RAC2 mutations define phenotype: constitutively active, RAS-like cause SCID, dominant-negative resembleLAD, dominant-activating cause CID.
RAC2 mutant proteins exhibit aberrant function although no singular test is sufficient to determine functional consequence.
Visual Abstract
Mutations in the small Rho-family guanosine triphosphate hydrolase RAC2, critical for actin cytoskeleton remodeling and intracellular signal transduction, are associated with neonatal severe combined immunodeficiency (SCID), infantile neutrophilic disorder resembling leukocyte adhesion deficiency (LAD), and later-onset combined immune deficiency (CID). We investigated 54 patients (23 previously reported) from 37 families yielding 15 novel RAC2 missense mutations, including one present only in homozygosity. Data were collected from referring physicians and literature reports with updated clinical information. Patients were grouped by presentation: neonatal SCID (n = 5), infantile LAD-like disease (n = 5), or CID (n = 44). Disease correlated to RAC2 activity: constitutively active RAS-like mutations caused neonatal SCID, dominant-negative mutations caused LAD-like disease, whereas dominant-activating mutations caused CID. Significant T- and B-lymphopenia with low immunoglobulins were seen in most patients; myeloid abnormalities included neutropenia, altered oxidative burst, impaired neutrophil migration, and visible neutrophil macropinosomes. Among 42 patients with CID with clinical data, upper and lower respiratory infections and viral infections were common. Twenty-three distinct RAC2 mutations, including 15 novel variants, were identified. Using heterologous expression systems, we assessed downstream effector functions including superoxide production, p21-activated kinase 1 binding, AKT activation, and protein stability. Confocal microscopy showed altered actin assembly evidenced by membrane ruffling and macropinosomes. Altered protein localization and aggregation were observed. All tested RAC2 mutant proteins exhibited aberrant function; no single assay was sufficient to determine functional consequence. Most mutants produced elevated superoxide; mutations unable to support superoxide formation were associated with bacterial infections. RAC2 mutations cause a spectrum of immune dysfunction, ranging from early onset SCID to later-onset combined immunodeficiencies depending on RAC2 activity. This trial was registered at www.clinicaltrials.gov as #NCT00001355 and #NCT00001467.
Introduction
RAC2 is a RAS–related guanosine triphosphate hydrolase (GTPase) within the highly conserved Rho subfamily. GTPases cycle between guanosine diphosphate (GDP)-bound (inactive) and GTP-bound (active) states acting as molecular switches regulating multiple downstream processes. Under basal conditions, the majority of RAC2 is held in an inactive state bound to a guanine dissociation inhibitor by C-terminal geranyl geranylation. Upon activation by G-protein coupled receptors or receptor tyrosine kinases, RAC2 releases GDP and binds GTP with the assistance of guanine nucleotide exchange factors. The 2 switch domains then undergo conformational changes, bind to downstream effectors, and drive multiple cellular functions, including reduced NAD phosphate (NADPH) oxidase activation (p67phox),1 actin polymerization leading to cell motility, and intracellular rearrangement (p21-activated kinase 1 [PAK1])2 and increased intracellular Ca++ levels (PLCγ2).3
The first RAC2 mutation, D57N, was identified in an infant with severe bacterial infections, impaired wound healing, and defective neutrophil chemotaxis.4 The mutant protein acted in a dominant-negative (DN) manner, failing to bind GTP.5 A decade later, a second D57N patient was identified through newborn screening (NBS).6,7 Two siblings with homozygous null mutations (p.W56∗) presented within the first 2 years of life with recurrent sinopulmonary infections, lymphopenia, and hypogammaglobulinemia.8 Although not tested at birth, both siblings had low T-cell receptor excision circles for their age, a hallmark of reduced thymic output.8 Since that time, several patients presenting with similar symptoms were found to carry dominant-activating mutations.9-14 More recently, 4 infants presenting in the first week of life with sepsis because of absence of neutrophils and lymphocytes, similar to reticular dysgenesis, were reported to have dominant, constitutively active mutations G12R15 and Q61R,16 equivalent to well-known somatic, oncogenic RAS mutations.17 Most patients reported, regardless of genotype, had significant T-lymphopenia and decreased recent thymic emigrants, including 26,9 identified by NBS. In keeping with these findings, RAC2 mutations were recently classified as leaky/atypical severe combined immunodeficiency (SCID).18
We investigated 54 patients from 37 families, including 23 previously reported, yielding 15 novel RAC2 missense mutations, including 1 presenting only in homozygosity.
Methods
Data collection
Patients were recruited by referral after discovery of RAC2 mutation from clinical and research centers in Canada, Europe, Asia, New Zealand, and the United States. Patients were evaluated by their home clinicians and data were extracted to a standard form with defined fields for demographics, clinical manifestations with age of onset, and free-text recording of uncategorized findings by the physicians. Previously reported cases were identified through PubMed search using the search terms, “RAC2 and mutation” with filter from 1998-2023. Inclusion criteria included data availability from publication or updated information provided by clinicians. All identified cases were reviewed to consolidate duplications.
All physicians confirmed that their patients had signed an informed consent under local ethics-approved protocols and in accordance with the Declaration of Helsinki.
Mutation nomenclature
Mutation information obtained from original publications and provided by physicians was reconciled according to the Human Genome Variation Society nomenclature using reference sequences NM_002872.5 and NP_002863.1 for gene and protein mutations, respectively.
Plasmids
pCMV6 expression vector for RAC2 wild-type (WT) (Origene, Rockville, MD) was used for targeted mutagenesis to produce patient-specific RAC2 mutations (BioInnovatise, Rockville, MD). pcDNA3.1 expression vectors for gp91phox/NOX2, p67phox, p47phox, and green fluorescent protein (GFP) have previously been reported.1
Cell culture and transfections
COS-7 and HEK293 cells were maintained in Dulbecco modified Eagle medium media (Gibco) with 10% fetal bovine serum plus penicillin-streptomycin in an incubator at 5% CO2.
Patient-specific RAC2 mutation expression constructs were generated by site-directed mutagenesis (BioInnovatise) performed on WT RAC2 complementary DNA in pcDNA3.1 (Origene). Patient or WT RAC2, gp91phox/NOX2, p67phox, p47phox, and GFP were transfected into HEK293 cells using Lipofectamine-LTX-Plus, as previously reported.1
Reactive oxygen species assay
Cells were harvested 48 hours after transfection. Cell suspension (2 × 105), diluted with Diogenes reagent with or without 1 mM phorbol myristate acetate (PMA) stimulation (Invitrogen), was measured at 1-minute intervals for 30 minutes on a Luminoskan Ascent plate reader (Thermo Fisher Scientific).
GST-PAK1 binding
Transfected COS-7 cell lysates were immunoprecipitated with GST-PAK1-pleckstrin homology domain beads. Isolated protein was electrophoresed, transferred to nitrocellulose membrane, and blotted with α-RAC2, as previously reported.1
AKT phosphorylation
Isolated protein from transfected COS-7 cell lysates was electrophoresed, transferred to nitrocellulose membranes, and probed with α-phospho-AKT (Ser473, Cell Signaling, 4060S) followed by rabbit horseradish peroxidase–linked secondary immunoglobulin G (Cytiva Life Sciences, NA934V), as previously reported.1
Protein stability
Protein isolated from COS-7 cell lysates 48 hours after transfection was electrophoresed, transferred to nitrocellulose membranes (Thermo Fisher Scientific, IB23001), and probed with α-RAC2 (Millipore, 07-604-I) and glyceraldehyde-3-phosphate dehydrogenase (R&D Systems, 2275-PC-100) followed by horseradish peroxidase–linked secondary antibody (Cytiva Life Sciences, NA934V). Protein stability was determined by calculating the amount of RAC2 protein relative to glyceraldehyde-3-phosphate dehydrogenase for each cell lysate. For each experiment, results were normalized to the corresponding WT RAC2–transfected cells.
Densitometry
Immunoblots were developed using SuperSignal West Dura ECL-imaging solution (Thermo Fisher Scientific, 35075) and imaged using an iBrightCL1000 Imaging System (Thermo Fisher Scientific) imager; bands were quantified by densitometry using ImageJ software.
Confocal microscopy
COS-7 cells were cotransfected with GFP and WT or patient-specific RAC2 expression constructs. After 48 hours, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (ChemCruz), washed in phosphate-buffered saline, stained for RAC2, followed by Alexa Fluor 594 goat antirabbit immunoglobulin G (Life Technologies, A11037), and stained for F-actin using Alexa Fluor 647 phalloidin (Thermo Fisher Scientific, A22287).
Results
Cohort characteristics
We compiled clinical data for 54 patients (26 males, 28 females) from 37 families.
Three groups were identified based on clinical presentation: Group 1 consisted of 5 patients who presented within the first 10 days of life with reticular dysgenesis-like SCID due to constitutively-active RAC2,15 characterized by profound neutropenia and lymphopenia with sepsis.15,16 Group 2 consisted of 5 patients who presented within the first month of life with omphalitis, abscesses, and periumbilical erythema with decreased T cells and B cells and normal or elevated neutrophil counts due to DN D57N mutation.4-7 Group 3 consisted of 44 patients grouped as combined immune deficiency (CID) based on decreased T cells, low B cells, low immunoglobulins, and recurrent respiratory infections (Figure 1A). One parent who was mutation positive lacks clinical data. Among the 8 patients with CD27+ memory B-cell enumeration, 7 of 8 were below the interquartile reference range for age (supplemental Table 1, available on the Blood website). Regardless of presentation, T-lymphopenia was a consistent finding (Figure 1B), affecting CD4+ more than CD8+ cells. B and natural killer cells were usually low, and most patients had low immunoglobulins, frequently receiving immunoglobulin replacement therapy. Reflective of the T-lymphopenia observed, 4 patients were diagnosed by NBS (Figure 1C). It is noteworthy that 1 patient in the constitutively active group (patient 4) failed NBS because of low total cells (<500 white blood cells per μL) leading to failure of the assay control causing an indeterminate result.
Clinical findings in patient with RAC2 mutation. (A) Age at clinical presentation across groups: CA, reticular dysgenesis without deafness caused by constitutively active mutations; DN, dominant-negative, D57N; N, homozygous null; A, CID caused by activating mutations; NBS, detected by TREC NBS; AR, autosomal recessive. (B) Lymphocyte counts by age for each patient with data. Circles colored by presentation group, gray shading indicates normal range for age. (C) Patient cohort sorted by presentation group including sex (M, male; F, female), mutations, and presence of clinical findings. “H” denotes homozygosity for listed mutation. Gray shading of RAC2 function indicates identification by NBS. Clinical manifestations denoted by filled box. % CID, percent patients with CID-manifesting specified phenotype; LRTI, lower respiratory tract infection; nd, no data; URTI, upper respiratory tract infection; X, deceased. ∗Forty three of 44 patients with CID had available clinical data, where a indicates streptococcal abscesses; b, necrotizing pneumonia and pulmonary abscesses; c, Escherichia coli skin; d, Serratia marcescens; e, bacterial skin; f, Staphylococcus aureus and Streptococcus pyogenes.
Clinical findings in patient with RAC2 mutation. (A) Age at clinical presentation across groups: CA, reticular dysgenesis without deafness caused by constitutively active mutations; DN, dominant-negative, D57N; N, homozygous null; A, CID caused by activating mutations; NBS, detected by TREC NBS; AR, autosomal recessive. (B) Lymphocyte counts by age for each patient with data. Circles colored by presentation group, gray shading indicates normal range for age. (C) Patient cohort sorted by presentation group including sex (M, male; F, female), mutations, and presence of clinical findings. “H” denotes homozygosity for listed mutation. Gray shading of RAC2 function indicates identification by NBS. Clinical manifestations denoted by filled box. % CID, percent patients with CID-manifesting specified phenotype; LRTI, lower respiratory tract infection; nd, no data; URTI, upper respiratory tract infection; X, deceased. ∗Forty three of 44 patients with CID had available clinical data, where a indicates streptococcal abscesses; b, necrotizing pneumonia and pulmonary abscesses; c, Escherichia coli skin; d, Serratia marcescens; e, bacterial skin; f, Staphylococcus aureus and Streptococcus pyogenes.
Clinical findings were largely consistent across the cohort (Figure 1C). Most patients with constitutively active and DN disease underwent hematopoietic stem cell transplantation (HSCT) within the first months (constitutively active) to first year (DN D57N) of life (8 of 9 surviving; supplemental Table 2) and did not develop additional disease phenotypes (during 61.5 patient-years of follow-up). Patients with constitutively active disease frequently had sepsis (4 of 5) whereas those with DN D57N had abscesses and bacterial infections (5 of 5). In contrast, patient with CID had upper and lower respiratory tract infections (36 of 43 [83.7%] and 33 of 43 [76.7%], respectively) with bronchiectasis (15 of 43, 34.9%). Viral infections were seen in 24 of 43 patients with CID (55.8%) with herpes simplex virus (n = 9) and human papillomavirus (n = 5) the most common. Although patients with DN D57N disease had bacterial infections and abscesses consistent with neutrophil dysfunction, bacterial infections were less common among patients with CID: 2 had abscesses, 1 streptococcal and 1 necrotizing pneumonia with lung abscesses. Six patients developed 8 malignancies including basal cell carcinoma (n = 2), and 1 case each of human papillomavirus–driven anogenital neoplasia, cutaneous squamous cell carcinoma, diffuse large B-cell lymphoma, B-cell non-Hodgkin lymphoma, Hodgkin lymphoma, and littoral cell angioma (a rare splenic tumor). HSCT was curative for 15 of 18 patients (83.3%). Eight patients are deceased (supplemental Table 3); 3 after HSCT graft failure,12,14 2 because of recurrent infections,19 2 from failure of solid organ transplantation (kidney8 and lung,11 respectively), and 1 unrelated to disease.
Although patient presentations defined clinical groups, several patients defied rigid categories. Two brothers with R174W had delayed umbilical cord separation but neither had abscesses nor the impaired inflammation seen in DN D57N disease. The older sibling, diagnosed at 7 years, had recurrent upper and lower respiratory tract infections and was placed on prophylactic cotrimoxazole, as was the younger after diagnosis at age 10 months. Consequently, the younger brother has not experienced recurrent pulmonary infections. One patient with E62K/D63N presented at 5 days with omphalitis then developed bacterial skin infections and abscesses, clinically appearing as DN D57N but with peripheral neutropenia similar to that of patients with constitutively active RAC2. She underwent successful HSCT at 1 year.
Identified RAC2 mutations
All patient mutations were novel in gnomAD (gnomad.broadinstitute.org, Table 1) except c.202C>T, p.R68W (n = 2 of 250 998, 0 homozygotes), which was phenotypic only in homozygosity, and c.79G>A, p.A27T (n = 1 of 31 382), which affects a conserved residue; both are predicted deleterious by CADD scores of >20. Several mutations occurred in multiple unrelated patients, including E62K (8 patients, 6 families), D57N (5 patients, 4 families), and G12R, P34H, W56∗, D63N, N92S, and R174W seen in 2 unrelated families each.
Patients, mutations, and current status
| Pt . | Sex . | Mutation . | CADD . | Inher . | RAC2 function . | Age∗ . | Transplant, age . | Status, age . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | c.34G>A; p.G12R | 31 | AD | CA | 3 d | HSCT ×2, 3 mo | Alive, 23 y | 15 |
| 2a | F | c.34G>A; p.G12R | 31 | AD | CA | 10 d | HSCT, 2 mo | Alive, 32 y | 15 |
| 2b | F | c.34G>A; p.G12R | 31 | AD | CA | 9 d | HSCT ×2, 2 mo | Dec, 5.5 mo | 15 |
| 3 | M | c.182A>G; p.Q61R | 27.9 | AD | CA | 2 d | HSCT, 45 d | Alive, 3 y | 16 |
| 4 | M | c.181C>A; p.Q61K | 26.5 | AD | CA | 7 d | HSCT, 3.5 mo | Alive, 18 mo | |
| 5 | M | c.169C>A; p.D57N | 28.8 | AD | DN | 1 mo | HSCT, 3.5 mo | nd | 5,20 |
| 6 | M | c.169C>A; p.D57N | 28.8 | AD | DN | NBS | HSCT, 3.5 mo | Alive, 25 mo | 6,7 |
| 7 | F | c.169C>A; p.D57N | 28.8 | AD | DN | NBS | No | Alive, 4 y | |
| 8a | F | c.169C>A; p.D57N | 28.8 | AD | DN | 1 mo | HSCT, 3 mo | Alive, 2 y | |
| 8b | F | c.169C>A; p.D57N | 28.8 | AD | DN | 1 mo | HSCT, 3 mo | Alive, 2 y | |
| 9a | F | c.168G>A; p.W56∗ | 39 | AR | N | 6 mo | Kidney, 21 y | Dec, 21 y | 8 |
| 9b | M | c.168G>A; p.W56∗ | 39 | AR | N | 2 y | No | Dec, 34 y | 8,19 |
| 37 | M | c.168G>A; p.W56∗ | 39 | AR | N | 2 y | No | Alive, 40 y | |
| 10 | F | c.202C>T; p.R68W | 28.2 | AR | A | 18 mo | No | Alive, 46 y | |
| 11a | M | c.44G > A; p.G15D | 31 | AD | A | 39 y† | No | Alive, 39 y | 14 |
| 11b | F | c.44G > A; p.G15D | 31 | AD | A | 1 y | No | Alive, 11 y | 14 |
| 11c | F | c.44G > A; p.G15D | 31 | AD | A | 9 y† | No | Alive, 9 y | 14 |
| 12 | M | c.54C>G; p.C18W | 26.1 | AD | A | 4 y† | No | Alive, 4 y | |
| 31a | F | c.62T>G; p.I21S | 29.6 | AD | A | nd | No | Alive, 43 y | |
| 31b | F | c.62T>G; p.I21S | 29.6 | AD | A | 6 y† | No | Alive, 7 y | |
| 31c | M | c.62T>G; p.I21S | 29.6 | AD | A | 8 y† | No | Alive, 9 y | |
| 31d | M | c.62T>G; p.I21S | 29.6 | AD | A | 9 y† | No | Alive, 10 y | |
| 32 | M | c.79G>A; p.A27T | 24.2 | AD | A | 35 y | No | Alive, 51 y | |
| 13 | F | c.86C>G; p.P29R | 25.1 | AD | A | 4 mo | No | Alive, 12 y | 13 |
| 14 | M | c.88G>A; p.G30R | 20.6 | AD | A | 1 y | No | Alive, 15 y | |
| 15a | M | c.101C>A; p.P34H | 30 | AD | A | 2 y | No | Alive, 43 y | 10 |
| 15b | F | c.101C>A; p.P34H | 30 | AD | A | 2 y | No | Alive, 6 y | 10 |
| 15c | F | c.101C>A; p.P34H | 30 | AD | A | 2 y | No | Alive, 11 y | 10 |
| 16 | F | c.101C>A; p.P34H | 30 | AD | A | 4 y | No | Dec, 36 y | |
| 17a | F | c.175G>T; p.A59S | 25.2 | AD | A | 1 y | No | Alive, 37 y | |
| 17b | F | c.175G>T; p.A59S | 25.2 | AD | A | 1 y | No | Alive, 10 y | |
| 18 | F | c.184G>A; p.E62K | 29.7 | AD | A | 2 mo | HSCT, 42 y | Alive, 44 y | 9 |
| 19 | F | c.184G>A; p.E62K | 29.7 | AD | A | NBS | HSCT, 2 y | Alive, 4 y | 9 |
| 20 | M | c.184G>A; p.E62K | 29.7 | AD | A | 4 y | HSCT, 17 y | Dec, 17 y | 9 |
| 21a | M | c.184G>A; p.E62K | 29.7 | AD | A | nd | Lung | Dec | 11 |
| 21b | M | c.184G>A; p.E62K | 29.7 | AD | A | 9 y | Lung | Alive, 41 y | 11 |
| 21c | M | c.184G>A; p.E62K | 29.7 | AD | A | 1.5 y | HSCT, 2 y | Alive, 5 y | 11 |
| 22 | F | c.184G>A; p.E62K | 29.7 | AD | A | 5 mo | No | Alive, 10.5 y | |
| 23 | F | c.184G>A; p.E62K | 29.7 | AD | A | 13 mo | No | Alive, 16 y | |
| 24 | F | c.184G>A,c.187G>A; p.E62K/D63N | AD | A | 2 wks | HSCT, 1 y | Alive, 6.7 y | ||
| 25a | F | c.187G>A; p.D63N | 29.2 | AD | A | 1 y | No | Alive, 36 y | |
| 25b | F | c.187G>A; p.D63N | 29.2 | AD | A | 1 y | No | Alive, 7 y | |
| 35a | F | c.187G>A; p.D63N | 29.2 | AD | A | nd | |||
| 35b | M | c.187G>A; p.D63N | 29.2 | AD | A | 1y | HSCT, 15 y | Alive, 17 y | |
| 26 | M | c.203G>A; p.R68Q | 30 | AD | A | 1 y | No | Alive, 6 y | |
| 27 | F | c.275A>C; p.N92T | 26 | AD | A | 4 mo | HSCT ×2, 9 y | Dec, 10 y | 12 |
| 28 | M | c.275A>G; p.N92S | 23.9 | AD | A | 2 y | No | Alive, 8 y | |
| 29 | M | c.275A>G; p.N92S | 23.9 | AD | A | 15 mo | No | Alive, 17 y | |
| 30 | F | c.276C>G; p.N92K | 23.1 | AD | A | NBS | Yes | Alive, 3 y | |
| 33 | M | c.520C>T; p.R174W | 28.1 | AD | A | 7 y | No | 13 y | |
| 36a | M | c.520C>T; p.R174W | 28.1 | AD | A | 2 wk | No | Alive, 7 y | |
| 36b | M | c.520C>T; p.R174W | 28.1 | AD | A | 2 wk | No | Alive, 10 mo | |
| 34a | M | c.536C>T; p.P179L | 24.6 | AD | A | 20 y | No | Alive, 60 y | |
| 34b | F | c.536C>T; p.P179L | 24.6 | AD | A | nd | No | Dec, 45 y |
| Pt . | Sex . | Mutation . | CADD . | Inher . | RAC2 function . | Age∗ . | Transplant, age . | Status, age . | Reference . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | c.34G>A; p.G12R | 31 | AD | CA | 3 d | HSCT ×2, 3 mo | Alive, 23 y | 15 |
| 2a | F | c.34G>A; p.G12R | 31 | AD | CA | 10 d | HSCT, 2 mo | Alive, 32 y | 15 |
| 2b | F | c.34G>A; p.G12R | 31 | AD | CA | 9 d | HSCT ×2, 2 mo | Dec, 5.5 mo | 15 |
| 3 | M | c.182A>G; p.Q61R | 27.9 | AD | CA | 2 d | HSCT, 45 d | Alive, 3 y | 16 |
| 4 | M | c.181C>A; p.Q61K | 26.5 | AD | CA | 7 d | HSCT, 3.5 mo | Alive, 18 mo | |
| 5 | M | c.169C>A; p.D57N | 28.8 | AD | DN | 1 mo | HSCT, 3.5 mo | nd | 5,20 |
| 6 | M | c.169C>A; p.D57N | 28.8 | AD | DN | NBS | HSCT, 3.5 mo | Alive, 25 mo | 6,7 |
| 7 | F | c.169C>A; p.D57N | 28.8 | AD | DN | NBS | No | Alive, 4 y | |
| 8a | F | c.169C>A; p.D57N | 28.8 | AD | DN | 1 mo | HSCT, 3 mo | Alive, 2 y | |
| 8b | F | c.169C>A; p.D57N | 28.8 | AD | DN | 1 mo | HSCT, 3 mo | Alive, 2 y | |
| 9a | F | c.168G>A; p.W56∗ | 39 | AR | N | 6 mo | Kidney, 21 y | Dec, 21 y | 8 |
| 9b | M | c.168G>A; p.W56∗ | 39 | AR | N | 2 y | No | Dec, 34 y | 8,19 |
| 37 | M | c.168G>A; p.W56∗ | 39 | AR | N | 2 y | No | Alive, 40 y | |
| 10 | F | c.202C>T; p.R68W | 28.2 | AR | A | 18 mo | No | Alive, 46 y | |
| 11a | M | c.44G > A; p.G15D | 31 | AD | A | 39 y† | No | Alive, 39 y | 14 |
| 11b | F | c.44G > A; p.G15D | 31 | AD | A | 1 y | No | Alive, 11 y | 14 |
| 11c | F | c.44G > A; p.G15D | 31 | AD | A | 9 y† | No | Alive, 9 y | 14 |
| 12 | M | c.54C>G; p.C18W | 26.1 | AD | A | 4 y† | No | Alive, 4 y | |
| 31a | F | c.62T>G; p.I21S | 29.6 | AD | A | nd | No | Alive, 43 y | |
| 31b | F | c.62T>G; p.I21S | 29.6 | AD | A | 6 y† | No | Alive, 7 y | |
| 31c | M | c.62T>G; p.I21S | 29.6 | AD | A | 8 y† | No | Alive, 9 y | |
| 31d | M | c.62T>G; p.I21S | 29.6 | AD | A | 9 y† | No | Alive, 10 y | |
| 32 | M | c.79G>A; p.A27T | 24.2 | AD | A | 35 y | No | Alive, 51 y | |
| 13 | F | c.86C>G; p.P29R | 25.1 | AD | A | 4 mo | No | Alive, 12 y | 13 |
| 14 | M | c.88G>A; p.G30R | 20.6 | AD | A | 1 y | No | Alive, 15 y | |
| 15a | M | c.101C>A; p.P34H | 30 | AD | A | 2 y | No | Alive, 43 y | 10 |
| 15b | F | c.101C>A; p.P34H | 30 | AD | A | 2 y | No | Alive, 6 y | 10 |
| 15c | F | c.101C>A; p.P34H | 30 | AD | A | 2 y | No | Alive, 11 y | 10 |
| 16 | F | c.101C>A; p.P34H | 30 | AD | A | 4 y | No | Dec, 36 y | |
| 17a | F | c.175G>T; p.A59S | 25.2 | AD | A | 1 y | No | Alive, 37 y | |
| 17b | F | c.175G>T; p.A59S | 25.2 | AD | A | 1 y | No | Alive, 10 y | |
| 18 | F | c.184G>A; p.E62K | 29.7 | AD | A | 2 mo | HSCT, 42 y | Alive, 44 y | 9 |
| 19 | F | c.184G>A; p.E62K | 29.7 | AD | A | NBS | HSCT, 2 y | Alive, 4 y | 9 |
| 20 | M | c.184G>A; p.E62K | 29.7 | AD | A | 4 y | HSCT, 17 y | Dec, 17 y | 9 |
| 21a | M | c.184G>A; p.E62K | 29.7 | AD | A | nd | Lung | Dec | 11 |
| 21b | M | c.184G>A; p.E62K | 29.7 | AD | A | 9 y | Lung | Alive, 41 y | 11 |
| 21c | M | c.184G>A; p.E62K | 29.7 | AD | A | 1.5 y | HSCT, 2 y | Alive, 5 y | 11 |
| 22 | F | c.184G>A; p.E62K | 29.7 | AD | A | 5 mo | No | Alive, 10.5 y | |
| 23 | F | c.184G>A; p.E62K | 29.7 | AD | A | 13 mo | No | Alive, 16 y | |
| 24 | F | c.184G>A,c.187G>A; p.E62K/D63N | AD | A | 2 wks | HSCT, 1 y | Alive, 6.7 y | ||
| 25a | F | c.187G>A; p.D63N | 29.2 | AD | A | 1 y | No | Alive, 36 y | |
| 25b | F | c.187G>A; p.D63N | 29.2 | AD | A | 1 y | No | Alive, 7 y | |
| 35a | F | c.187G>A; p.D63N | 29.2 | AD | A | nd | |||
| 35b | M | c.187G>A; p.D63N | 29.2 | AD | A | 1y | HSCT, 15 y | Alive, 17 y | |
| 26 | M | c.203G>A; p.R68Q | 30 | AD | A | 1 y | No | Alive, 6 y | |
| 27 | F | c.275A>C; p.N92T | 26 | AD | A | 4 mo | HSCT ×2, 9 y | Dec, 10 y | 12 |
| 28 | M | c.275A>G; p.N92S | 23.9 | AD | A | 2 y | No | Alive, 8 y | |
| 29 | M | c.275A>G; p.N92S | 23.9 | AD | A | 15 mo | No | Alive, 17 y | |
| 30 | F | c.276C>G; p.N92K | 23.1 | AD | A | NBS | Yes | Alive, 3 y | |
| 33 | M | c.520C>T; p.R174W | 28.1 | AD | A | 7 y | No | 13 y | |
| 36a | M | c.520C>T; p.R174W | 28.1 | AD | A | 2 wk | No | Alive, 7 y | |
| 36b | M | c.520C>T; p.R174W | 28.1 | AD | A | 2 wk | No | Alive, 10 mo | |
| 34a | M | c.536C>T; p.P179L | 24.6 | AD | A | 20 y | No | Alive, 60 y | |
| 34b | F | c.536C>T; p.P179L | 24.6 | AD | A | nd | No | Dec, 45 y |
A, activating; AD, autosomal dominant; AR, autosomal recessive; CA, constitutively active; CADD, combined annotation dependent depletion; Dec, deceased; F, female, Inher, inheritance; M, male; nd, no data; Pt, patient number.
Age at clinical presentation.
Age taken from publication.
Critical RAC2 sequences include 3 regions that harbor most of the identified mutations: the guanine nucleotide binding P-loop, and switch I and switch II, which undergo critical conformational changes depending on the state of the bound nucleotide (supplemental Figure 1). Altered switch region conformations allow binding to guanine nucleotide exchange factors, which drive GDP-to-GTP exchange, GTPase activating proteins (GAPs) that accelerate GTP hydrolysis, as well as downstream effectors driving diverse functions such as superoxide production (p67phox),1 F-actin assembly (PAK1),2 and intracellular Ca++ release (PLCγ2).3 The C-terminus includes a prenylation motif for posttranscriptional geranyl geranylation.
RAC2 is highly conserved, having 92% identity (96% conserved) to ubiquitously expressed RAC1, 89% identity (95% conserved) to neuronally expressed RAC3, and 69% identity (80% conserved) to ubiquitously expressed CDC42; in contrast to RAC1, RAC3, and CDC42, RAC2 expression is limited primarily to immune cells and lymphoid tissue. The P-loop and switch II are identical across the 4 proteins (supplemental Figure 2) with CDC42 switch I differing by only 3 amino acids, which specify effector interactions including RAC2-p67phox.21 The first reported germ line mutations in CDC4222,23 presented with developmental delay and thrombocytopenia. Subsequent patients have had developmental delay,24 pancytopenia and infections,25 and autoinflammation (reviewed in Coppola et al26). RAC127 and RAC328 mutations were also identified in patients with developmental and neurodevelopmental disorders, respectively. Interestingly, mutations across RAC2, RAC1, RAC3, and CDC42 are clustered similarly in the switch regions and P-loop (supplemental Figure 2; supplemental Tables 4-6).
Functional characterization of RAC2 mutations
Given the spectrum of phenotypes of patients with RAC2 mutation, we hypothesized that different mutations caused different downstream effects. Because neutrophil survival during shipping may confound direct ex vivo studies, we developed heterologous expression and functional assays to investigate the molecular bases of RAC2 mutation phenotypes.
RAC2 is a critical component of the phagocyte NADPH oxidase complex, with mutations causing either absent or increased levels of stimulus-induced superoxide (reviewed in Hsu29). Given the initial identification of RAC2 DN D57N with neutrophil abnormalities,4,5 we compiled neutrophil functional data across the cohorts. Patients with constitutively active mutations had no peripheral neutrophils.15,16 All patients with DN D57N tested (3 of 3) had absent or severely reduced neutrophil respiratory burst in response to the formyl-Met-Leu-Phe peptide (fMLF)5,6 whereas all 5 patients had absent or drastically impaired chemotaxis to fMLF.5,6 Whereas PMA bypasses receptor-mediated pathways, fMLF signals through the G-protein coupled receptor, FPR1, leading to RAC2 activation. Patients with CID frequently had normal PMA-induced dihydrorhodamine assays but increased fMLF-induced superoxide.9,10,13,14
Similar to PAK1 binding and pAKT levels, RAC2-GTP is required for superoxide generation. We assessed superoxide production of RAC2 mutants both at basal state and after PMA stimulation. Using an established heterologous transfection system in which the NADPH oxidase components, gp91phox, p67phox, p47phox, along with RAC2 variants are expressed,9 we obtained kinetic measurements of NADPH oxidase-derived superoxide production. Under basal conditions, there was little measurable superoxide from WT RAC2, whereas after PMA addition, increasing levels were observed over 30 minutes (Figure 2A). In contrast, several RAC2 mutants produced superoxide without exogenous stimulation, increasing further after PMA stimulation (Figure 2A). Cumulative superoxide production was quantified and normalized to basal WT RAC2 for unstimulated and PMA-stimulated cells (Figure 2B; supplemental Figure 3). Mutations in, and near, the switch II region caused increased basal superoxide production with further increases after PMA stimulation. Mutations located near switch I failed to support PMA-induced increase of superoxide production. Consistent with impaired phagocyte superoxide production, patients with C18W, I21S, and G30R had bacterial infections (Figure 1C) some of which are associated with chronic granulomatous disease.
Functional assessment of patient variants. (A) Cumulative luminescence of cells cotransfected with NADPH oxidase components (gp91phox, p67phox, p47phox) and specific RAC2 mutants (30-minute time course). Unstimulated cells (basal) or after addition of 1 μM PMA (PMA). Bottom row of lower set shows expanded y-axis to detect minor activation levels. (B) Summary bar graphs from integrated kinetics of unstimulated (left) or PMA-stimulated (right) superoxide production normalized to WT basal. Dashed and solid lines correspond to WT basal and WT PMA-stimulated levels, respectively. Bars shows average ± standard of the mean (SEM), n = 3-5 independent experiments. (C) Summary of RAC2 protein stability quantified by Western blot densitometry. Graph shows RAC2/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein levels, normalized to WT, and expressed as mean ± SEM, n = 3 to 5. (D) Summary of RAC2-PAK1 binding expressed as bound/total mutant RAC2 normalized to WT bound/total, determined by densitometry of Western blots, displayed as mean ± SEM, n = 3-5 independent experiments. (E) Summary of pAKT levels determined by densitometry of Western blots. pAKT levels were normalized to RAC2 expression to control for protein stability and GAPDH for cell loading. Values are mean ± SEM, n = 3 to 4 independent experiments. In all plots, significance determined by Kruskal-Wallis test using 2-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli comparing RAC2 variants with WT RAC2; ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Functional assessment of patient variants. (A) Cumulative luminescence of cells cotransfected with NADPH oxidase components (gp91phox, p67phox, p47phox) and specific RAC2 mutants (30-minute time course). Unstimulated cells (basal) or after addition of 1 μM PMA (PMA). Bottom row of lower set shows expanded y-axis to detect minor activation levels. (B) Summary bar graphs from integrated kinetics of unstimulated (left) or PMA-stimulated (right) superoxide production normalized to WT basal. Dashed and solid lines correspond to WT basal and WT PMA-stimulated levels, respectively. Bars shows average ± standard of the mean (SEM), n = 3-5 independent experiments. (C) Summary of RAC2 protein stability quantified by Western blot densitometry. Graph shows RAC2/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein levels, normalized to WT, and expressed as mean ± SEM, n = 3 to 5. (D) Summary of RAC2-PAK1 binding expressed as bound/total mutant RAC2 normalized to WT bound/total, determined by densitometry of Western blots, displayed as mean ± SEM, n = 3-5 independent experiments. (E) Summary of pAKT levels determined by densitometry of Western blots. pAKT levels were normalized to RAC2 expression to control for protein stability and GAPDH for cell loading. Values are mean ± SEM, n = 3 to 4 independent experiments. In all plots, significance determined by Kruskal-Wallis test using 2-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli comparing RAC2 variants with WT RAC2; ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Because it was previously suggested that some mutations may affect protein stability,15 we quantified RAC2 protein levels 48 hours after transfection. Normalizing to WT RAC2, the majority of mutant RAC2 proteins displayed decreased stability (Figure 2C; supplemental Figure 4). Constitutively active, RAS-like Q61 mutations displayed increased stability similar to previously reported G12R15; those patients presented in the first week of life with SCID resembling reticular dysgenesis, although lacking deafness.15,16 By contrast, W56∗,8 predicted to induce nonsense-mediated messenger RNA decay, and the unstable R68W, presented clinically only in homozygosity. One ClinVar mutation lacking associated patient data, R163∗, is highly unstable (supplemental Figure 5) and would be expected to produce a clinical phenotype only in homozygosity. Although the low stability of C18W would suggest a phenotype only in homozygosity, in fact, the increased PAK1 binding and pAKT produce a clinical presentation similar to other heterozygous, CID RAC2 mutations.
RAC2 mutations causing delayed GTP hydrolysis had increased levels of PAK1 binding and pAKT.9 Because only GTP-bound RAC2 interacts with PAK1, we normalized PAK1-bound RAC2 levels to input lysate (total RAC2) across RAC2 variants, providing adjustment for the stability of specific RAC2 mutations, and compared with WT RAC2 (Figure 2D; supplemental Figure 4A). The mutations at A27, E62K/D63N, and P179 exhibited PAK1 binding similar to that of WT RAC2 whereas neither G30 nor R163∗ showed detectable PAK1 binding. By contrast, the remainder of RAC2 mutants exhibited increased PAK1 binding.
RAC2 regulates activation of AKT (pAKT) downstream of phosphatidylinositol-3 kinase signaling.30 We assessed RAC2-induced pAKT in unstimulated, transfected COS-7 cells. Although transfection itself may induce pAKT (supplemental Figure 6), many mutations caused significant, further increase in pAKT (9 of 17) whereas A27T, A59S, Q61K, N92S and P179L were equivalent to that of WT RAC2 (Figure 2E; supplemental Figure 4B). Correcting for RAC2 stability demonstrates that unstable proteins, such as C18W and R68W, are activating mutations, despite the inability of C18W to support normal levels of PMA-induced superoxide. Notably, RAC2[G30R] caused increased pAKT despite failing to bind PAK1, suggesting differential effector impacts for that mutation.
Cellular effects of RAC2 variants
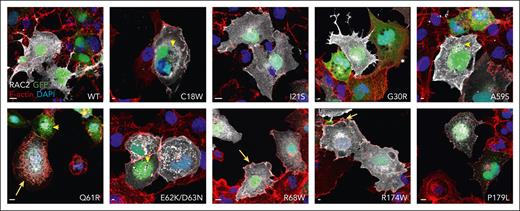
Altered RAC2 activity and stability can be seen by confocal microscopy as altered actin dynamics, membrane ruffling, macropinosomes, as well as RAC2 localization.9 Cells transfected with WT RAC2 exhibit diffuse RAC2 cytoplasmic localization with small granular aggregates and a smooth plasma membrane (PM) outline (Figure 3, upper left; supplemental Figure 7). By contrast, several RAC2 variants demonstrated characteristics of active RAC2 including macropinosomes (Figure 3, yellow arrowheads; supplemental Figures 7-8) and PM ruffling, (Figure 3, yellow arrows; supplemental Figures 7 and 9), which were highest for the canonical RAS-like mutation, Q61R, which is both stable and active in all assays (Figure 4A). Increased macropinosomes were observed for most mutations (Figure 4A; supplemental Figure 8), although most notably for the Q61, D63, and N92K mutations.
Effects of RAC2 mutations on cellular localization and morphology. COS-7 cells cotransfected with GFP (green) and various RAC2 mutants were stained for F-actin (red) and RAC2 (white), nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). Representative merged images are shown. Scale bar, 10 μm. Yellow arrows, PM ruffling; yellow arrowheads, macropinosomes.
Effects of RAC2 mutations on cellular localization and morphology. COS-7 cells cotransfected with GFP (green) and various RAC2 mutants were stained for F-actin (red) and RAC2 (white), nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). Representative merged images are shown. Scale bar, 10 μm. Yellow arrows, PM ruffling; yellow arrowheads, macropinosomes.
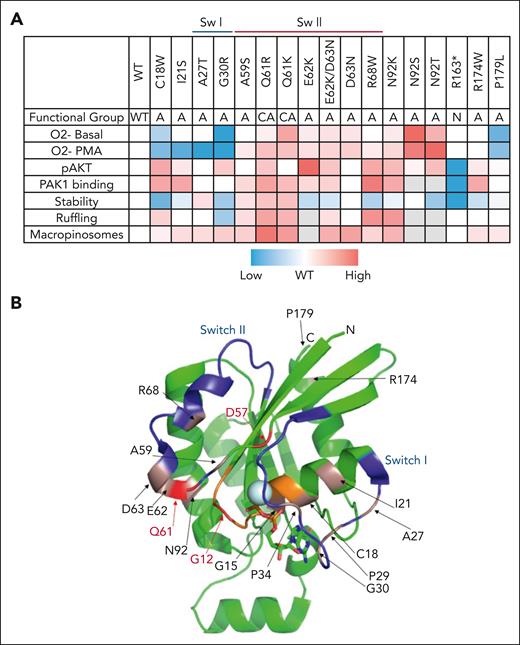
Summary of mutation functional assays and location on RAC2 structure. (A) Summary of each mutant tested across functional assays. Functional groups: A, activating; CA, constitutively active; and N, null. Values derived from functional studies were row normalized and colored. Red and blue correspond to increased or decreased activity, respectively, relative to WT RAC2; gray indicates variant not tested in specified assay. (B) Identified patient mutations shown on 3-dimensional structure of RAC1 amino acids 2-177 (3TH5)31 with switch regions (blue) and P-loop (orange) highlighted. Constitutively active and D57N mutations are noted in red; CID mutations in pink.
Summary of mutation functional assays and location on RAC2 structure. (A) Summary of each mutant tested across functional assays. Functional groups: A, activating; CA, constitutively active; and N, null. Values derived from functional studies were row normalized and colored. Red and blue correspond to increased or decreased activity, respectively, relative to WT RAC2; gray indicates variant not tested in specified assay. (B) Identified patient mutations shown on 3-dimensional structure of RAC1 amino acids 2-177 (3TH5)31 with switch regions (blue) and P-loop (orange) highlighted. Constitutively active and D57N mutations are noted in red; CID mutations in pink.
The highly stable Q61 mutations as well as lower stability C18W and R68W mutations, appear to support increased PM ruffling compared with that for WT (supplemental Figures 7 and 9), consistent with the high PAK1 binding. G30R, which did not bind PAK1, showed cytoplasmic localization with reduced PM staining. Several variants, (C18W, A59S, and D63N) displayed intense PM localization, whereas E62K/D63N showed both PM staining and protein aggregates. Lastly, P179L exhibited punctate PM staining with low levels of diffuse cytoplasmic staining.
Summarizing the functional assays performed (Figure 4A), both gain (red) and loss (blue) of function were seen with switch I region mutations. Switch II region mutations had predominantly gain-of-function effects; decreased stability of the mutant protein was frequently observed. All mutant proteins examined by confocal microscopy except G30R showed decreased cytoplasmic and increased PM-bound RAC2 compared with that of WT RAC2. Examination of RAC2 structure (Figure 4B) shows physical clustering of mutations around Switch I (lower right) and switch II (left side). N92, while not located in switch II, is in close proximity in 3-dimensional space to the switch II region.
Discussion
Dominant and recessive RAC2 mutations differentially perturb RAC2 functions leading to a spectrum of clinical phenotypes. We report 23 unique RAC2 mutations among 54 patients, including 15 novel variants from 31 previously unreported individuals. Initial patient presentations varied from sepsis due to reticular dysgenesis-like SCID in the first days of life caused by constitutively active mutations,15,16 to LAD-like SCID appearing within the first month of life due to DN D57N,4-7,20 to CID, presenting most frequently after 6 months of age caused by homozygous null or heterozygous activating mutations with variable stability. Almost all patients had severely decreased T cells, predominantly in the CD4+ compartment, low to low-normal B cells and low immunoglobulins. NBS for T-cell receptor excision circles identified 4 patients, allowing early genetic diagnosis.6,9 Consistent with T-lymphopenia, viral infections were seen in >50% of the CID group. Two adult patients received lung transplantation for severely impaired pulmonary function.11 Three patients with CID with early diagnosis were treated prophylactically and, to date, remain clinically healthy. Eighteen patients underwent HSCT with 15 of 18 having successful outcomes. Within the constitutively active and DN D57N groups, early HSCT prevented disease sequelae among the 8 of 9 surviving patients, including the recurrent respiratory infections and subsequent bronchiectasis seen in many patients with CID. These clinical groupings, based on initial presentation, demonstrate a RAC2 genotype–phenotype correlation driven by protein activity and stability.
Mutations clustered in the switch I and switch II regions undergo conformational changes depending on the bound nucleotide state. We assessed the ability of various mutant RAC2 proteins to drive downstream effector functions. Each mutation affected RAC2 function differently: switch I region mutations inhibited PMA-induced superoxide production, variably increased PAK1 binding and pAKT, and did not exhibit PM ruffling. By contrast, switch II region mutations, including nearby N92 mutations, increased superoxide under basal and PMA-induced conditions, elevated PAK1 binding, and increased pAKT. Switch II region mutations showed increased macropinosome formation and membrane ruffling. Of the 3 mutations at the C-terminus, R174W did not affect either basal or PMA-induced superoxide production, however it did have increased PAK1 binding. P179L was unique in its effects, showing reduced superoxide production, WT levels of PAK1 binding and pAKT, and no PM ruffling or macropinosomes. Lastly, R163∗ was profoundly unstable, suggesting that it would cause disease only in homozygosity similar to W56∗ or R68W. It has been hypothesized that, in the absence of RAC2, effectors may use RAC1 although much less efficiently.9 All mutations tested except G30R had increased membrane RAC2 staining compared with that of WT RAC2.
Functionally, the switch I region binds p67phox to activate phagocyte NADPH oxidase32 with specificity being attributed to A27 and G30,21 both of which are mutated in this cohort. The switch II region binds GAPs33 promoting rapid, GAP-mediated hydrolysis whereas homodimerization mediated by the carboxy-terminal polybasic domain facilitates slower, intrinsic hydrolysis.34 The switch II mutation, E62K, blocks GAP-mediated GTP hydrolysis while maintaining intrinsic hydrolysis.9 The increased superoxide production, PAK1 binding and pAKT observed across switch II region mutations support loss of GAP binding as a common mechanism.
Murine Rac2−/− neutrophils have aberrant migration and superoxide production. Transduction with RAC2 variants demonstrated that ability to bind either PAK1 or p67phox but not both is insufficient to rescue both migration and superoxide production.34 Similarly, mutations among this cohort did not affect effectors equally, such as loss of superoxide production with increased pAKT and PAK1 binding (C18W) or normal pAKT but increased PAK1 binding (Q61K). Decreased stability of RAC2 mutants may limit the effect of the mutant protein although constitutively active mutations at G12 or Q61 were both highly active and stable. Cells transfected with G12R showed significant mitochondrial disruption and decreased cell survival 8 days after transduction,15 a possible mechanism for the severe hypocellularity observed in the bone marrow and periphery among those patients. Recently, Mishra et al35 demonstrated murine Rac2+/E62K bone marrow–derived macrophages engulf and destroy Rac2+/E62K T cells, providing an additional potential mechanism for the observed T-lymphopenia.
Within the Rho family of Ras–related small GTPases, the RAC/CDC42 subgroup (which includes RAC1, RAC3, CDC42, and RhoG), regulates actin polymerization and turnover.36 Mutations in each of these have been reported in neurodevelopmental (RAC1,27 RAC3,37 and CDC4222-24) and immune (RAC2,5,9-16 CDC42,38 and RHOG39) disorders. Highlighting the conservation across the RAC/CDC42 subgroup, the majority of reported mutations occur in the switch regions; mutations in each disrupted PAK1 binding, actin assembly, or cellular migration. Consistent with our RAC2 findings, RAC1,40 RAC3,37 or CDC4224 mutations differentially affect downstream effectors.
To date, most reported mutations increase RAC2 activity, with the exceptions of DN D57N, and the homozygous null mutation, W56∗. Disparate functional assays have been used to demonstrate altered mutant RAC2 function. Here, we summarize all known phenotypes of patient with RAC2 mutation and functionally characterize the mutations. Examination of any single RAC2 functional assay is insufficient, as either gain or loss of function may occur, depending on the downstream effector examined. Our cohort, in contrast, confirms that RAC2 mutations affect hematopoietic cell functions, leading to an immunodeficiency that is detectable by NBS, treated with prophylactic antimicrobials and immunoglobulin replacement, and potentially curable by HSCT.
Acknowledgments
This research was supported, in part, by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID). S.B. is supported by the NIH, NIAID under award number K23AI163350. G.H. received funding from NIH, NIAID T32 training grant (T32AI007062) through the Duke University School of Medicine Division of Pediatric Allergy and Immunology. M.R.J.S. is supported by The Foundation for Pediatric Research, Finland, Pediatric Research Center Funds, HUS Group Finland, and Jane and Aatos Erkko Foundation, Finland. S.K. was funded by the French state (via the Agence Nationale de la Recherche’s “Investissments d’avenir” program (ANR-10-IAHU-01), and grant ANR-19-CE17-0012-01 [ANR-AID]). T.M. is supported by the NIH, NIAID under award number U54 AI082973. J.W. receives grant, research and clinical trial support from Octapharma, X4-Pharmaceuticals, ADMA Biologicals, Bristol Myers Squibb, Janssen, and Takeda.
Authorship
Contribution: Å.D. and S.G. performed experiments; S.O.S. facilitated acquisition of clinical data; Å.D., J.K., T.L.L., and A.P.H. analyzed the data; M.S.A. assisted with figures and data analysis; F.H.H., J.R.E.B., L.M., J.A., D.M., K.W.W., N.C., P.L.M., C.L.-P., T.T., N.B.K., E.A.D., E.V.R., H.A., V.B., C.M., G.H., T. Mousallem, J.C., N.K., G.C., H.C., C.F.-J., I.L.-L., M.M., J.W.L., C.K., M.J.S., A.F., K.-C.H., T. Martelius, M.R.J.S., S.B., J.W., T.N.M., A.A.M., E.L.F., S.K., A.S., and S.M.H. performed genetic diagnostics, patient care, and provided patient data; A.P.H. and T.L.L. supervised the study; A.P.H. wrote the manuscript; Å.D., S.M.H., T.L.L., and A.P.H. revised the manuscript; and all authors contributed to editing and approving the final manuscript.
Conflict-of-interest disclosure: S.K. is a Centre National de la Recherche Scientifique staff researcher. J.W. is a consultant on the Advisory Board of Octapharma, X4-Pharmaceuticals, Pharming, CSL-Behring, Takeda, and Enzyvant. J.W. is a medical writer for UptoDate. The remaining authors declare no competing financial interests.
Correspondence: Amy P. Hsu, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Building 10, Room 11S257, 10 Center Dr, MSC 1960, Bethesda, MD 20892-1960; email: amy.hsu@nih.gov; and Thomas L. Leto, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Building 10, Room 11S257, 10 Center Dr, MSC 1960, Bethesda, MD 20892-1960; email: letot@niaid.nih.gov.
References
Author notes
A.D. and S.O.S. contributed equally to this study.
T.L.L. and A.P.H. contributed equally to this study.
Deidentified data will be shared with other researchers upon reasonable request to corresponding author Amy P. Hsu (amy.hsu@nih.gov).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal