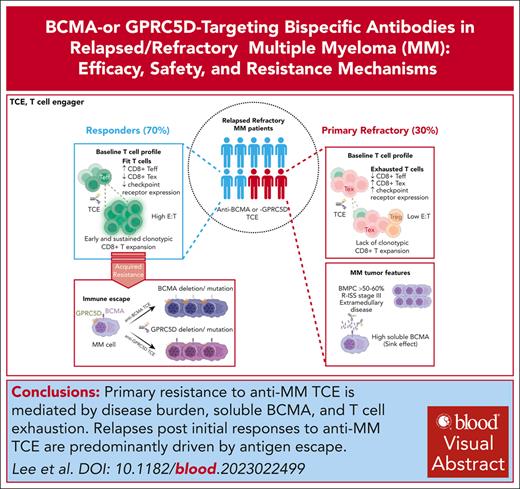
Visual Abstract
Bispecific antibodies that engage T cells to target B-cell maturation antigen or G-protein–coupled receptor class C group 5 member D have demonstrated remarkable efficacy in heavily pretreated relapsed or refractory multiple myeloma (MM), leading to the recent accelerated approval of teclistamab, elranatamab, and talquetamab by health agencies. Future challenges, however, remain to define their optimal dosing schedule and duration, sequencing, and integration with established anti-MM therapeutics as well as delineating the biological and clinical mediators of immune escape.
Introduction
Targeted immunotherapies have ushered in a transformative era in multiple myeloma (MM) treatment, with chimeric antigen receptor T cells (CAR T) and bispecific T-cell engagers (TCEs) at the forefront. These T-cell immunotherapies are rapidly advancing to address unmet needs within the MM treatment landscape, in particular for patients with triple-class and pentarefractory disease.1,2 The unique dual specificity of TCE design enables the formation of a near physiological immunologic synapse by engaging CD3 on T cells and surface antigens on MM cells (Figure 1A). In MM, 3 TCEs have been approved by regulatory agencies, including teclistamab and elranatamab, aimed at B-cell maturation antigen (BCMA), and talquetamab, which targets G-protein–coupled receptor class C group 5 member D (GPRC5D). This article will discuss the current use of TCEs in MM, providing a review of the clinical effectiveness and safety data, and a detailed exploration of the mechanisms underlying MM resistance to TCEs. We will focus on strategies to overcome these challenges, offering insights into the sequencing of immunotherapies and the development of innovative therapeutic approaches.
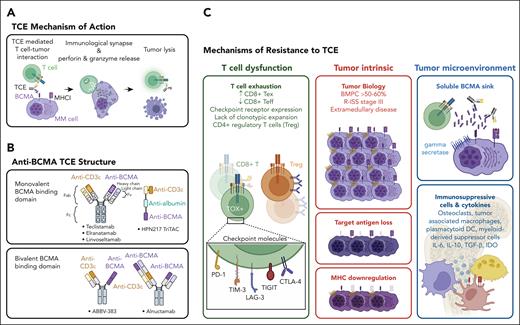
Anti-BCMA TCE mechanism of action and resistance. (A) TCEs redirect host T cells to enhance tumor killing by engaging CD3 on T cells and BCMA (or other target antigens) on MM cell surface. TCEs induce close-contact immune synapse and promote T-cell activation and granzyme and perforin release to target cells. (B) Distinct TCE molecular designs include those that feature monovalent vs bivalent BCMA binding domains as well as cis or trans orientation of CD3 and BCMA binding domains. (C) Mechanisms of MM resistance to TCE are broadly categorized into T-cell dysfunction, tumor intrinsic, and tumor microenvironment related features. Host T-cell dysfunction marked by the abundance of exhausted T cells (Tex), reduced effector T cells (Teff), expression of checkpoint molecules, and lack of clonotypic T-cell expansion contribute to primary refractoriness to TCE. Recruitment of regulatory T cells (Tregs) may also hinder TCE-mediated cytotoxic activity. Tumor intrinsic features leading to resistance include increased disease burden, R-ISS stage III, and extramedullary plasmacytoma. MM immune evasion occurs through mutation and/or deletions of BCMA or GPRC5D. Major histocompatibility complex I (MHCI) loss has also noted in some cases at relapse. MM cells release soluble BCMA into the TME, generating a ligand sink that attenuates TCE efficacy. The MM bone marrow milieu consists of immunosuppressive cells and cytokines. CTLA-4, cytotoxic T-lymphocyte associated protein 4; IDO, indoleamine 2,3-dioxygenase 1; IL, interleukin; LAG-3, lymphocyte activating 3; PD-1, programmed cell death protein 1; TGF-β, transforming growth factor-β; TIGIT, T-cell immunoreceptor with Ig and ITIM domains; TIM-3, T-cell immunoglobulin mucin 3; TOX, thymocyte selection associated high mobility group box.
Anti-BCMA TCE mechanism of action and resistance. (A) TCEs redirect host T cells to enhance tumor killing by engaging CD3 on T cells and BCMA (or other target antigens) on MM cell surface. TCEs induce close-contact immune synapse and promote T-cell activation and granzyme and perforin release to target cells. (B) Distinct TCE molecular designs include those that feature monovalent vs bivalent BCMA binding domains as well as cis or trans orientation of CD3 and BCMA binding domains. (C) Mechanisms of MM resistance to TCE are broadly categorized into T-cell dysfunction, tumor intrinsic, and tumor microenvironment related features. Host T-cell dysfunction marked by the abundance of exhausted T cells (Tex), reduced effector T cells (Teff), expression of checkpoint molecules, and lack of clonotypic T-cell expansion contribute to primary refractoriness to TCE. Recruitment of regulatory T cells (Tregs) may also hinder TCE-mediated cytotoxic activity. Tumor intrinsic features leading to resistance include increased disease burden, R-ISS stage III, and extramedullary plasmacytoma. MM immune evasion occurs through mutation and/or deletions of BCMA or GPRC5D. Major histocompatibility complex I (MHCI) loss has also noted in some cases at relapse. MM cells release soluble BCMA into the TME, generating a ligand sink that attenuates TCE efficacy. The MM bone marrow milieu consists of immunosuppressive cells and cytokines. CTLA-4, cytotoxic T-lymphocyte associated protein 4; IDO, indoleamine 2,3-dioxygenase 1; IL, interleukin; LAG-3, lymphocyte activating 3; PD-1, programmed cell death protein 1; TGF-β, transforming growth factor-β; TIGIT, T-cell immunoreceptor with Ig and ITIM domains; TIM-3, T-cell immunoglobulin mucin 3; TOX, thymocyte selection associated high mobility group box.
Anti-BCMA TCEs: teclistamab and elranatamab
Teclistamab and elranatamab are bispecific antibodies designed to target BCMA, featuring a symmetrical antibody design with CD3 and BCMA binding domains in trans orientation (Figure 1B).3,4 These 2 anti-BCMA TCEs have received accelerated approval from regulatory agencies for the treatment of patients with MM who have received ≥4 lines of therapies, including proteasome inhibitors, immunomodulatory drugs, and anti-CD38 monoclonal antibody. In their respective single-agent clinical trials in patients with triple-class or pentarefractory MM, MajesTEC-1 (NCT03145181 and NCT04557098), and MagnetisMM-1 (NCT03269136), teclistamab and elranatamab demonstrated an overall response rate (ORR) of ≈60% and a median progression-free survival of ≈11 months,5-7 as summarized in Table 1.
BCMA targeting bispecific antibodies in relapsed/refractory MM
| Variable . | Teclistamab5,6 . | Elranatamab7 . | Elranatamab8 . | Linvoseltamab10 . | Alnuctamab11,12 . | ABBV-38313,15 . | HPN-217-300114 . |
|---|---|---|---|---|---|---|---|
| Patients, n | 165 | 55 | 123 | 117 | 73 | 124 | 97 |
| Dosing schedule | Weekly/q2w SC | Weekly/q2w SC | Weekly/q2w IV | Weekly/q2w or q4w IV | Weekly/q2-4w IV/SC | Q3w-q4w IV | Weekly/q2w |
| Median prior LOT | 5 | 6 | 5 | 5 | 4 | 5 | 6 |
| ISS III/ ↑↑ PC, % | 12.3/11.2 | 20/— | 15.4/21.1 | 18.8/22.2 | 16/— | 31/— | 38/— |
| HR/EMD, % | 25.7/17 | 29.1/30.9 | 25.2/31.7 | 35.9/13.7 | 26/21 | 18/— | 19/10 |
| TCR, % | 77.6 | 90.9∗ | 100 | 73.5 | 63 | 82 | 78 |
| ORR/≧ CR, % at RP2D | 63/45.5 1500 μg/kg SC | 64/38.2 76 mg SC | 61/35 76 mg SC | 71/30 200 mg IV | 69/43 30 mg SC | 57/28 40-60 mg IV | 63/21 12 mg |
| Median DOR | 21.6 mo | 17.1 mo | 71.5% at 15 mo | — | — | 72.2% at 12 mo† | 20.5 mo |
| Median PFS | 11.3 mo ≦ 3 LOT 18.1 mo | 11.8 mo | 50.9% at 15 mo | 72.7% at 6 mo | 53% at 12 mo | 10.4 mo 57.9% at 12 mo† | — |
| Median OS | 21.9 mo | 21.2 mo | 56.7% at 15 mo | — | — | — | — |
| Hematological AE (any grade/G3-4) | Neutropenia 72/65 Anemia 54/38 ↓Plt 42/22 | Neutropenia 74.5/71 Anemia 67.3/50.9 ↓Plt 50.9/29.1 | Neutropenia 48.8/48.8 Anemia 48.8/37. 4 ↓Plt 30.9/23. 6 | Neutropenia 32.5/30.8 Anemia 27.4/23.9 ↓Plt 17.1/13.7 | Neutropenia 55/45 Anemia 47/27 ↓Plt 37/16 | Neutropenia 37/34 Anemia 29/16 ↓Plt 23/12 | Neutropenia 40/34 Anemia 44/34 ↓Plt 28/18 |
| Infections, % | 80 (55.2 G3-4) | 74.5 (27.3 G3-4) | 69.9 (39.8 G3-4) | 59.8 (36.8 G3-4) | 62 (16 G3-4) | 41 (5 G3-4) | 59 (25 G3-4) |
| Hypogammaglobulinemia, % | 74.5 | — | 98.6 | — | — | 14 | — |
| CRS, % | 72.1 (0.6 G3) | 87.3 (0 G3) | 56.3 (0 G3) | 45.3 (0.9 G3) | 56 (0 G3) | 57 (2 G3) | 30 (2 G3) |
| ICANS, % | 3 (0 G3-4) | 6.7 (0 G3-4) | 3.4 (0 G3-4) | 5.9 (1.8 G3-4) | 0.03 (0 G3-4) | 0.02 | 0.03 (0 G3-4) |
| Variable . | Teclistamab5,6 . | Elranatamab7 . | Elranatamab8 . | Linvoseltamab10 . | Alnuctamab11,12 . | ABBV-38313,15 . | HPN-217-300114 . |
|---|---|---|---|---|---|---|---|
| Patients, n | 165 | 55 | 123 | 117 | 73 | 124 | 97 |
| Dosing schedule | Weekly/q2w SC | Weekly/q2w SC | Weekly/q2w IV | Weekly/q2w or q4w IV | Weekly/q2-4w IV/SC | Q3w-q4w IV | Weekly/q2w |
| Median prior LOT | 5 | 6 | 5 | 5 | 4 | 5 | 6 |
| ISS III/ ↑↑ PC, % | 12.3/11.2 | 20/— | 15.4/21.1 | 18.8/22.2 | 16/— | 31/— | 38/— |
| HR/EMD, % | 25.7/17 | 29.1/30.9 | 25.2/31.7 | 35.9/13.7 | 26/21 | 18/— | 19/10 |
| TCR, % | 77.6 | 90.9∗ | 100 | 73.5 | 63 | 82 | 78 |
| ORR/≧ CR, % at RP2D | 63/45.5 1500 μg/kg SC | 64/38.2 76 mg SC | 61/35 76 mg SC | 71/30 200 mg IV | 69/43 30 mg SC | 57/28 40-60 mg IV | 63/21 12 mg |
| Median DOR | 21.6 mo | 17.1 mo | 71.5% at 15 mo | — | — | 72.2% at 12 mo† | 20.5 mo |
| Median PFS | 11.3 mo ≦ 3 LOT 18.1 mo | 11.8 mo | 50.9% at 15 mo | 72.7% at 6 mo | 53% at 12 mo | 10.4 mo 57.9% at 12 mo† | — |
| Median OS | 21.9 mo | 21.2 mo | 56.7% at 15 mo | — | — | — | — |
| Hematological AE (any grade/G3-4) | Neutropenia 72/65 Anemia 54/38 ↓Plt 42/22 | Neutropenia 74.5/71 Anemia 67.3/50.9 ↓Plt 50.9/29.1 | Neutropenia 48.8/48.8 Anemia 48.8/37. 4 ↓Plt 30.9/23. 6 | Neutropenia 32.5/30.8 Anemia 27.4/23.9 ↓Plt 17.1/13.7 | Neutropenia 55/45 Anemia 47/27 ↓Plt 37/16 | Neutropenia 37/34 Anemia 29/16 ↓Plt 23/12 | Neutropenia 40/34 Anemia 44/34 ↓Plt 28/18 |
| Infections, % | 80 (55.2 G3-4) | 74.5 (27.3 G3-4) | 69.9 (39.8 G3-4) | 59.8 (36.8 G3-4) | 62 (16 G3-4) | 41 (5 G3-4) | 59 (25 G3-4) |
| Hypogammaglobulinemia, % | 74.5 | — | 98.6 | — | — | 14 | — |
| CRS, % | 72.1 (0.6 G3) | 87.3 (0 G3) | 56.3 (0 G3) | 45.3 (0.9 G3) | 56 (0 G3) | 57 (2 G3) | 30 (2 G3) |
| ICANS, % | 3 (0 G3-4) | 6.7 (0 G3-4) | 3.4 (0 G3-4) | 5.9 (1.8 G3-4) | 0.03 (0 G3-4) | 0.02 | 0.03 (0 G3-4) |
—, Not reported; AE, adverse event; CR, complete response; CRS, cytokine release syndrome; DOR, duration of response; EMD, extramedullary disease; G3, grade 3; HR, high-risk cytogenetics; ICANS, immune effector cell–associated neurotoxicity; ISS, International Staging System; LOT, line of therapy; ↑↑ PC, >50% to 60% bone marrow plasma cells; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; ↓Plt, thrombocytopenia; q2w, every 2 weeks; q3w, every 3 weeks; q4w, every 4 weeks; RP2D, recommended phase 2 dose; SC, subcutaneously; TCR, triple-class refractory.
At 23.6% before anti-BCMA.
Median PFS at ≥ 40-mg dose level.
Although these trials established anti-BCMA TCEs as a standard of treatment of relapsed/refractory MM, the trials also pinpointed high-risk subgroups, mainly those with high disease burden, who are less likely to benefit from such therapies. Subgroup analyses in MajesTEC-1 demonstrated lower responses with teclistamab in patients with Revised International Staging System (R-ISS) stage III, extramedullary disease (EMD), and increased bone marrow plasma cells of ≥60%.5 Similarly in MagnetisMM-17 and MagnetisMM-38 trials with elranatamab, patients with R-ISS stage III, EMD, and pentarefractory disease had lower ORR, with a trend for lower response in patients with ≥50% baseline bone marrow plasma cell and high-risk cytogenetics.
Common treatment-emergent adverse events with anti-BCMA TCEs include cytopenia, infections, and cytokine release syndrome (CRS), which are summarized in Table 1. Hypogammaglobulinemia, an on-target toxicity of anti-BCMA TCE, is observed in most patients. CRS severity and frequency are mitigated by the implementation of step-up dosing and priming with low-dose corticosteroids. More important, shifting from a weekly to a biweekly dosing regimen after achieving an initial durable response with teclistamab and elranatamab did retain the efficacy of anti-BCMA TCEs and reduced the occurrence of grade 3 to 4 adverse events.8,9
Other anti-BCMA TCEs currently under clinical development include those with distinct geometries with cis or trans orientations as well as monovalency or bivalency for BCMA10-14 (Figure 1B). These TCEs demonstrate comparable therapeutic efficacies, with ORRs of 60% to 70% (Table 1), including the BCMA monovalent linvoseltamab10 and HPN217,14 the BCMA bivalent symmetrical TCE ABBV-383,13,15 and the bivalent asymmetrical TCE alnuctamab.11,12 These distinctive designs and geometries may lead to divergent therapeutic profiles and efficacies for these anti-BCMA TCEs, as explored in the subsequent discussions.
Talquetamab
Talquetamab is currently the only approved TCE targeting GPRC5D,16,17 with forimtamig following suite in development18,19 (supplemental Table 1, available on the Blood website) as well as anti-GPRC5D CAR T therapies.20 Updated results of MonumenTAL-1 phase 1/2 trial (NCT03399799),16,17 with talquetamab administered weekly or biweekly, resulted in ORRs of 74% and 73%, respectively. Of interest, responses were consistent across subgroups, including baseline International Staging System (ISS) stage III, high-risk cytogenetics, prior lines of therapy, and anti-BCMA drug exposure, with a lower ORR (49%) in patients with EMD. In patients with prior treatment with T-cell redirection therapy, including prior anti-BCMA CAR T and/or TCE,21 ORRs were 72.9% (CAR T) and 56.5% (TCE) compared with 67.1% in the overall population and did vary based on the interval from the last dose of the prior T-cell redirecting therapy to the first dose of talquetamab.21
The safety profile of talquetamab is overall similar to that of anti-BCMA TCEs, with respect to hematological toxicities, CRS, and immune effector cell–associated neurotoxicity.17 However, talquetamab resulted in a lower incidence of infections, possibly due to GPRC5D restricted expression to plasma cells sparing terminally differentiated B lymphocytes, which are also targeted by anti-BCMA TCEs.22 Additional on-target toxicities observed with talquetamab due to GPRC5D expression on keratinocytes included dysgeusia, onychodystrophic changes, and skin rashes.
Mechanisms of resistance to TCE
Notwithstanding the efficacy of BCMA- and GPRC5D-directed TCEs, responses, however, are not universal. Nearly one-third of patients are primary refractory to TCEs, and relapses eventually develop among responders. Factors mediating these resistance mechanisms are broadly categorized into tumor intrinsic or immune dependent (Figure 1C).
High disease burden and sBCMA sink effect
Across anti-BCMA TCE monotherapy trials, advanced ISS stage, high disease burden, and EMD were associated with lower responses.5,7,8 These clinical factors are characterized by high serum levels of soluble BCMA (sBCMA), which has been shown in univariate and multivariate regression analyses to be an independent predictor of poor response to anti-BCMA TCEs.10,23-27 Furthermore, in vitro studies confirmed this “sink effect,” with increased concentrations sBCMA attenuating the binding and cytolytic activity of anti-BCMA antibodies in cocultures of MM cells with healthy donor mononuclear cells.4,28,29 To circumvent this sink effect of sBCMA, TCE designs incorporated anti-BCMA binders with higher affinity to full-length BCMA rather than to sBCMA.26 In addition, increasing TCE concentrations does partially overcome the in vitro sink effect of sBCMA on TCE-mediated cytotoxicity,28 and this dose-response effect relative to sBCMA levels was also clinically validated.10,27,30 Furthermore, targeting γ-secretases, responsible for cleaving BCMA transmembrane domain, has shown therapeutic promise. This approach increases the density of BCMA molecules on MM cells while reducing sBCMA sink effect, enhancing the cytotoxicity of anti-BCMA TCEs in preclinical settings.3,4,31 Ongoing trials (MajesTEC-2, MagnetisMM-4, NCT05137054, and NCT05259839) are investigating the safety and efficacy of the combination of anti-BCMA TCEs with γ-secretase inhibitors.32,33
It is apparent that high disease burden may also affect the efficacy of TCEs independently of the sBCMA sink effect. This is supported by the observation in patients with MM treated with non-BCMA targeting TCEs, such as talquetamab, where advanced ISS or the presence of EMD also results in lower responses.16,17 It is plausible that this reduced efficacy stems from a lower effector/target ratio with ISS III disease or reduced T-cell fitness with impaired T-cell infiltration of large lesions with hypoxic milieu in the case of EMD. Therefore, therapeutic strategies encompassing the integration of anti-MM TCEs following disease debulking or in combination with MM backbone agents (including immunomodulatory drugs, cereblon E3 ligase modulatory drugs (CELMoDs), proteasome inhibitors, or anti-CD38 antibodies) are currently being investigated.3,34-38
T-cell fitness
The preexisting fitness of T cells is a critical determinant of response to TCEs in MM.39 Nonresponders exhibit an abundance of TOX+ CD8+ terminally exhausted T cells and a reduced frequency of CX3CR1+ CD8+ effector cells, reflecting a spent immune response before TCE initiation.39 This was corroborated in the correlative study for teclistamab wherein higher baseline frequency of T cells expressing checkpoint molecules PD-1 and TIM-3 was associated with primary refractoriness.24 In addition to T-cell fitness, the triple-class refractory (TCR) repertoire is also implicated in responses to TCE. Clinical responders consistently exhibit early and sustained increase in clonality, primarily in CD8+ T cells.39 This clonotypic expansion suggests that following the initial TCR-independent T-cell activation by TCE, a subsequent secondary response, contingent on the expression of MM antigen-specific TCR by endogenous T cells, has the potential to enhance and sustain TCE-mediated anti-tumor immunity.
Although T-cell dysfunction plays a significant role in primary refractoriness to TCE, it does not appear to constitute a prominent mechanism of acquired resistance. This is evidenced by emerging observations that patients who relapse after durable remissions on TCE do respond to a subsequent immediate TCE that targets an alternative antigen.21,40,41 Further work is required to understand the extent to which TCEs mediate the pooling of nonexhausted postthymic peripheral blood T cells to the tumor site39 in addition to engaging existing marrow infiltrated T cells.
Given the indiscriminate engagement of CD3 molecules by TCEs, regulatory T cells (Tregs) may potentially be activated and pooled to the tumoral milieu attenuating cytotoxic T-cell activity.42 This concern lead to the design of TCEs that can preferentially activate CD8+ cells over Tregs.43 High proportion of Tregs was also noted in nonresponders to anti-MM TCEs.24,44,45 Ongoing studies are exploring various strategies to enhance T-cell fitness by combining TCE with anti-CD38 antibodies46 and/or immunomodulatory drugs,47 novel CELMoDs (NCT06163898), or PD-1 inhibitors (TRIMM-3, NCT05338775).
Antigenic escape
Antigenic escape is the predominant mechanism of acquired resistance to anti-BCMA and anti-GPRC5D TCEs.20,48-53 Although monoallelic copy number losses in TNFRSF17 are present in 4% to 6% of T-cell immunotherapy-naïve patients with MM,49,54 BCMA antigenic loss, resulting from biallelic or monoallelic deletions coupled with BCMA extracellular domain mutations, is observed in ≈40% of relapse cases after anti-BCMA TCE.48 Such BCMA null clones may be newly detected at the time of relapse after TCE, whereas in other cases, preexisting subclones (<1%) harboring biallelic deletion of TNFRSF17 can clonally expand under prolonged TCE-mediated immunoselection.48 A clustering of mutations mapping to BCMA extracellular domain between amino acids in positions 27 (arginine) and 34 (proline) coupled with monoallelic loss of TNFRSF17 (chromosome 16p) was observed in patients progressing on teclistamab and elranatamab, with these mutations abrogating the binding of these TCEs to BCMA. However, these mutations did not universally negate the efficacy of all anti-BCMA TCEs in vitro, in particular those with different geometry and valency, such as alnuctamab.48 Indeed, designing TCE with reduced interdomain distance by the tandem arrangement of CD3 and tumor antigen binding domains (cis orientation) has been reported to be more potent in vitro and in vivo,55 particularly for targeting tumor cells with low antigen expression, and has also demonstrated the ability to overcome resistance mediated by low effector/target ratios.56
Although GPRC5D monoallelic loss is reported in 15% of TCE naïve patients,49 a similar pattern of convergent evolution leading to biallelic antigenic exclusion was also noted in nearly all patients progressing on talquetamab.48,53 This higher incidence of GPRC5D antigenic loss compared with BCMA is consistent with the oncogenic dependency and survival signaling mediated by BCMA ligands.
Therapeutically, the emergence of these mutant clones highlights the need for dynamic surveillance of antigenic escape with adapted interventions or the use of multiantigenic targeting modalities to minimize the risk of clonal escape and efficiently target low antigen density reservoirs preceding clinical relapse. As such, the RedirecTT-1 trial (NCT04586426)57 trial is investigating the combination of talquetamab with teclistamab every 2 weeks in triple-class therapy exposed patients with MM. Preliminary results showed an encouraging ORR of 96.3%, including an 85.7% response in patients with EMD.57 This enhanced effectiveness achieved through dual targeting may reflect the eradication of MM clonal variants expressing low or no antigens. Furthermore, talquetamab, unaffected by sBCMA, may diminish tumor bulk and subsequently reduce sBCMA sink, potentiating the cytolytic effect of teclistamab. Other novel trial designs are exploring the sequential combination of targeted immunotherapies, with the use of anti-GPRC5D TCEs as consolidation or maintenance strategy after BCMA-targeting CAR T cells (NCT06066346) to eliminate BCMA low or null minimal residual clones. Moreover, exploration of alternative targets beyond BCMA or GPRC5D, such as FcRL5, is also underway58 (supplemental Table 1).
Sequencing and duration of therapy
Recent data reveal distinct clinical outcomes based on the timing of TCE administration in relation to CAR T therapy. Patients experiencing disease relapse after anti-BCMA TCE followed by anti-BCMA CAR T exhibit suboptimal response and progression-free survival rates.59,60 Conversely, favorable responses are observed when anti-BCMA TCE is administered after anti-BCMA CAR T.7,61,62 Non-BCMA targeting TCEs, such as talquetamab and cevostamab (targeting FcRL5), have also demonstrated durable responses after anti-BCMA agents.16,21,61,63,64 This stark difference in efficacy between the 2 sequencing approaches may be partly attributed to the potentially higher likelihood of antigen escape as well as skewing of T-cell repertoires following sustained immunotherapeutic pressure with continuous anti-BCMA TCE administration.
Preclinical data suggest that T-cell exhaustion induced by continuous TCE exposure can be ameliorated by treatment-free intervals.65 It is, however, important to note that unlike an artificial in vitro system, physiological T-cell repertoires are replenished daily by continuous supply of postthymic T cells. Nevertheless, even if treatment-free intervals do not affect TCE efficacy, they are likely to reduce the frequency and severity of infections.9,13 Furthermore, emerging data with cevostamab support fixed duration of therapy with maintained responses after treatment discontinuation.66
Conclusion
In MM, the rapidly evolving landscape of targeted immunotherapies with TCE offers a great promise with unprecedented responses in heavily pretreated patients. Ongoing studies to identify the optimal dosing schedule, duration of therapy, and combinations with other anti-MM agents will permit the optimization of their efficacy while minimizing their toxicities. The evolving understanding of tumor immune biology not only sheds light on the mechanisms of therapy resistance but also paves the way for developing rational therapeutic approaches.
Acknowledgments
H.L., P.N., and N.J.B. are supported by grants from the Terry Fox Foundation, International Myeloma Society, Multiple Myeloma Research Foundation, Myeloma Canada, and Leukemia Lymphoma Society.
Authorship
Contribution: H.L., P.N., and N.J.B. wrote the article.
Conflict-of-interest disclosure: N.J.B. has received research funding from Pfizer; has received speaker’s bureau honoraria from Amgen, BMS, Sanofi, Pfizer, and Janssen; and is a consultant/advisory board member for BMS, Janssen, and Pfizer. P.N. has received speaker’s bureau honoraria from BMS, Janssen, and Sanofi; and is a consultant/advisory board member for BMS and Janssen. H.L. declares no competing financial interests.
Correspondence: Nizar J. Bahlis, Arnie Charbonneau Cancer Institute, Heritage Medical Research Bldg, Room 328, 3330 Hospital Dr NW, Calgary, AB, Canada T2N 4N1; email: nbahlis@ucalgary.ca.
References
Author notes
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal