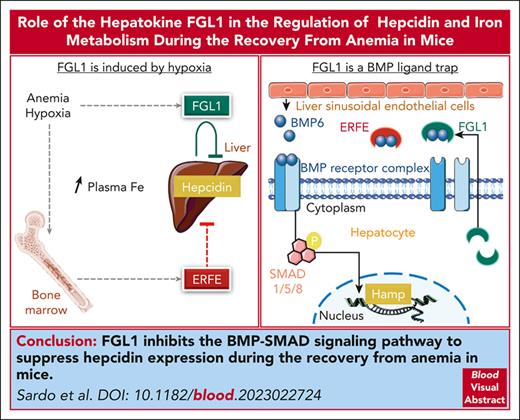
FGL1 regulates hepcidin expression during the recovery from anemia.
FGL1 is an antagonist of the BMP/SMAD signaling pathway.
Visual Abstract
As a functional component of erythrocyte hemoglobin, iron is essential for oxygen delivery to all tissues in the body. The liver-derived peptide hepcidin is the master regulator of iron homeostasis. During anemia, the erythroid hormone erythroferrone regulates hepcidin synthesis to ensure the adequate supply of iron to the bone marrow for red blood cell production. However, mounting evidence suggested that another factor may exert a similar function. We identified the hepatokine fibrinogen-like 1 (FGL1) as a previously undescribed suppressor of hepcidin that is induced in the liver in response to hypoxia during the recovery from anemia, and in thalassemic mice. We demonstrated that FGL1 is a potent suppressor of hepcidin in vitro and in vivo. Deletion of Fgl1 in mice results in higher hepcidin levels at baseline and after bleeding. FGL1 exerts its activity by directly binding to bone morphogenetic protein 6 (BMP6), thereby inhibiting the canonical BMP-SMAD signaling cascade that controls hepcidin transcription.
Introduction
Anemia, defined as a decreased number of functional red blood cells, is a major cause of morbidity and mortality, affecting one-third of the worldwide population.1 Sustained delivery of iron for erythropoiesis in the bone marrow is required to maintain the erythrocyte hemoglobin synthesis and tissue oxygenation.2,3 Iron is released from iron-recycling macrophages, enterocytes, and hepatocytes by the sole iron exporter ferroportin. The liver-derived hormone hepcidin regulates body iron content by occluding and triggering the degradation of ferroportin.4,5
Hepcidin synthesis is regulated by the canonical bone morphogenetic protein (BMP)-SMAD signaling pathway.6-8 Binding of BMP2/6 to a large receptor complex leads to the phosphorylation of SMAD effectors that translocate into the nucleus to activate hepcidin transcription.9 Hepcidin is rapidly suppressed by the erythroid regulator erythroferrone (ERFE) in conditions associated with expanded erythropoiesis such as anemia caused by bleeding or inflammation.10,11 Conversely, excessive release of ERFE in inherited anemias (eg, β-thalassemia and congenital dyserythropoietic anemia) or in myelodysplastic syndromes12-14 causes iron overload and, if untreated, lethal clinical complications. In response to erythropoietin (EPO), ERFE is secreted by erythroid precursors in the bone marrow and spleen and sequesters BMP2/6 in the liver perisinusoidal space to prevent receptor engagement and inhibit the signaling cascade directing hepcidin expression.15
Although ERFE is essential for the suppression of hepcidin within the first hours after an erythropoietic stress, Erfe-deficient mice recover from anemia induced by hemorrhage and chronic inflammation.10,11 In thalassemic mice, ablation or neutralization of ERFE16,17 raises hepcidin levels and mitigates the systemic iron overload. However, restoration of physiological levels of hepcidin is not sufficient to correct the iron overload, and hepcidin synthesis remains inappropriately low relative to the liver siderosis. Collectively, these data indicated that one or more ERFE-independent mechanisms repressed hepcidin during anemia.
We therefore examined hepcidin regulation during the recovery from hemorrhage-induced anemia in wild-type (WT) and Erfe-deficient mice. Here, we describe the identification of a new BMP antagonist and hepcidin suppressor: the hepatokine fibrinogen-like 1 (FGL1).
Methods
Animal models
Erfe−/− and WT mice on a C57BL/6J background were housed in a specific and opportunistic pathogen–free facility in the animal facilities of INSERM US006. Fgl1−/− and WT controls on a C57Bl/6N background were obtained from The European Mouse Mutant Archive, bred by Janvier Laboratories (Le Genest St Isle, France) and transferred to the animal facilities of INSERM US006 at age 4 to 5 weeks. Mice were housed under a standard 12-hour light-dark cycle with water and standard laboratory mouse chow diet (Ssniff, 200 mg iron per kg) ad libitum, in accordance with the European Union guidelines. The study was approved by the Midi-Pyrénées animal ethics committee.
Statistical analysis
Statistical significance was assessed by the Student t test or the 1- or 2-way analysis of variance using Prism 10 (GraphPad). Results of statistical analysis are reported in supplemental Table 5, available on the Blood website. Statistics shown for 2-way analyses of variance are results of the Holm-Šidák multiple comparisons test.
Results
ERFE-independent repression of hepcidin during anemia
We first delineated the timeline of the recovery from anemia induced by phlebotomy in WT and Erfe-deficient mice. In both genotypes, we observed that hemoglobin and hematocrit levels decreased for 3 days after phlebotomy and were significantly improved by day 6 (Figure 1A-B). Serum ERFE levels were maximally increased 24 hours after phlebotomy but decreased below detection level within 3 to 4 days (Figure 1C). Kidney Epo and bone marrow and spleen Erfe messenger RNA (mRNA) expression were also highest after 24 hours and progressively decreased toward normal (supplemental Figure 1). However, a significant reduction in liver hepcidin mRNA expression was maintained from 1 to 5 days after phlebotomy in WT mice (Figure 1D). In Erfe−/− mice, Hamp mRNA expression did not decrease at 24 hours but 2 to 3 days after phlebotomy dropped to levels comparable to those of WT mice before returning toward normal after 5 to 6 days. Similar results were observed in phlebotomized female mice (supplemental Figure 2). Liver Id1 and Smad7 mRNA expression was unchanged throughout the time course in males (Figure 1E-F), but Smad7 expression was reduced in both WT and Erfe−/− females 2 days after phlebotomy (supplemental Figure 2C). We did not detect any statistically significant increase in Gdf1518 and Twsg119 mRNA expression in the bone marrow and the spleen of phlebotomized WT and Erfe−/− mice (supplemental Figure 3). Serum hepcidin concentration and hepcidin/liver iron content ratio mirrored liver Hamp mRNA expression 1 and 2 days after phlebotomy in WT and Erfe−/− mice, but the liver iron content and Bmp6 mRNA expression were unchanged (supplemental Figure 4A-D). We therefore decided to focus on the mechanisms triggered within 24 to 48 hours after phlebotomy that could contribute to hepcidin suppression in Erfe−/− mice. In Erfe−/− mice, hepcidin repression occurred between 1 and 2 days after bleeding without any alteration in serum iron concentration, transferrin saturation, and SMAD5 phosphorylation compared to control mice (supplemental Figure 4E-H). These data indicate that by 48 hours after phlebotomy, hepcidin expression is negatively regulated by an ERFE-independent mechanism.
Recovery from hemorrhage-induced anemia in WT and Erfe−/− mice. Hemoglobin (A) and hematocrit (B) levels in 7- to 9-week-old WT (blue) and Erfe−/− (red) male mice 0, 1, 2, 3, 4, 5, and 6 days after phlebotomy (500 μL). (C) Time course of serum ERFE concentration in phlebotomized WT mice. mRNA expression of Hamp (D), Id1 (E), and Smad7 (F) in the liver of phlebotomized WT and Erfe−/− mice. Data shown are mean ± standard error of the mean (SEM) and were compared for each time point with values for control mice at t = 0 (n = 5-8) for each genotype by 2-way (A,B,D,E,F) or 1-way (C) analysis of variance (ANOVA) and corrected for multiple comparisons by the Holm-Šidák method. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05.
Recovery from hemorrhage-induced anemia in WT and Erfe−/− mice. Hemoglobin (A) and hematocrit (B) levels in 7- to 9-week-old WT (blue) and Erfe−/− (red) male mice 0, 1, 2, 3, 4, 5, and 6 days after phlebotomy (500 μL). (C) Time course of serum ERFE concentration in phlebotomized WT mice. mRNA expression of Hamp (D), Id1 (E), and Smad7 (F) in the liver of phlebotomized WT and Erfe−/− mice. Data shown are mean ± standard error of the mean (SEM) and were compared for each time point with values for control mice at t = 0 (n = 5-8) for each genotype by 2-way (A,B,D,E,F) or 1-way (C) analysis of variance (ANOVA) and corrected for multiple comparisons by the Holm-Šidák method. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05.
ERFE was originally identified by searching for transcripts that were induced in the bone marrow 9 to 15 hours after bleeding.11 We hypothesized that another erythroid regulator derived from the bone marrow3 or a factor directly derived from the liver could repress hepcidin 24 to 48 hours after phlebotomy. We thus analyzed by microarray the transcriptomic profiles of phlebotomized Erfe−/− mice 1 and 2 days after phlebotomy compared to control mice.
Fgl1 mRNA expression is induced in mouse livers during anemia
We found that, compared to control mice, 63 and 38 transcripts were induced (fold change >2; P < .05) 24 hours after phlebotomy in the liver and the bone marrow, respectively (supplemental Table 2 and 3). Six transcripts in the liver and 23 in the bone marrow were still induced 48 hours after phlebotomy compared to control mice (Figure 2A-B). In the liver, only Fgl1, Gdf15, and Cxcl1 encoded secreted proteins. Interestingly, fibrinogen-like 1 (Fgl1) mRNA expression was increased in both the liver and the bone marrow. We confirmed by qRT-PCR that Fgl1 mRNA expression was significantly induced in these tissues (Figure 2C-D) 1 to 3 days after phlebotomy in WT and Erfe−/− mice but was several orders of magnitude higher in the liver. In contrast, Gdf15 mRNA expression was mildly induced 1 to 2 days after phlebotomy, but Gdf15 was reported not to contribute to hepcidin regulation during hemorrhage-induced anemia.20,21,Cxcl1 mRNA expression was only induced in Erfe−/− mice after 24 hours (supplemental Figure 5), which did not correlate with the time course of hepcidin repression. Moreover, unlike Fgl1, the stimulation of Gdf15 and Cxcl1 was restricted to the liver. We therefore focused on FGL1 as the remaining potential candidate. Indeed, FGL1, also known as hepassocin22 or HFREP-1,23 is a member of the fibrinogen family of proteins mostly produced by hepatocytes that share structural homologies to angiopoietin-like proteins,24 including a C-terminal globular domain homologous to fibrinogen β and γ subunits. In contrast to other fibrinogen-related factors, FGL1 lacks the platelet-binding and thrombin-sensitive sites involved in clot formation.23,25 Intraperitoneal injection of EPO in WT mice led to a significant reduction in liver Hamp mRNA expression and an increase in bone marrow Erfe mRNA expression, but no change in Fgl1 expression was detected (Figure 3A-C). However, Fgl1 mRNA expression was upregulated in the liver of thalassemic Th3/+ mice and Th3/+ mice deficient for Erfe (Figure 3D). Mouse Fgl1-promoter analysis revealed 2 hypoxia-inducible transcription factor (HIF) binding sites (supplemental Table 4). To examine whether Fgl1 expression was stimulated by the decreased oxygen saturation in the liver of anemic mice, we compared Fgl1 expression in mouse primary hepatocytes incubated at low-oxygen conditions (2% oxygen) or in the presence prolyl-hydroxylases inhibitor dimethyloxalylglycine, N-(methoxyoxoacetyl)-glycine methyl ester (DMOG) to its expression in mouse primary hepatocytes incubated under control conditions. We observed that the expression of HIF target genes Vegfa, Gapdh, and Angptl1 was increased in cells incubated for 15 hours in presence of DMOG or at low-oxygen (2%) conditions (supplemental Figure 6) compared with expression in untreated cells. Similarly, Fgl1 mRNA expression was induced in mouse primary hepatocytes incubated in hypoxic conditions or with DMOG (Figure 3E). A trend toward an increase was observed in the livers of Albumin-Cre/VHLflox/flox mice26 (Figure 3F), and a significant increase in Fgl1 mRNA expression was detected in the livers of mice treated chronically with prolyl-hydroxylase inhibitor vadadustat27 (Figure 3G). These results suggest that Fgl1 expression is regulated by HIF. In accordance with a previous study,28 WT mice fed a 10-50-200 or 8000 mg/kg iron diet for 2 weeks did not exhibit any change in liver Fgl1 mRNA expression, whereas Hamp and Id1 mRNA expression followed the dietary iron content (supplemental Figure 7), indicating that Fgl1 is not regulated by iron. FGL1 was described as an acute-phase protein,29 and similar to hepcidin, its expression was mildly induced and repressed by interleukin 6 (IL-6) and tumor necrosis factor α, respectively (supplemental Figure 8).
Fgl1 mRNA expression is induced in the mouse liver during anemia. Representative heat maps of the transcripts induced in the liver (A) and the bone marrow (B) at 24 and 48 hours compared to control mice at t = 0. Time course of Fgl1 mRNA expression in the liver (C) and the bone marrow (D) 1 to 6 days after phlebotomy in WT and Erfe−/− mice (n = 5-8). WT and Erfe-/- as shown in Figure 1. Data shown are mean ± standard error of the mean (SEM) and were compared for each time point with values for control mice at t = 0 by 2-way ANOVA and were corrected for multiple comparisons by the Holm-Šidák method (C-D). ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗P < .05.
Fgl1 mRNA expression is induced in the mouse liver during anemia. Representative heat maps of the transcripts induced in the liver (A) and the bone marrow (B) at 24 and 48 hours compared to control mice at t = 0. Time course of Fgl1 mRNA expression in the liver (C) and the bone marrow (D) 1 to 6 days after phlebotomy in WT and Erfe−/− mice (n = 5-8). WT and Erfe-/- as shown in Figure 1. Data shown are mean ± standard error of the mean (SEM) and were compared for each time point with values for control mice at t = 0 by 2-way ANOVA and were corrected for multiple comparisons by the Holm-Šidák method (C-D). ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗P < .05.
Fgl1 mRNA expression is induced by hypoxia.Hamp (A) and Fgl1 (B) mRNA expression in the liver, and Erfe and Fgl1 (C) mRNA expression in the bone marrow of 7-week-old mice at t = 0 to 20 hours after a single intraperitoneal injection of EPO (200 U; n = 5). (D) Fgl1 mRNA expression in the liver of 8-week-old WT, Th3/+, and Erfe−/−; Th3/+ mice (n = 7-9). (E) Fold change of Fgl1 mRNA expression in mouse primary hepatocytes in serum-free media and incubated for 15 hours in low-oxygen condition (2%) or in presence of prolyl-hydroxylases inhibitor DMOG compared with untreated cells. (F) Fgl1 mRNA expression in the liver of Vhl-deficient mice (Albumin-Cre/VHLflox/flox), and (G) mice treated with prolyl-hydroxylase inhibitor vadadustat. Data shown are mean ± SEM and were compared for each time point with values for control mice at t = 0 (A-C) or to WT mice (D) by 1-way ANOVA and were corrected for multiple comparisons by the Holm-Šidák method or with WT or vehicle-treated mice by the Student t test (F-G). Data shown for the experiment in primary hepatocytes are means of 3 independent experiments and were compared with control cells by the Student t test (E). ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05.
Fgl1 mRNA expression is induced by hypoxia.Hamp (A) and Fgl1 (B) mRNA expression in the liver, and Erfe and Fgl1 (C) mRNA expression in the bone marrow of 7-week-old mice at t = 0 to 20 hours after a single intraperitoneal injection of EPO (200 U; n = 5). (D) Fgl1 mRNA expression in the liver of 8-week-old WT, Th3/+, and Erfe−/−; Th3/+ mice (n = 7-9). (E) Fold change of Fgl1 mRNA expression in mouse primary hepatocytes in serum-free media and incubated for 15 hours in low-oxygen condition (2%) or in presence of prolyl-hydroxylases inhibitor DMOG compared with untreated cells. (F) Fgl1 mRNA expression in the liver of Vhl-deficient mice (Albumin-Cre/VHLflox/flox), and (G) mice treated with prolyl-hydroxylase inhibitor vadadustat. Data shown are mean ± SEM and were compared for each time point with values for control mice at t = 0 (A-C) or to WT mice (D) by 1-way ANOVA and were corrected for multiple comparisons by the Holm-Šidák method or with WT or vehicle-treated mice by the Student t test (F-G). Data shown for the experiment in primary hepatocytes are means of 3 independent experiments and were compared with control cells by the Student t test (E). ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05.
FGL1 is a suppressor of hepcidin in vivo and in vitro
We next evaluated whether FGL1 can suppress hepcidin. HAMP mRNA expression was induced 600- and 150-fold in response to BMP6 (25 ng/mL; 6 hours) in Hep3B and HepG2 cells, respectively (Figure 4A). Treatment with recombinant Fc-tagged mouse FGL1 for 6 hours under serum-free conditions led to a significant reduction in HAMP and ID1 (Figure 4B-C) expression in the presence of BMP6 in both hepatoma cell lines. A higher dose of FGL1 was required to repress HAMP and ID1 expression in serum-containing media (supplemental Figure 9). Mouse and human FGL1 share 82% identity and human FGL1 also suppressed HAMP and ID1 mRNA expression in Hep3B cells but at higher concentrations (supplemental Figures 10 and 11). We therefore decided to use mouse FGL1 in this study. Injection of recombinant FGL1 (10 mg/kg) into WT male mice led to a significant reduction in hepatic Hamp mRNA expression and serum hepcidin concentration (Figure 4D-E) compared to Fc-treated mice (n = 5) 6 hours after injection, but no change in liver Id1 and Smad7 mRNA expression was observed (Figure 4F-G). Consistent with hepcidin repression, serum and liver iron concentration were respectively increased and decreased after FGL1 treatment (Figure 4H-I). In female WT mice, treatment with FGL1 did not repress hepcidin expression. A marked inflammatory reaction to FGL1 administration that was not seen in male mice, as shown by increased Saa1 levels, may hinder the effect of FGL1. However, hepcidin expression was robustly suppressed by FGL1 in hepatocytes isolated from female mice (supplemental Figure 12). Collectively, these results indicate that FGL1 is a potent suppressor of hepcidin.
FGL1 is a suppressor of hepcidin in vitro and in vivo.HAMP mRNA expression in Hep3B and HepG2 cells cultured in serum-free medium in response to BMP6 (25 ng/mL, 6 hours) (A) or BMP6 + recombinant mouse FGL1 (1-10 μg/mL) (B). (C) ID1 expression in hepatoma cell lines treated with BMP6 and FGL1. Hepatic Hamp mRNA expression (D), serum hepcidin concentration (E), and liver Id1 (F) and Smad7 (G) mRNA expression, serum (H) and liver iron (I) concentration in mice treated for 6 hours with saline, Fc, or recombinant mouse FGL1 (10 mg/kg; n = 5). Data shown are mean ± SEM of 3 independent experiments (A-C) and were compared with untreated cells using the Student t test (A) or 1-way ANOVA (B-I) and corrected for multiple comparisons using the Holm-Šidák method. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05.
FGL1 is a suppressor of hepcidin in vitro and in vivo.HAMP mRNA expression in Hep3B and HepG2 cells cultured in serum-free medium in response to BMP6 (25 ng/mL, 6 hours) (A) or BMP6 + recombinant mouse FGL1 (1-10 μg/mL) (B). (C) ID1 expression in hepatoma cell lines treated with BMP6 and FGL1. Hepatic Hamp mRNA expression (D), serum hepcidin concentration (E), and liver Id1 (F) and Smad7 (G) mRNA expression, serum (H) and liver iron (I) concentration in mice treated for 6 hours with saline, Fc, or recombinant mouse FGL1 (10 mg/kg; n = 5). Data shown are mean ± SEM of 3 independent experiments (A-C) and were compared with untreated cells using the Student t test (A) or 1-way ANOVA (B-I) and corrected for multiple comparisons using the Holm-Šidák method. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05.
Fgl1-/- mice exhibit a blunted response to phlebotomy
To determine whether FGL1 contributes to hepcidin regulation during the recovery from anemia, we compared WT and Fgl1-/- mice 36 hours after phlebotomy. We first confirmed that Fgl1 mRNA expression was significantly increased in livers of male and female WT mice 36 hours after bleeding compared to control mice (Figure 5A; supplemental Figure 13A). Red blood cell count and hemoglobin (Figure 5B-C) levels were reduced 36 hours after phlebotomy in WT and Fgl1-/- mice. A comparable increase in Erfe mRNA expression in the bone marrow and the spleen was detected 36 hours after bleeding in both genotypes (Figure 5D-E). Interestingly, Fgl1-deficient mice exhibited higher hepcidin expression than WT mice at baseline in both male and female mice (Figure 5F; supplemental Figure 13F). As expected, given the contribution of ERFE, liver Hamp mRNA expression was reduced in both phlebotomized WT and Fgl1−/− mice compared to control mice but to a lesser extent in Fgl1−/− mice (Figure 5F), suggesting that FGL1 also contributes to hepcidin suppression in vivo. No significant difference in Id1 (Figure 5G) and Smad7 (Figure 5H) mRNA expression was observed between genotypes. Female Fgl1−/− mice had a similar response to bleeding as WT mice but Smad7 mRNA expression was decreased in both WT and Fgl1−/− female mice after bleeding (supplemental Figure 13). No significant difference in serum, liver, and spleen iron concentration or in hematological parameters was observed between WT and Fgl1−/− mice (supplemental Figures 14 and 15) nor in the expression of erythroid markers Tfr1 and Gypa mRNA in the bone marrow and spleen (supplemental Figure 16). These results indicate that FGL1 is increased during recovery from anemia and contributes to hepcidin suppression.
Fgl1−/− mice exhibit a blunted response to phlebotomy. (A) Fgl1 mRNA expression in the liver of male WT mice 36 hours after bleeding compared to control mice. Red blood cell count (RBC) (B) and hemoglobin (Hb) (C); Erfe mRNA expression in the bone marrow (D) and the spleen (E); and liver Hamp (F), Id1 (G), and Smad7 (H) mRNA expression in WT and Fgl1−/− mice at t = 0 or 36 hours after phlebotomy. Data shown are mean ± SEM (n = 5-8) and were compared between control and phlebotomized mice using the Student t test (A) or between WT and Fgl1−/− mice by 2-way ANOVA and corrected for multiple comparisons using the Holm-Šidák method (B-H). ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05.
Fgl1−/− mice exhibit a blunted response to phlebotomy. (A) Fgl1 mRNA expression in the liver of male WT mice 36 hours after bleeding compared to control mice. Red blood cell count (RBC) (B) and hemoglobin (Hb) (C); Erfe mRNA expression in the bone marrow (D) and the spleen (E); and liver Hamp (F), Id1 (G), and Smad7 (H) mRNA expression in WT and Fgl1−/− mice at t = 0 or 36 hours after phlebotomy. Data shown are mean ± SEM (n = 5-8) and were compared between control and phlebotomized mice using the Student t test (A) or between WT and Fgl1−/− mice by 2-way ANOVA and corrected for multiple comparisons using the Holm-Šidák method (B-H). ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05.
The globular domain of FGL1 is responsible for hepcidin suppression
Mouse FGL1 is composed of a signal peptide for secretion, a short coil-coil N-terminal domain, and a C-terminal globular domain homologous to fibrinogen β and γ chains (Figure 6A). To identify the active domain of FGL1, we produced the N-terminal and globular domains of FGL1 (Figure 6B) and treated Hep3B cells and mouse primary hepatocytes with Fc, full length FGL1 (FL), or its N-terminal and globular domains. We found that FGL1 FL and the globular domain repressed HAMP and ID1 expression (Figure 6C-D), whereas the N-terminal domain was inactive.
The globular domain of FGL1 mediates hepcidin suppression. (A) AlphaFold structure prediction of mouse FGL1. (B) Schematic representation of the recombinant mouse FGL1 protein constructs. HAMP (C) and ID1 (D) expression in Hep3B cells and mouse primary hepatocytes treated for 6 hours with BMP6 and either Fc, FL, or the N-terminal (Nter) or globular (glob) domains of FGL1. Data shown are mean ± SEM of 3 independent experiments (C-D) and were compared with Fc-treated cells using 1-way ANOVA and corrected for multiple comparisons using the Holm-Šidák method. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05.
The globular domain of FGL1 mediates hepcidin suppression. (A) AlphaFold structure prediction of mouse FGL1. (B) Schematic representation of the recombinant mouse FGL1 protein constructs. HAMP (C) and ID1 (D) expression in Hep3B cells and mouse primary hepatocytes treated for 6 hours with BMP6 and either Fc, FL, or the N-terminal (Nter) or globular (glob) domains of FGL1. Data shown are mean ± SEM of 3 independent experiments (C-D) and were compared with Fc-treated cells using 1-way ANOVA and corrected for multiple comparisons using the Holm-Šidák method. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05.
FGL1 is a BMP antagonist
Next, we examined the mechanism by which FGL1 represses hepcidin. Because treatment of hepatic cells with FGL1 leads to a downregulation of ID1 mRNA expression, we tested whether FGL1 could act as a BMP antagonist. In mouse primary hepatocytes, we observed that FGL1 could repress the induction of Hamp and Id1 mRNA expression by BMP6 and BMP7 but not by BMP2 and BMP4 (Figure 7A-B) in comparison to control cells. However, we did not observe any significant change in Smad7 mRNA expression when cells were treated with FGL1 (Figure 7C). Pretreatment of cells with BMP6 before the addition of FGL1 confirmed its ability to repress hepcidin and BMP-target genes (supplemental Figure 17). In addition, we found that FGL1 led to a significant reduction in SMAD5 phosphorylation in BMP6-treated Hep3B cells (Figure 7D). Finally, we incubated Fc or Fc-FGL1 (FL, globular domain, and N-terminal domain) with BMP6 and performed pull-down assays using protein A magnetic beads. We found that FGL1 FL and globular domain and, to a lesser extent, the N-terminal domain but not the Fc fragment, interacted with BMP6 (Figure 7E). Altogether, these results indicate that, similar to erythroferrone, FGL1 acts as a ligand trap for BMP6 to repress hepcidin transcription during recovery from hemorrhage.
FGL1 is a BMP antagonist. Relative expression of Hamp (A), Id1 (B), Smad7 (C) mRNA expression in mouse primary hepatocytes treated with BMP ligands (10 ng/mL) and human Fc immunoglobulin G2 (IgG2; control [CTRL], 10 μg/mL) or Fc-FGL1 (mouse FGL1, 10 μg/mL) for 15 hours relative to untreated cells in serum-free conditions. Data shown are mean ± SEM of 3 independent experiments and were compared for each BMP between FGL1-treated cells and Fc-treated cells using the Student t test. ∗∗∗∗P < .0001. (D) Western blotting of Hep3B cells treated for 6 hours with BMP6 (10 ng/mL), ERFE (1 μg/mL), or FGL1 (10 μg/mL) for P-SMAD5, SMAD5, and GAPDH. (E) Western blotting of pull-down assay of BMP6 and FGL1 (FL, glob, and Nter) or human Fc IgG2.
FGL1 is a BMP antagonist. Relative expression of Hamp (A), Id1 (B), Smad7 (C) mRNA expression in mouse primary hepatocytes treated with BMP ligands (10 ng/mL) and human Fc immunoglobulin G2 (IgG2; control [CTRL], 10 μg/mL) or Fc-FGL1 (mouse FGL1, 10 μg/mL) for 15 hours relative to untreated cells in serum-free conditions. Data shown are mean ± SEM of 3 independent experiments and were compared for each BMP between FGL1-treated cells and Fc-treated cells using the Student t test. ∗∗∗∗P < .0001. (D) Western blotting of Hep3B cells treated for 6 hours with BMP6 (10 ng/mL), ERFE (1 μg/mL), or FGL1 (10 μg/mL) for P-SMAD5, SMAD5, and GAPDH. (E) Western blotting of pull-down assay of BMP6 and FGL1 (FL, glob, and Nter) or human Fc IgG2.
Discussion
The observation that, in humans, iron absorption is regulated by the extent of the iron stores and the rate of erythropoiesis was first published in 1958.30 During expanded erythropoiesis, iron consumption by erythroid precursors and hemoglobin synthesis can increase up to 10-fold.31 To fulfill these iron requirements, liver hepcidin synthesis is suppressed to stabilize ferroportin and increase availability. Over the last decade, ERFE11 has emerged as the erythron-related regulator3 of hepcidin. Under the influence of EPO, ERFE is secreted by erythroblasts and binds to soluble BMP ligands in the liver perisinusoidal space to prevent the activation of their cognate receptors. This, in turn, decreases the phosphorylation of SMAD effectors and hepcidin transcription. As a stress hormone, ERFE promotes the recovery from anemia induced by hemorrhage,11 chronic inflammation,10 chronic kidney disease,32 and malarial infection.33 However, mice deficient for Erfe do recover from anemia with a few days delay compared to their WT counterparts. One possible explanation would be that, without the rapid (24 hour) compensatory suppression of hepcidin by ERFE, increased erythropoietic rate and iron consumption by erythroid precursors result in a transient hypoferremia which, in turn, decreases the activation of the BMP-SMAD signaling and hepcidin transcription.34,35 A comparable observation has been made in Erfe−/− mice subjected to acute34 or chronic EPO treatment.36 Another likely possibility is that prolonged anemia and hypoxia stimulate the production of another, not yet identified, repressor of hepcidin.
In pathologies associated with ineffective erythropoiesis such as non–transfusion dependent β-thalassemia,12,16 pathological overproduction of ERFE leads to iron overload and severe clinical complications associated with increased mortality and morbidity. Indeed, in thalassemic mice, the genetic ablation of ERFE16 or its inhibition using neutralizing antibodies17 elevated hepcidin to levels comparable to those of control WT mice. However, the increase in hepcidin relative to the liver iron content was still blunted compared to that of WT mice, further supporting the hypothesis of an additional hepcidin repressor.
Here, we studied the recovery from a single blood withdrawal in mice. The nadir hemoglobin was observed 3 days after phlebotomy with partial recovery after 5 to 6 days (Figure 1). Interestingly, we demonstrated that an ERFE-independent mechanism contributed to hepcidin regulation between 24 and 48 hours after phlebotomy in Erfe-deficient mice (Figure 1; supplemental Figure 1). The downregulation of hepcidin was not preceded by any change in liver and serum iron concentration, transferrin saturation, and hepatic BMP-SMAD signaling.
We therefore initiated a search for potential hepcidin suppressors derived from the liver and the bone marrow by microarray. In the liver, acute-phase proteins (Saa1, Saa2, and Lcn2) were induced 24 hours after phlebotomy, indicating a transient inflammatory response in the liver after bleeding (supplemental Table 2). We focused specifically on transcripts encoding secreted proteins that were induced 24 and 48 hours after phlebotomy in Erfe-deficient mice. We found that FGL1 (Fgl1) mRNA expression was the only transcript induced in both the liver and the bone marrow 1 to 3 days after phlebotomy in WT and Erfe−/− mice. FGL1 is a member of the fibrinogen superfamily, produced mostly by hepatocytes and its hepatic expression is ∼40 000-fold higher than in the bone marrow, suggesting that bone marrow–derived FGL1 may not participate in systemic hepcidin regulation (Figure 2). FGL1 has been described as an acute-phase protein that is induced by IL-6,29 but we did not observe a significant increase in expression of IL-6 target gene Socs3 (supplemental Table 2), suggesting that Fgl1 expression is not induced by inflammation in our model. Similarly, our data indicate that Fgl1 is not an EPO-responsive gene, but its expression was increased in the liver of thalassemic mice compared to control mice (Figure 3). We found HIF-binding sites in murine Fgl1 promoter and observed that Fgl1 mRNA expression was increased in hepatocytes incubated in hypoxic conditions or treated with the prolyl-hydroxylase inhibitor DMOG and in mice treated with the prolyl-hydroxylase inhibitor vadadustat (Figure 3). An induction of Fgl1 mRNA expression was also reported in the liver of mice subjected to hypoxic conditions for 32 days.37 In humans, FGL1 mRNA expression was reduced in MCF-7 breast cancer cells deficient in HIF1α or HIF2α and induced when WT MCF-7 cells were treated with DMOG.38 Nothing is known about a potential tissue-specific function of FGL1 in hypoxia-sensitive organs (eg, the kidney and muscle).
Treatment of human hepatoma cells lines or primary mouse hepatocytes with recombinant mouse FGL1 resulted in a robust suppression of hepcidin and Id1 mRNA expression when cells were incubated in serum-free medium and hepcidin stimulated by BMP6 (Figure 4). A higher dose of FGL1 was necessary to repress hepcidin in serum-containing media (supplemental Figure 9). Because FGL1 is known to bind fibrin39 and fibrin matrix of a plasma clot,25 we speculate that FGL1 may bind and/or be chelated by components of fetal bovine serum. In contrast to in vitro data, although mice treated with FGL1 exhibited decreased liver and serum hepcidin levels, decreased liver iron content, and increased serum iron concentration, no significant change in Id1 and Smad7 mRNA expression was observed. Administration of FGL1 to female mice did not repress hepcidin but this batch of protein induced an inflammatory response, which, in combination with higher iron stores and BMP signaling activation status in female than in male mice,40 might have increased the required doses of FGL1 to observe an effect in female mice. However, FGL1 suppressed hepcidin expression in hepatocytes isolated from female mice. Adjustments in the injection protocol (injection route, dose, timing, dietary iron content, etc) will be necessary to compare the response to FGL1 in the 2 sexes. Importantly, hepcidin levels were elevated at baseline in both sexes in mice with ablated Fgl1 compared to their WT controls, indicating that FGL1 can repress hepcidin in female mice (Figure 5; supplemental Figure 13).
In contrast with the BMP agonist property of fibrinogen,41,42 we show that FGL1 acts as a BMP antagonist, directly binding BMP6 to repress hepcidin transcription (Figure 7). Although the hepcidin-suppressive activity is conferred by its C-terminal globular domain, the N-terminal domain seems to bind BMP6 but to a lesser extent than the full length protein or its globular domain. Similar to ERFE,43 FGL1 may contain multiple potential contact points for BMPs and the N-terminal domain may increase the avidity of the protein for BMP ligands. Unlike ERFE, FGL1 cannot repress the expression of hepcidin induced by BMP2 and BMP4. Interestingly, we did not observe any change in SMAD signaling in phlebotomized mice (Figure 1; supplemental Figure 4) or in mice treated with FGL1 (Figure 4). This is in line with previous studies11,44,45,46 describing that EPO/ERFE-mediated suppression of hepcidin was not paralleled by a downregulation in Id1 mRNA expression in mice treated with EPO or ERFE or in Erfe-overexpressing transgenic mice. In vivo, hepcidin suppression is accompanied by a mobilization of iron that stimulates the SMAD pathway through the HFE/TFR2 axis independently of BMP ligands.47,48 The FGL1 effect may thus not be discernible in the context of erythropoietic drive and serum and liver iron concentration, all converging on BMP-SMAD signaling to regulate hepcidin.
Fgl1-deficient mice do not harbor any obvious phenotype with the exception of an increased body weight and glucose intolerance.24 Interestingly, female WT mice exhibit higher Fgl1 levels than male WT mice, but the physiological relevance of this sexual dimorphism is unknown. We did not observe any difference in iron and hematological parameters between WT and Fgl1−/− mice at 8 weeks of age. Because the repression of hepcidin occurs between 24 and 48 hours after phlebotomy in Erfe−/− mice, we compared the hepcidin response to bleeding in WT and Fgl1−/− mice 36 hours after phlebotomy. Although Erfe mRNA expression was similarly upregulated in the bone marrow and spleen of both genotypes, hepcidin expression was higher in Fgl1−/− mice than in WT mice, confirming that FGL1 contributes to hepcidin regulation in physiological conditions and during the recovery from anemia. A fully blunted repression in absence of FGL1 could not be expected given the hepcidin-suppressive activity of ERFE. Consistent with these results at baseline, single-cell RNA sequencing experiments revealed that the hepatic zonation of Fgl1 expression was inversely correlated with hepcidin mRNA expression but, similar to other HIF target genes, its expression was not proportional to the lobule oxygen gradient at baseline.49 Indeed, the liver is subjected to a metabolic zonation, with oxygen concentration and the rate of gluconeogenesis and β-oxidation decreasing from the portal vein to the central vein50 whereas glycolysis, lipogenesis, and triglycerides synthesis51 increase. The adaptation to hypoxia triggers a metabolic switch from mitochondrial oxidative phosphorylation to glycolysis for energy production, but the potential contribution of FGL1 to the liver reprogramming and the zonation of Fgl1 expression during hypoxia is unknown.
In humans, increased circulating levels of FGL1 have been reported in patients with nonalcoholic fatty liver disease,52 obesity,53 or diabetes.54 Increased expression or circulating levels of FGL1 also correlated with unfavorable prognosis in patients with non–small cell lung cancer, metastatic melanoma,55,56 gastric cancer,57 and clear cell renal cell carcinoma.58 Only 1 single-nucleotide polymorphism has been associated with the cytotoxicity of hepatitis C virus.59 Nothing is known about FGL1 levels in patients with various forms of anemia. However, the contribution of FGL1 in humans remains difficult to appreciate because no validated assay to measure plasma FGL1 has been reported. Moreover, highly variable measurements of FGL1 are found in the literature with normal ranges in healthy patients differing by several thousand-fold when measured with the same assays.
The regulation of iron metabolism during hemorrhage-induced anemia can be interpreted as a 2-wave mechanism. ERFE first acts as a stress hormone within 24 hours to rapidly sustain the iron needs for erythropoiesis whereas FGL1 is induced after 24 hours as EPO expression starts to return to normal before the nadir hemoglobin is reached. FGL1 assay validation and clinical studies including measurements of plasma FGL1 for patients with chronic anemias will be necessary to determine its contribution in human pathologies.
Acknowledgments
The authors thank the members of the INSERM US006 facility (Toulouse, France) and the Janvier Laboratories (Le Genest St. Isle, France) for their technical assistance and help in the mouse breeding; Anthony Emile and Yannick Lippi for their contribution to microarray fingerprints acquisition and microarray data analysis carried out at GeT-TRiX Genopole Toulouse Midi-Pyrénées facility; and the platform Aninfimip, an EquipEx (Equipement d’Excellence) supported by the French government through the Investments for the Future program.
Support for this work was provided by the French National Research Agency (ANR-16-ACHN-0002-01 and ANR-22-CE14-0076-01); the Fondation Maladie Rare; the region Occitanie; and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 715491 and 101113536) (L.K.); the French Society of Hematology (SFH) (U.S.); the Fondation pour la recherche sur le cancer; and the Fondation pour la Recherche Médicale (J.P.); the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01DK126680 [T.G.] and R01DK107670 and DK095112 [Y.Z.G.]); the Fondation pour la Recherche Médicale EQ202103012630; the Laboratory of Excellence GR-Ex (ANR-11-LABX-0051); and the program Investissements d’avenir of the French National Research Agency (reference ANR-11-IDEX-0005-02) (C.P.).
Authorship
Contribution: U.S. and P.P. performed experiments and analyzed data; K.C., M.S., J.P., T.M., A.D., L.C., M.R.-M., J.T., B.B., G.J., and E.A. performed experiments; C.P. and Y.Z.G. collected data and edited the manuscript; E.N. and T.G. provided reagents, collected data, and edited the manuscript; and L.K. designed and supervised the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: T.G. and E.N. are scientific cofounders of Intrinsic LifeSciences and Silarus Therapeutics. T.G. is a consultant for ADARx, Akebia, Pharmacosmos, Ionis, Gossamer Bio, Global Blood Therapeutics, American Regent, Disc Medicine, RallyBio, and Rockwell Scientific. E.N. is a consultant for Protagonist, Vifor, RallyBio, Ionis, GlaxoSmithKline, Novo Nordisk, AstraZeneca FibroGen, and Disc Medicine. Y.Z.G. is a consultant for Ionis, Protagonist, Denali/Takeda, and Bay Clinical. The remaining authors declare no competing financial interests.
Correspondence: Léon Kautz, Université de Toulouse, INSERM U1220 Institut de Recherche en Santé Digestive, CHU Purpan, Place du Docteur Baylac, 31024 Toulouse, France; email: leon.kautz@inserm.fr.
References
Author notes
U.S. and P.P. contributed equally to this study.
Microarray data and experimental details are available in the NCBI Gene Expression Omnibus database (accession number GSE229041).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.







![FGL1 is a BMP antagonist. Relative expression of Hamp (A), Id1 (B), Smad7 (C) mRNA expression in mouse primary hepatocytes treated with BMP ligands (10 ng/mL) and human Fc immunoglobulin G2 (IgG2; control [CTRL], 10 μg/mL) or Fc-FGL1 (mouse FGL1, 10 μg/mL) for 15 hours relative to untreated cells in serum-free conditions. Data shown are mean ± SEM of 3 independent experiments and were compared for each BMP between FGL1-treated cells and Fc-treated cells using the Student t test. ∗∗∗∗P < .0001. (D) Western blotting of Hep3B cells treated for 6 hours with BMP6 (10 ng/mL), ERFE (1 μg/mL), or FGL1 (10 μg/mL) for P-SMAD5, SMAD5, and GAPDH. (E) Western blotting of pull-down assay of BMP6 and FGL1 (FL, glob, and Nter) or human Fc IgG2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/13/10.1182_blood.2023022724/1/m_blood_bld-2023-022724-gr7.jpeg?Expires=1764964918&Signature=DHtVYVlBli5rSTTkTNlFWneYobct3UYTtsxsveUTBDdosaymWZSG5fT1a24uLofpInvsCY7cgjDJNNIbGHw31esdQz1~F8BzkZtBjshRdLaWS2Sf3QQVaMU8KhZhdJh1OJGChbMdNntFCzVurAYVKp3NMOjuhIMAOCP3JJdv2e7eeGCbrVZCqcP2LaTRpuWXv6wtfpvsbMPM-zvO~4c00VFFguE6z1rgMErDuxjFq~rznKvRHe3uHIzMT1xMYbdVfVMgZe1FT6HgvzxaKa0o4FRB11xq0qpy36OgtSLE~SpoCdNNK9~F8pkLPibObgOCOmauIjPvNT4rW94DPbDFGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal