In this issue of Blood, Granel and colleagues1 take advantage of a large observational cohort of children and adolescents with immune cytopenias to examine the subset of these patients who have positive antinuclear antibodies (ANA+) and then to follow the fate of these patients over time. They specifically address the question “Among those patients who have presented with chronic ITP [immune thrombocytopenia], isolated AIHA [autoimmune hemolytic anemia], or the combination, and who are either concurrently ANA+, or develop ANA positivity over time, what is the risk for, and time-to-progression to, systemic lupus erythematosus (SLE)?”
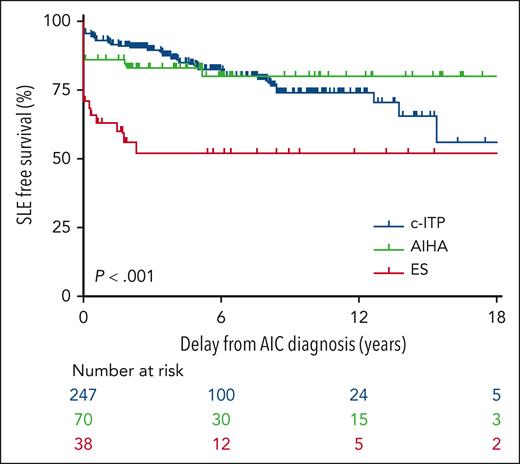
To answer the question would be a daunting task but for an existing, longitudinal, prospective, multicenter study. The OBS’CEREVANCE prospective cohort (clinicaltrials.gov NCT05937828) is based at a national reference center for autoimmune cytopenias in Bordeaux, France, led by Nathalie Aladjidi and her colleagues. It has been fertile ground for studies (eg, Ducassou et al2 and Pincez et al3) that depend on unbiased primary observations of cytopenia patients with long-term longitudinal follow-up. Many cohort patients enrolled since 2004 are now adults. The available sample size was substantial: CEREVANCE has tracked more than 1800 patients with immune cytopenias for the present study, and of these, 355 have had at least 1 ANA titer of 160 or greater, with the longest follow-up nearly 40 years, at 30 centers. The median follow-up was 5.1 years. The figure demonstrates cohort characteristics and the key time-to-progression analysis from the Granel article, sorted into patients who presented initially with chronic ITP, AIHA, or the 2 concurrently (Evans syndrome).
In the OBS’CEREVANCE longitudinal cohort, 355 of 1803 subjects with autoimmune cytopenias (AIC) had at least 1 ANA titer ≥ 160. Among this group, the time course of SLE-free survival differed markedly by the initial cytopenia: chronic ITP (c-ITP) is shown in blue, AIHA is shown in green, and Evans syndrome (ES) is shown in red. Only a fraction of the patients in each group went on to develop lupus, with a median delay of 3.4 years (range, 0.3-15.5). See Figure 1 in the article by Granel et al, which begins on page 1576.
In the OBS’CEREVANCE longitudinal cohort, 355 of 1803 subjects with autoimmune cytopenias (AIC) had at least 1 ANA titer ≥ 160. Among this group, the time course of SLE-free survival differed markedly by the initial cytopenia: chronic ITP (c-ITP) is shown in blue, AIHA is shown in green, and Evans syndrome (ES) is shown in red. Only a fraction of the patients in each group went on to develop lupus, with a median delay of 3.4 years (range, 0.3-15.5). See Figure 1 in the article by Granel et al, which begins on page 1576.
What can these results tell us? The association of autoimmune cytopenias with SLE is not new. As experienced clinicians have known for decades, these data convincingly redemonstrate that the cohort of pediatric patients with immune cytopenias most likely to progress to lupus are in the older age range (age > 10 years, relative risk 3.67) and have higher ANA titers (>160, relative risk 5.28; see figure). None of the patients with ANA titers < 160 went on to develop lupus. The progression time was substantially fastest in those with Evans syndrome, consistent with the idea that the biology of multilineage autoimmune cytopenias is distinct from ITP alone.4,5
About one-fourth of the nonlupus, ANA+ patients at initial observation were subsequently treated with hydroxychloroquine, and the remaining patients were not. The rate of progression to lupus was much lower in the treated group (5%) than in the group of those not treated (18%) (see Figure 4 in Granel et al). The difference is striking, but this was not a randomized trial, and confirmation of this result will be vital. Although the authors call reasonably for a randomized trial, it seems unlikely that a placebo-controlled randomization could occur today in France, because hydroxychloroquine use is widespread in those at risk, especially those with “incomplete SLE” who have not met criteria for the disease. But in countries where hydroxychloroquine use is not routine, therapeutic equipoise may present a chance to examine this question cleanly. An alternative assessment of benefit of hydroxychloroquine in this setting might be a comparative effectiveness study, using propensity scores to account for why a treating physician chooses particular therapies.6 For example, if it could be established that French hematologists were reserving hydroxychloroquine, over the time frame of CEREVANCE, to those at highest risk of lupus (in their estimations), then it is remarkable that the eventual SLE prevalence was threefold lower in the treated group.
Among other important points, Granel and colleagues note that development of lupus can lag after immune cytopenia by many years (although the lag is notably shorter in Evans syndrome; see figure). Because the lag in chronic ITP can be well over a decade, patients at risk who present as older children or teens may transition from pediatric hematologists to adult care while they are still at risk for lupus. If their ITP is quiescent, it may be especially important to arrange follow-up for them, with either hematologists or rheumatologists who treat adults.
Readers should keep in mind some caveats to interpretation of the Granel study. First, these results do not apply to all childhood ITP patients at diagnosis, but only those whose ITP becomes “chronic” (after 1 year). This delay enriches the population with patients at higher risk of other immune disorders than, for example, acute or persistent childhood ITP in toddlers, which is less commonly chronic and most often self-limited (though young children are found in this cohort). Second, almost 10% of the patients in the cohort with ANA+ had already received a diagnosis of overt SLE at or near the time initial presentation; these patients should be considered distinct from those with only immune cytopenias in the risk assessments (as the authors have done). Third, the study entry criterion for the Granel assessment was ANA titer at least 160 (ie, ≥160), and the progression risk factor they identified was ANA > 160 (ie, ≥320, or the next highest measurable titer in common practice); this is quite a fine line of distinction, and it is possible that different reagents or testing regimens could yield slightly different results. Fourth, ANA testing was not required across all patients in the cohort, and negative ANAs, if any, were not recorded, so there is something of a “missing denominator” in evaluating the test results. Nevertheless, those without positive tests did not go on to develop lupus (ie, negative tests would apparently be highly specific).
This convincing article is also timely for the field. The international study group that produced the landmark publication for standardization of ITP terminology, definitions, and outcome criteria7 is considering updated and revised guidelines at this time. The moment may be right to express more clearly what is known about progression of ANA+ ITP and recommend appropriate follow-up for patients that may be at high risk. This work will certainly be of value to the international consensus group.
Conflict-of-interest disclosure: E.J.N. serves on advisory boards for Genentech, Takeda, and Saliogen; serves on data safety monitoring boards for Merck, Swedish Orphan Biovitrum, Laboratoire français du Fractionnement et des Biotechnologies, and Agios; and has received stock options from Saliogen.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal