biological replicates in 1-2 technical replicates). (F) 6-hour VITAL assay. (n = 3 biological replicates in 3-4 technical replicates; two-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance.). (G) Top: relative expansion of CAR+ T cells. Bottom: CAR+ frequency within T-cell products. (n = 4 biological replicates). (H) Cell surface expression of inhibitory receptors (LAG-3, PD-1, TIM-3; means of n = 4 biological replicates). (I) In vivo CAR-T-cell efficacy tested in Nalm-6 acute lymphoblastic leukemia xenograft mouse model (n = 5-6 mice/group; multiple log-rank tests).
We next evaluated the impact of the different CD19-CAR-expression levels during repeated leukemia challenges (Figure 2E-H) which were performed once per week at a CAR+ T cell to tumor cell ratio of 1:1. After serial coculture, all 3 conditions retained their physiological CAR-expression dynamics, but basal CAR expression did not differ anymore between CD3ζ-truncCARGSG and TRAC, while the original CD3ζ-truncCAR cells still showed lower CAR expression (Figure 2E). Interestingly, all 3 conditions showed similar cytotoxicity (Figure 2F) and proliferation (Figure 2G). CD3ζ-edited conditions displayed slightly lower expression of inhibitory markers in the CD8 compartment after serial leukemia rechallenges (Figure 2H; detailed analysis in supplemental Figure 5). Serial coculture resulted in a similar shift towards a more differentiated phenotype in all conditions (supplemental Figure 6a) with a trend towards a CD8 polarization in the CD3ζ-truncCARGSG condition (supplemental Figure 6b). Of note, the differences in cytokine production were preserved (supplemental Figure 6c).
Finally, we assessed the in vivo antitumor efficacy in a Nalm-6 mouse model (Figure 2I). Here, ex vivo expansion of CAR-T cells was shortened to 6 days due to a preferable phenotype with a high proportion of central memory (TCM) and naïve-like (TN) cells and a physiological CD4/CD8 ratio (supplemental Figure 7). TRAC-CAR-T cells and CD3ζ-truncCAR both resulted in a similarly prolonged, statistically significant survival compared to mock-electroporated T cells. Expression-tuned CD3ζ-truncCARGSG-T cells showed the highest survival benefit which was statistically significant to the other treatment groups.
CD3ζ-targeting allows redirection of more immune cell types than TRAC-editing
Nonconventional T cells and NK cells have emerged as important CAR carriers for adoptive cell transfer.2,3,47-49 To test the suitability of CD3ζ-editing for different cell therapy applications, we compared CD3ζ-truncCAR and TRAC-CAR integration in TCRγ/δ T cells, Treg and primary NK cells (Figure 3). Like TRAC, CD3ζ is expressed in all TCRα/β T cells and gene editing of the respective loci led to similar frequencies of HLA-A2-specific CARs in Treg cells (Figure 3A). Furthermore, CD3ζ is expressed in other immune cells which do not express TRAC and should therefore not be targetable by in-frame TRAC integration, notably TCRγ/δ T cells and NK cells. To our surprise, TRAC-editing in TCRγ/δ T cells resulted in substantial CAR+/TCRγ/δ+ double-positive fractions, suggesting mRNA transcription of the TRAC-gene in TCRγ/δ T cells (Figure 3B). As expected for NK cells, truncCAR integration into CD3ζ, but not TRAC, led to detectable CAR expression. Therefore, CD3ζ-gene editing may serve as a universal approach to redirect different conventional and nonconventional T cells as well as NK cells with CARs (Figure 3C).
CD3ζ truncCAR-integration facilitates CAR expression in different nonconventional T-cell subtypes and NK cells. (A) HLA-A2 CAR-integration in regulatory T-cells, n = 3 biological replicates. (B) CD19-CAR integration in TCRγ/δ T cells. TRAC integration generates CAR+/TCRγ/δ+ double positive T cells, n = 2 biological replicates. (C) Integration of a CD19-CAR in primary human NK cells, n = 6 biological replicates.
CD3ζ truncCAR-integration facilitates CAR expression in different nonconventional T-cell subtypes and NK cells. (A) HLA-A2 CAR-integration in regulatory T-cells, n = 3 biological replicates. (B) CD19-CAR integration in TCRγ/δ T cells. TRAC integration generates CAR+/TCRγ/δ+ double positive T cells, n = 2 biological replicates. (C) Integration of a CD19-CAR in primary human NK cells, n = 6 biological replicates.
CD3ζ-KO does not impede canonical functions of primary NK cells
In NK cells, CD3ζ is an adapter protein which assembles with activating killer cell immunoglobulin-like receptors (KIR) and Fc-receptors, such as CD16.49 These cells dynamically balance inhibitory and activating signals, favoring the elimination of target cells upon detecting elevated activating KIR signaling (triggered by stress or cancer markers such as MICA/B) or when CD16 mediates ADCC. Our knock-in approach impedes the expression of free CD3ζ-protein, which could potentially impair NK-cell activation and disturb canonical NK functions. To investigate these potential downsides, we disrupted CD3ζ in primary human NK cells, either via CRISPR-Cas9-mediated KO or CD3ζ-GFP-reporter knock-in that disrupts CD3ζ (Figure 4A). Measuring cytotoxicity (Figure 4B) and degranulation (Figure 4C) in simple cocultures, we did not observe major differences regarding missing-self activation, cancer-directed activation, and alloreactivity. Importantly, gene editing of CD3ζ did not alter CD16 expression. (Figure 4D). We also did not detect differences in anti-CD20-antibody–induced ADCC towards the CD20+ cell line Jeko-1 (Figure 4E) which is partially resistant to NK-cell cytotoxicity (supplemental Figure 8).
CD3ζ-disuption does not impede canonical NK cell functions in vitro. (A) CD3ζ editing outcomes assessed by flow cytometry. (B) Cytotoxicity of primary CD3ζ disrupted NK cells in simple 16 hours coculture assay with K562 cells, Nalm-6 cells or allogeneic PBMC (n = 3 biological replicates). (C) Degranulation of primary CD3ζ disrupted NK cells assessed by flow cytometry (n = 3 biological replicates; two-way ANOVA followed by Dunnett’s multiple comparison test with a single pooled variance). (D) Expression of CD16 and CD3ζ in wild-type NK-92 cells and primary NK cells after CD3ζ-disruption. (E) ADCC of primary CD3ζ-disrupted NK cells against CD20+ bGal− Jeko-1 cells at different concentrations of antibodies specific for CD20 (rituximab) or bGal (n = 3 biological replicates, each in 3 technical replicates).
CD3ζ-disuption does not impede canonical NK cell functions in vitro. (A) CD3ζ editing outcomes assessed by flow cytometry. (B) Cytotoxicity of primary CD3ζ disrupted NK cells in simple 16 hours coculture assay with K562 cells, Nalm-6 cells or allogeneic PBMC (n = 3 biological replicates). (C) Degranulation of primary CD3ζ disrupted NK cells assessed by flow cytometry (n = 3 biological replicates; two-way ANOVA followed by Dunnett’s multiple comparison test with a single pooled variance). (D) Expression of CD16 and CD3ζ in wild-type NK-92 cells and primary NK cells after CD3ζ-disruption. (E) ADCC of primary CD3ζ-disrupted NK cells against CD20+ bGal− Jeko-1 cells at different concentrations of antibodies specific for CD20 (rituximab) or bGal (n = 3 biological replicates, each in 3 technical replicates).
CD3ζ-truncCAR knock-in conveys cytotoxicity in primary NK cells and NK-92 cells
Using PBMC-derived NK cells, we next sought to characterize and compare CD3ζ-truncCAR-NK cells with LV-transduced NK cells (Figure 5). CD3ζ-truncCAR knock-in rates remained below 10% and were thus considerably lower than in T cells (Figure 5A). Despite using a high multiplicity of infection (MOI = 5) for LV CAR transfer, transduction rates were higher only in some replicates. While CAR MFI did not significantly differ between the conditions, the coefficient of variation of the CAR MFI was significantly lower after CD3ζ-integration indicating a more controlled and predictable transgene expression after targeted CD3ζ-integration (Figure 5B). Both conditions, but not a TRAC-CAR knock-in control, showed dose-dependent CAR-mediated killing in a VITAL assay, an internally controlled coculture assay which is less biased by the NK cells’ CAR-independent (background) killing (Figure 5C). CD3ζ-truncCAR-NK cells significantly outperformed the LV control at the lowest dose and kept this trend at high doses. Analysis of the degranulation marker CD107a showed similar CAR-mediated activation of both LV and CD3ζ-truncCAR NK cells when cocultured with CD19-expressing allogeneic B cells (Figure 5D). As for CD3ζ-KO cells (Figure 4), ADCC towards the CD20+ cell line Jeko-1 was not altered for TRAC, LV or CD3ζ-truncCAR-NK cells compared to mock-electroporated (wildtype) NK cells (Figure 5E). Thus, CD3ζ-gene editing may be used to redirect primary NK cells with CARs while retaining their canonical functions.
CD3ζ-editing enables redirection of NK cells with CARs and does not impede canonical NK cell functions in vitro. CAR editing in primary NK cells via LV CAR transfer, TRAC-CAR or CD3ζ-truncCAR-integration: (A) CAR+ frequencies after editing (n = 6 biological replicates, mixed-effects analysis with Geisser-Greenhouse correction followed by Tukey’s multiple comparison test with individual variances computed for each comparison). (B) mean CAR expression (MFI) normalized to CD3ζ-truncCAR integrated NK cells and robust coefficient of variation in CAR+ cells (n = 6 biological replicates; t test). (C) CAR-dependent cytotoxicity detected in a VITAL assay (data normalized to mock-electroporated (wildtype) NK cells; n = 6 biological replicates each in 3-4 technical replicates; 2-way ANOVA followed by Tukey’s multiple comparison test with a single pooled variance). (D) Degranulation as indicator of NK effector function via flow cytometric detection of CD107a (n = 6 biological replicates; two-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance). (E) ADCC of primary (CAR) NK cells against CD20+ bGal− Jeko-1 cells. Bars represent killing for each condition in the presence of a CD20-targeting monoclonal antibody (0.5 μg/mL) normalized to the respective condition without supplemented antibody (n = 5 biological replicates; mixed-effects analysis with Geisser-Greenhouse correction followed by Tukey’s multiple comparison test with individual variances computed for each comparison). (F-H) CD19-CAR (2) transfer to NK-92 cells via AAVS1 integration of a CMV promotor-controlled, full-length CAR or CD3ζ integration of a truncCAR. CAR+ fractions were enriched using MACS. (F) CAR expression in flow cytometry histograms. (G) CAR-dependent cytotoxicity in a 4-hour VITAL-assay (n = 6 technical replicates; two-way ANOVA with Tukey’s multiple comparison test with a single pooled variance. (H) CAR-independent cytotoxicity towards the MHC I deficient, CD19− K562 (control) cell line (n = 15 technical replicates; two-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance).
CD3ζ-editing enables redirection of NK cells with CARs and does not impede canonical NK cell functions in vitro. CAR editing in primary NK cells via LV CAR transfer, TRAC-CAR or CD3ζ-truncCAR-integration: (A) CAR+ frequencies after editing (n = 6 biological replicates, mixed-effects analysis with Geisser-Greenhouse correction followed by Tukey’s multiple comparison test with individual variances computed for each comparison). (B) mean CAR expression (MFI) normalized to CD3ζ-truncCAR integrated NK cells and robust coefficient of variation in CAR+ cells (n = 6 biological replicates; t test). (C) CAR-dependent cytotoxicity detected in a VITAL assay (data normalized to mock-electroporated (wildtype) NK cells; n = 6 biological replicates each in 3-4 technical replicates; 2-way ANOVA followed by Tukey’s multiple comparison test with a single pooled variance). (D) Degranulation as indicator of NK effector function via flow cytometric detection of CD107a (n = 6 biological replicates; two-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance). (E) ADCC of primary (CAR) NK cells against CD20+ bGal− Jeko-1 cells. Bars represent killing for each condition in the presence of a CD20-targeting monoclonal antibody (0.5 μg/mL) normalized to the respective condition without supplemented antibody (n = 5 biological replicates; mixed-effects analysis with Geisser-Greenhouse correction followed by Tukey’s multiple comparison test with individual variances computed for each comparison). (F-H) CD19-CAR (2) transfer to NK-92 cells via AAVS1 integration of a CMV promotor-controlled, full-length CAR or CD3ζ integration of a truncCAR. CAR+ fractions were enriched using MACS. (F) CAR expression in flow cytometry histograms. (G) CAR-dependent cytotoxicity in a 4-hour VITAL-assay (n = 6 technical replicates; two-way ANOVA with Tukey’s multiple comparison test with a single pooled variance. (H) CAR-independent cytotoxicity towards the MHC I deficient, CD19− K562 (control) cell line (n = 15 technical replicates; two-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance).
Low CAR gene transfer rates in primary NK cells are a challenge in the field.50,51 The use of immortal cell lines does not require high CAR-integration rates because the edited cells can be enriched prior to a potentially unlimited expansion. One example is the NK-cell-derived cancer cell line NK-92, which has been used as the cell source for CAR-NK therapy in multiple clinical trials.52 To test the feasibility of our approach in NK-92 cells, we generated CD19-specific CD3ζ-truncCAR-NK-92 cells and hAAVS1-CAR-NK-92 cells as controls (Figure 5F). CAR+ NK-92 cells were enriched via MACS. Compared to hAAVS1, CD3ζ-truncCAR-NK-92 cells displayed higher CAR-mediated cytotoxicity (Figure 5G) and superior (CAR-independent) missing-self activation towards the MHC-I-deficient cell line K562 (Figure 5H). As NK-92 cells do not express CD16, ADCC was not studied.
Discussion
Here, we propose a strategy for site-specific CAR gene transfer to T and NK cells. Truncated CAR transgenes lacking a TCR-like effector domain were precisely inserted into the CD3ζ-gene. Via in-frame integration, a complete CAR fusion gene (comprising an exogenous truncated CAR transgene and the endogenous CD3ζ-gene) is formed resulting in surface expression of functional CAR proteins. In T cells, this prevents TCR/CD3 complex assembly and brings the CAR under the transcriptional regulation of the CD3ζ-promoter. Despite its function as a signal transducer of activating NK-cell receptors, CD3ζ can be edited to generate functional CAR-NK cells without affecting their canonical functions.
The CD3ζ-locus is a CAR-integration site which limits necessary transgene size and shares features and advantages with the TRAC knock-in in T cells.17,27 Like in the TRAC approach, CD3ζ-editing causes TCR-ablation, because the CAR’s CD3ζ-domain cannot rescue TCR/CD3 expression in CD3ζ-KO T cells.53 Together, this circumvents alloreactivity in T cells and should minimize the risk for GvHD if residual TCR+ T cells are efficiently depleted before allogeneic application of CD3ζ-edited CAR-T cells.54,55 Therefore, the CD3ζ-approach may be preferentially suited for allogeneic applications. Further, the physiological TCR-like CAR downregulation after antigen-engagement (achieved via TRAC- or CD3ζ-integration) may enable transient resting, preventing terminal differentiation and exhaustion.17,56 When considering autologous manufacturing, transgene expression from TCR/NK-cell lineage genes, such as TRAC or CD3ζ, provides a safety advantage because it should prevent the inadvertent CAR expression in B-cell leukemic blasts which can cause B-ALL relapse.57
CAR-expression level influences CAR-T-cell performance, differentiation and exhaustion in preclinical and clinical settings.17,58,59 For viral gene transfer, CAR surface density may be modulated by variation of viral titers, aiming for different transgene copy numbers, as well as promoters60 or transgene designs.58 Exogenous promoters required for CAR expression after random integration can cause unphysiological CAR upregulation after antigen-encounter (Figure 2C) leading to cellular exhaustion.17 The promoters and respective 5′- or 3′-UTR could also contribute to the differences in transgene expression when comparing CD3ζ- or TRAC-editing. However, we have also observed transgene-related differences (CD19-CAR vs HER2-CAR; see supplemental Figure 3) that were locus-dependent which warrants further investigation. We show that basal CD19-CAR expression can be increased by insertion of a GSG-linker before the 2A-self-cleavage peptide (Figure 2). Increasing the CD19-CAR expression in CD3ζ-truncCARGSG-T cells was associated with enhanced cytokine production after antigen-engagement and improved antileukemia activity in vivo (Figure 2). Modulation of both, steady-state CAR expression and dynamic CAR regulation, may impact the activation threshold of the CAR-T cells. A lower CAR expression may be beneficial to mitigate antigen-independent differentiation of CARs prone to tonic signaling or reduce on-target off-tumor toxicity when targeting tumor-associated antigens upregulated in the tumor but not completely absent in normal tissue.61 Of note, all CARs used in this study employed the CD28 costimulatory domain. Future studies should revisit the contribution of other costimulatory domains to select the most efficacious CAR version for the targeted disease.
Serendipitously, TRAC-integration resulted in the generation of large fractions of CAR+/TCRγ/δ+ double-positive T cells (Figure 3), despite the apparent absence of a TRAC gene-product in this cell type. We hypothesize that this unexpected outcome arises from the interconnection between the genomic sites encoding the TCRα and TCRδ chains.62 Further investigation is warranted to explore potential synergies between CARs and certain γ/δ-TCRs in this distinct cell type.48
Unlike TRAC, CD3ζ-editing can be applied not only to all T-cell subsets but also to NK cells (Figure 3). Deleterious mutations of CD3ζ have been found to be a cause for severe combined immunodeficiency, and patient NK cells were hypo-responsive in tumor cocultures and after CD16 stimulation.63,64 This raised concerns regarding the impact of CD3ζ-editing on the functionality of resulting CAR-NK cells. However, in this study, CD3ζ-disruption in primary human NK cells from healthy donors did not impair crucial immune functions such as ADCC, cytotoxicity or degranulation (Figure 4). These findings align with previous research indicating that FcRγ compensates CD3ζ-loss after knockout, thereby enabling ADCC by primary NK cells.65
This study is the first to demonstrate nonviral CRISPR-Cas-mediated knock-in for functional redirection of primary human NK cells with CARs. In comparison to CAR-T cells, CAR-NK cells have a favorable safety profile as they lack alloreactivity and show a reduced incidence of severe cytokine release syndrome and neurotoxicity.2 CAR-NK cells can be combined with monoclonal antibodies for synergistic activity when targeting heterogenous tumors. For example, the CD19-specific CAR-NK cells generated by CD3ζ-editing (Figure 5) may be combined with the CD20-targeting antibody rituximab to overcome antigen-escape and relapse by CD19-negative cancer cells. However, allogeneic CAR-NK cells are generally short-lived and do not persist. Therefore, physiological CAR regulation which improves persistence in T cells may not confer similar biological advantages in NK cells. Consequently, the primary advantages of CD3ζ-editing in NK cells may be related to manufacturing and cost-aspects of miniaturized nonviral vectors. Before testing in suitable in vivo models and future clinical translation, the efficacy of nonviral reprogramming of primary NK cells should be further increased, for example by using pharmacological enhancers33 and/or end-modified ssDNA donor templates.66
CD3ζ-editing for CAR gene transfer should be combined with other edits to enhance the functionality of CAR-T/NK-cell products for autologous and allogeneic use. First clinical trials demonstrated that TCR-deleted allogeneic CAR-T cells can induce remissions in heavily pretreated B-ALL and B-lymphoma patients, but additional gene editing was needed to circumvent immunological barriers of HLA-mismatches between CAR-T-cell donor and patient.54,67,68 Therefore, CD3ζ-editing would benefit from those modifications to improve the efficacy of allogeneic CAR-T cells.54,69 Future studies may investigate the combination of CD3ζ-editing with additional KOs to improve functionality,70,71 safety72 as well as persistence67,73,74 of allogeneic T and NK cells. The respective additional edits required to improve the functionality of NK cells75,76 may differ from the ones proposed for T cells.71,77 Finally, complex editing may require the combination of nuclease-assisted gene transfer with other gene silencing modalities such as base editing78,79 to reduce the risk for genomic rearrangements with unknown biological impact.54,77,80
Acknowledgments
The authors express their gratitude to the following individuals for their valuable contributions: Laila Hassan (Charité, Berlin, Germany [deceased]) for her technical assistance with the experiment presented in Figure 1; Silke Schwiebert from Annette Künkele's laboratory (Charité, Berlin, Germany) for her assistance with lentivirus preparation; Andreas Schneider, Alina Pruene and Tobias Braun (Medizinische Hochschule Hannover, Hannover, Germany) for their support in animal model 2; Amanda Roswell-Shaw and Daniel Wang (Baylor College of Medicine, Houston, TX) for their assistance with HER2-CAR-T-cell cocultures; Geoffroy Andrieux (from the Institute of Medical Bioinformatics and Systems Medicine, Medical Center-University of Freiburg) for his help with the bioinformatic part in the CAST-Seq pipeline; and Chiara Romagnani and Timo Rückert (German Rheumatism Research Center, a Leibniz Institute, Berlin, Germany) for their expert advice on NK cells.
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 825392 (ReSHAPE: ReSHAPE-h2020.eu) to M.S.-H., H.-D.V., P.R., and D.L.W. Further, the project received funding from the European Union under grant agreement no. 101057438 (geneTIGA: genetiga-horizon.eu) to T.C., H.-D.V., P.R., and D.L.W. J.K. and D.L.W. thank the Einstein Center for Regenerative Therapies (ECRT) for support via the ECRT Research Grant (2020-2022) and the ECRT Young Scientist Kickbox Grant. J.K. and D.L.W. were supported by the SPARK-BIH Program by the Berlin Institute of Health, Germany. M.A. is partially supported by award no. P30CA014089 from the National Institutes of Health, National Cancer Institute. The R.S. laboratory was financed by grants from the German Cancer Aid (Deutsche Krebshilfe no. 70114234), by the Jackson Laboratory (LV-HLA2), and by a professorship funded by the Cancer Research Center Cologne Essen (CCCE).
Views and opinions expressed are those of the author(s) only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency (HADEA). Neither the European Union nor the granting authority can be held responsible for them.
Illustrations were created with BioRender.com.
Authorship
Contribution: J.K. designed the study, planned and performed experiments, analyzed results, interpreted the data, and wrote the manuscript; C.F., V.D., and W.D., planned and performed experiments, analyzed results, interpreted the data, and edited the manuscript; V.G., M. Stein, T.Z., S.S., C.E.P., L.A., and J.A. performed experiments and analyzed results; C.F.-G. performed and interpreted CAST-seq and provided respective sections for the manuscript; M. Suzuki, J.H., and R.S. planned experiments, interpreted data, and edited the manuscript; H.A. provided materials (HER2-CAR transgenes41), interpreted the data and edited the manuscript; A.K., M.A.-e.-E., and A.P. provided reagents, interpreted data and edited the manuscript; T.C. supervised work on CAST-seq, provided reagents, interpreted data, and edited the manuscript; H.-D.V., P.R., and M.S.-H. supervised parts of the study, provided reagents, interpreted data, and edited the manuscript; D.L.W. designed and led the study, planned experiments, analyzed results, interpreted data, and wrote the manuscript; and all authors reviewed and approved the manuscript in its final form.
Conflict-of-interest disclosure: J.K., H.-D.V., P.R., M.S.-H., and D.L.W. are listed as inventors on a patent application related to the work presented in this manuscript. J.A. and J.H. are employees of Experimental Pharmacology & Oncology Berlin Buch GmbH. H.-D.V. is founder and CSO at CheckImmune GmbH. P.R., H.-D.V., and D.L.W. are cofounders of the startup TCBalance Biopharmaceuticals GmbH focused on regulatory T-cell therapy. R.S. is a founding shareholder and scientific advisor of BioSyngen/ Zelltechs Pte Ltd (Republic of Singapore). The remaining authors declare no competing financial interests.
Correspondence: Dimitrios L. Wagner, Berlin Center for Advanced Therapies, Charité-Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany; email: dimitrios-l.wagner@charite.de.
References
Author notes
HER2-CARs were previously published.41 Other CAR/HDR-templates and sgRNA sequences are provided in supplemental Table 1. Plasmids encoding CD3ζ-HDR-templates will be distributed through Addgene (pUC19-HDRT-CD3ζ-truncCARGSG Addgene ID: 215758; pUC19-HDRT-CD3ζ-truncCARGSG Addgene-ID: 215759). All other data may be requested from the corresponding author, Dimitrios L. Wagner (dimitrios-l.wagner@charite.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Integration of a truncated CD19-specific CAR into CD3ζ, but not TRAC, conveys cytotoxicity in conventional T cells toward CD19+ leukemia cells. (A) full-length second-generation CAR protein (left) and virus-free knock-in strategies to integrate a full-length CAR into TRAC or a truncated CAR (truncCAR) into TRAC or CD3ζ. (B) Flow cytometry dot plots after knock-in. Transgene integration into TRAC or CD3ζ disrupts expression of the TCR/CD3 complex. (C) Relative cytotoxicity in coculture with (CD19+) Nalm-6 target cells and CD19 KO Nalm-6 control cells (VITAL assay). Calculation of relative cytotoxicity according to formula stated in methods section. (n = 2 biological replicates each in 2 technical replicates; ordinary one-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance). (D-G) Functional testing of CD3ζ truncCAR, T cells in comparison to TRAC CAR and LV CAR-T cells. (D) Mean fluorescence intensity (MFI) determined by flow cytometry as a measure of cellular CAR expression and normalized to each donor’s mean CAR MFI in the TRAC condition. (n = 7 biological replicates each in 2-5 technical replicates; mixed-effects analysis with Geisser-Greenhouse correction + Holm-Šídák multiple comparison test with individual variances computed for each comparison). (E) Relative cytotoxicity towards CD19+ cells assessed in a 6-hour VITAL assay. (mock-E′: mock-electroporated controls without ribonucleoproteins/HDR templates) (n = 4 biological replicates each in 1-3 technical replicates; two-way ANOVA followed by Holm-Šídák multiple comparison test with a single pooled variance (F) Changes in CAR-expression levels (MFI normalized to start) after target cell encounter. (TRAC and LV in 4 biological replicates; CD3ζ in 2 biological replicates). (G) Acute lymphoblastic leukemia xenograft mouse model using luciferase-labeled Nalm-6 (CD19+) tumor cells. 4 days post Nalm-6 administration, 1 × 106 cryopreserved, 14-day expanded TCR-deleted CAR+ T cells were injected systemically. Tumor burden was assessed via bioluminescence imaging. (n = 5-6; 2-way ANOVA with Geisser-Greenhouse correction of log-transformed bioluminescence imaging data followed by Holm-Šídák multiple comparison test, with individual variances computed for each comparison). Asterisks in this and all further figures represent different P values calculated in the respective statistical tests (not significant [ns], P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/25/10.1182_blood.2023020973/2/m_blood_bld-2023-020973-gr1.jpeg?Expires=1765885588&Signature=Im87~5bIB~8MDtlW1vaw-9INxtSogqbbRmZfNqzOi2t1NTA4vEIjhvzxM7fnt--0F49ddHLdPH5rZfxVYqKYxFVBZbln9LSGSIYLdJNiLhAXc7D10nueAqgowDcCFyVeQEi1xucQlli9-M2ayZYrw17xY3ZMgYhOCn8Gqlr1x74UonZNm1k4Bq1Uex1v85NVqLVnMTD1e15b6c0c1V4Uo8IDVTkEl4QEG5QyOZL3R1g~QaPnNWmAIHViJGeYh9VgUG4IMnP2AVxTIG~3zvchSx~9laDg2IDPBrEIgHhZiwL9ySjmM8sDVUimKtWpXRdvfa~o4p8GbhAkmx0tb9mynQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal