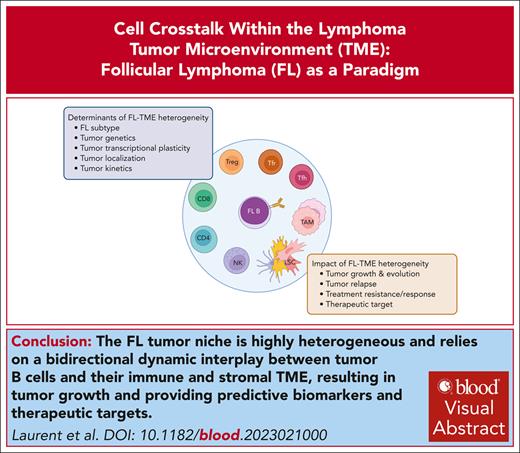
Visual Abstract
Follicular lymphoma (FL) is an indolent yet incurable germinal center B-cell lymphoma retaining a characteristic follicular architecture. FL tumor B cells are highly dependent on direct and indirect interactions with a specific and complex tumor microenvironment (TME). Recently, great progress has been made in describing the heterogeneity and dynamics of the FL TME and in depicting how tumor clonal and functional heterogeneity rely on the integration of TME-related signals. Specifically, the FL TME is enriched for exhausted cytotoxic T cells, immunosuppressive regulatory T cells of various origins, and follicular helper T cells overexpressing B-cell and TME reprogramming factors. FL stromal cells have also emerged as crucial determinants of tumor growth and remodeling, with a key role in the deregulation of chemokines and extracellular matrix composition. Finally, tumor-associated macrophages play a dual function, contributing to FL cell phagocytosis and FL cell survival through long-lasting B-cell receptor activation. The resulting tumor-permissive niches show additional layers of site-to-site and kinetic heterogeneity, which raise questions about the niche of FL-committed precursor cells supporting early lymphomagenesis, clonal evolution, relapse, and transformation. In turn, FL B-cell genetic and nongenetic determinants drive the reprogramming of FL immune and stromal TME. Therefore, offering a functional picture of the dynamic cross talk between FL cells and TME holds the promise of identifying the mechanisms of therapy resistance, stratifying patients, and developing new therapeutic approaches capable of eradicating FL disease in its different ecosystems.
Introduction
Follicular lymphoma (FL) is a B-cell neoplasm derived from germinal center (GC) B cells and involves primarily nodal sites.1 Normal GCs are transient and dynamic microanatomical structures dedicated to the antigen-driven expansion, selection, and differentiation of high-affinity B cells through a finely controlled process, ultimately conferring long-term humoral immunity.2 Recent studies have demonstrated that class switching to downstream immunoglobulin (Ig) isotypes IgG, IgE, and IgA essentially occurs early in pre-GC B cells.3 Within the GC, B-cell fate results from the integration of signals received from the B-cell receptor (BCR), T follicular helper (Tfh) cells, and specialized lymphoid stromal cell (LSC) subsets. In particular, follicular dendritic cells (FDCs) organize a spatial segregation of the GC. In the dark zone (DZ), centroblasts proliferate and undergo random somatic hypermutation in Ig-variable regions. In the light zone (LZ), high-affinity centrocytes (CCs) capture antigen from FDCs, thus receiving a BCR-dependent signal,4 and also receive survival and differentiation stimuli from cognate CD4+CXCR5+PD-1hiICOShi Tfh cells. Selected CCs recirculate to the DZ for additional cycles of proliferation and mutations or differentiate into memory B cells and plasma cells. Tfh help is counterbalanced by a subset of Foxp3+ GC-resident T cells called T follicular regulatory (Tfr) cells, acting as a critical modulating force in GC B-cell selection and contributing to GC contraction.5 The vast majority of GC B cells are not positively selected, and approximately half of them undergo apoptosis every 6 hours before being cleared by GC tingible-body macrophages.6 Finally, several subsets of non-GC LSCs, collectively referred as fibroblastic reticular cells (FRCs), sequentially regulate each step of B-cell activation within discrete lymph node (LN) 3-dimensional (3D) areas by recruiting naïve B and T cells, transferring antigens to B cells, organizing activated B- and T-cell traffic and interaction, and mediating plasmablast and plasma cell migration and survival.7,8 In turn, B cells affect both Tfh maturation and function through cognate antigen presentation, costimulatory signals,9 and FDC and FRC differentiation and maintenance, in particular through the production of tumor necrosis factor α (TNFα) and lymphotoxin-α1β2 (LT-α1β2).10-13 All these cell subsets are present within FL LNs and contribute to the organization of tumor-permissive niches while receiving FL B-cell–derived signals modulating their functional properties. A rich ecosystem of antitumor immune cells is also detected but displays altered phenotype and functions supporting immune escape mechanisms. The dynamic interplay between protumor and antitumor cell components, organized within a highly remodeled extracellular matrix (ECM), structures the FL TME, whose composition and organization has been largely associated with FL development, evolution, and prognosis, thus providing predictive biomarkers and putative therapeutic targets.
General FL niche organization
Histological examination of FL LNs classically reveals a follicular growth pattern, in which neoplastic follicles include malignant B cells, Tfh, Tfr, and a CD21+ FDC network of varying intensity1 (Figure 1). However, FL follicles display several altered features, including a low proliferation rate, a loss of DZ/LZ polarization, a lack of tingible-body macrophages, and attenuated mantle zones. Moreover, unlike their normal counterpart, FL follicles are not confined to the LN cortex but disseminated throughout chronically enlarged LNs with a disrupted architecture. Surrounding extrafollicular areas are reduced and display a dense network of activated FRCs and T cells with disseminated neoplastic CCs. CD8+ T cells are usually excluded from FL follicles.14 Subcapsular and medullary sinuses are typically obliterated. FL tissues contain numerous blood vessels, and neovascular sprouts are associated with tumor-infiltrating macrophages (TAMs) in extrafollicular areas.15 Besides conventional FL, early in situ lesions of FL, including in situ follicular neoplasia and duodenal-type FL, share with conventional FL a follicular pattern1 and the t(14;18) translocation, the earliest event initiating FL in ∼85% of cases by producing constitutive expression of the BCL2 antiapoptotic protein. In contrast, t(14;18)− CD23− FL was recently proposed as a distinct entity called follicle center lymphoma (FCL) and usually shows a diffuse growth pattern.16 In addition to BCL2 deregulation, FL is characterized by numerous recurrent genetic hits, particularly targeting the epigenetic machinery. The impact of the FL genetic landscape on the FL TME composition and organization, our understanding of which has recently been boosted by detailed analysis of genetically-engineered FL mouse models, has only recently emerged as a major determinant of FL pathogenesis and clinical outcome.17,18
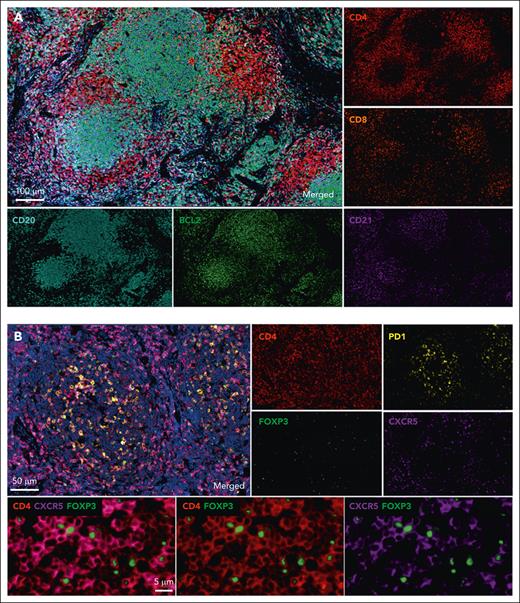
In situ phenotyping of FL grade 1/2 on formalin-fixed, paraffin-embedded sections using multiplex immunohistochemistry. (A) The normal architecture of the LN is effaced by neoplasia follicles of malignant B cells (CD20, clone L26, cyan) sustained by CD21+ FDCs (clone EP3093, magenta). Tumor cells strongly express BCL2 (clone SP66, green). Tumor-infiltrating T cells include a high number of CD4+ T cells (red) in interfollicular and intrafollicular areas, whereas CD8+ T cells (clone C8/144B, orange) are less numerous and usually observed at the follicle border. (B) Detection of Tfh and Tfr subpopulations in FL. Tfh cells are mostly observed in neoplastic follicles and coexpress CD4 (red), PD1 (clone NAT105, yellow), and CXCR5 (clone D6L3C, magenta). High magnification of follicle area shows Tfr positive for CD4 (red), CXCR5 (magenta), and FOXP3 (clone EP340, green).
In situ phenotyping of FL grade 1/2 on formalin-fixed, paraffin-embedded sections using multiplex immunohistochemistry. (A) The normal architecture of the LN is effaced by neoplasia follicles of malignant B cells (CD20, clone L26, cyan) sustained by CD21+ FDCs (clone EP3093, magenta). Tumor cells strongly express BCL2 (clone SP66, green). Tumor-infiltrating T cells include a high number of CD4+ T cells (red) in interfollicular and intrafollicular areas, whereas CD8+ T cells (clone C8/144B, orange) are less numerous and usually observed at the follicle border. (B) Detection of Tfh and Tfr subpopulations in FL. Tfh cells are mostly observed in neoplastic follicles and coexpress CD4 (red), PD1 (clone NAT105, yellow), and CXCR5 (clone D6L3C, magenta). High magnification of follicle area shows Tfr positive for CD4 (red), CXCR5 (magenta), and FOXP3 (clone EP340, green).
Extracapsular extension is common in conventional FL, with the invasion of the adipose perinodal tissue by ectopic GC-like structures and formation of fibrous bands in the extranodal tissue.7 Accordingly, FL is a disseminated disease with multiple nodal lesions and bone marrow (BM) involvement in ∼70% of cases.19 Within invaded BM, tumor cells are organized as follicle-like structures displaying a predominant paratrabecular localization and including ectopic functional FDCs and PD-1hi CD4+ T cells. As a whole, FL is characterized by its capacity to disseminate and the requirement of a follicle-like organization and cellular composition to support malignant B-cell growth, underlying the key role of tumor-TME cross talk in this neoplasia.
Protumoral vs antitumoral immune TME: T cells at the front
T cells are a major component of the FL TME (Figure 2). FL T cells are enriched for Tfh cells, exhausted cytotoxic T cells, and specific subsets of regulatory T cells (Tregs) compared with nonmalignant LN, creating a FL-specific T-cell signature.20-22 FL Tfh are not only expanded but overexpress interleukin 4 (IL-4), CD40L, and IL-21, which contribute to malignant B-cell survival and may also favor the commitment of a small fraction of the B-cell clone to plasma cell differentiation,20,23-25 challenging the concept of FL B cells being uniformly “frozen” at the GC stage.17 In addition, FL Tfh contribute indirectly to FL growth, as demonstrated in several in vitro studies. In particular, FL Tfh stimulate the capacity of FL B cells to respond to TAM-derived IL-1526 and to BCR activation mediated by dendritic-cell specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)–expressing TAMs.27 Indeed, N-glycosylation sites are introduced into FL BCR variable regions by somatic hypermutation and are occupied by unusual oligomannose glycans recognized by DC-SIGN, triggering antigen-independent BCR activation (as reviewed by Stevenson and Forconi28 in this review series). CD40L and IL-4 signaling also trigger the production of the Treg chemoattractant CCL22 by FL B cells, thereby promoting immune escape.29 Finally, FL Tfh–derived IL-4, TNF, and LT participate in the differentiation of FL cancer-associated fibroblasts (FL CAFs)30 and in their overexpression of CXCL12, which is involved in B-cell recruitment, adhesion, and activation.31 The major role of Tfh-derived IL-4 in FL pathogenesis is further supported by the demonstration that the gain-of-function phenotype of STAT6 mutations frequently found in FL is strictly dependent on the presence of IL-4.32 FL has been largely recognized as an immune-responsive neoplasia, infiltrated by cytotoxic CD8 and CD4 T cells, γδ T cells, and natural killer (NK) cells, and responding to vaccination strategies and immunomodulatory agents. However, FL-infiltrating CD4 and CD8 T-cell effectors are characterized by the coexpression of numerous immune checkpoint inhibitors, including LAG-3, TIM-3, and TIGIT.33-37 In contrast, programmed cell death protein 1 (PD-1) is not a relevant exhaustion marker in FL because of its high expression on fully functional Tfh23 and Treg/Tfr cell subsets.38 In addition, intratumoral T cells lacking CD27 and CD28 costimulatory receptor expression are enriched in FL.39 Besides reduced proliferation and cytokine production, FL T cells also exhibit an impaired lymphocyte function-associated antigen (LFA-1)–dependent motility,40 and a defective capacity to form mature immunological synapses with malignant B cells.41 Expansion of immunosuppressive cells is another hallmark of the FL TME with a strong amplification of Tregs, including classical Treg and Tfr subsets, which could both contribute to immune escape.38,42
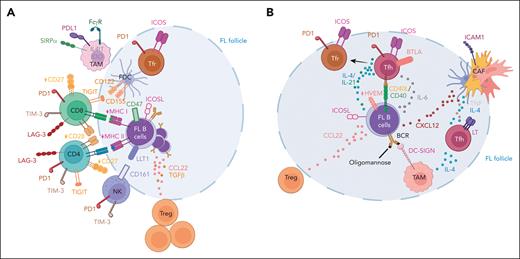
T-cell subsets within the FL microenvironment. (A) FL is characterized by a defective antitumor immune TME including amplified Treg/Tfr cells and exhausted CD4 and CD8 T cells expressing high amounts of immune checkpoint inhibitors, such as PD1, T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), lymphocyte activation gene-3 (LAG-3), and T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT), and reduced CD27 and CD28 costimulatory receptors. Ligands of immune checkpoint inhibitors and immunosuppressive enzymes are mainly expressed by TAMs and FDCs. However, tumor B cells play a central role in the induction of the altered immune TME, by overexpressing CCL22, inducible T-cell costimulator ligand (ICOSL), and transforming growth factor (TGFβ), involved in T-cell exhaustion and Treg amplification. FL B cells also frequently display reduced MHC I and MHC II expression, thus abrogating immune recognition by cytotoxic T cells. In addition, the cytotoxic activity of TAMs and NK cells is inhibited, particularly through CD47 and lectin-like transcript 1 (LLT1) expression by tumor B cells. (B) Tfh are expanded in FL and overexpress CD40L, IL-4, IL-21, and TNF/LT, thus contributing to tumor B-cell survival and transcriptomic plasticity. FL Tfh cells also favor B-cell–TAM cross talk, trigger CXCL12 expression in FL CAFs, and increase CCL22 expression by tumor B cells. FL Tfh could differentiate into FL Tfr that contribute to tumor immune escape. FL B cells contribute to FL Tfh amplification and reprogramming, notably by expressing CD40, ICOSL, and IL-6, and by losing herpes virus entry mediator (HVEM) expression, thus alleviating the B and T lymphocyte attenuator (BTLA)-mediated inhibitory signal on Tfh. Figure created with BioRender.com.
T-cell subsets within the FL microenvironment. (A) FL is characterized by a defective antitumor immune TME including amplified Treg/Tfr cells and exhausted CD4 and CD8 T cells expressing high amounts of immune checkpoint inhibitors, such as PD1, T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), lymphocyte activation gene-3 (LAG-3), and T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT), and reduced CD27 and CD28 costimulatory receptors. Ligands of immune checkpoint inhibitors and immunosuppressive enzymes are mainly expressed by TAMs and FDCs. However, tumor B cells play a central role in the induction of the altered immune TME, by overexpressing CCL22, inducible T-cell costimulator ligand (ICOSL), and transforming growth factor (TGFβ), involved in T-cell exhaustion and Treg amplification. FL B cells also frequently display reduced MHC I and MHC II expression, thus abrogating immune recognition by cytotoxic T cells. In addition, the cytotoxic activity of TAMs and NK cells is inhibited, particularly through CD47 and lectin-like transcript 1 (LLT1) expression by tumor B cells. (B) Tfh are expanded in FL and overexpress CD40L, IL-4, IL-21, and TNF/LT, thus contributing to tumor B-cell survival and transcriptomic plasticity. FL Tfh cells also favor B-cell–TAM cross talk, trigger CXCL12 expression in FL CAFs, and increase CCL22 expression by tumor B cells. FL Tfh could differentiate into FL Tfr that contribute to tumor immune escape. FL B cells contribute to FL Tfh amplification and reprogramming, notably by expressing CD40, ICOSL, and IL-6, and by losing herpes virus entry mediator (HVEM) expression, thus alleviating the B and T lymphocyte attenuator (BTLA)-mediated inhibitory signal on Tfh. Figure created with BioRender.com.
Importantly, FL T-cell polarization relies on a dynamic cross talk with FL B cells, as highlighted by the impact of FL genetics on T-cell features (Figure 2). The loss of HVEM expression by somatic mutations in 40% of patients with FL disrupts the inhibitory signal delivered to BTLAhi Tfh, favoring their amplification, capacity to deliver survival signal to healthy and malignant GC B cells, and overexpression of TNF/LT.30 Mutations of HVEM are essentially mutually exclusive to gain-of-function mutations of the cysteine protease cathepsin S, which similarly result in increased tumor-supportive Tfh infiltration in both mouse models and patients with FL by favoring major histocompatibility complex class II (MHC II)-restricted antigen presentation.43,44 These data further underscore the central role of Tfh cells in FL pathogenesis. High expression of CD40, IL-6, and ICOSL by tumor B cells has also been implicated in Tfh recruitment, activation, and proliferation.45,46 Although FL B cells do not express PD-1 or TIGIT ligands,35,47 in vitro experiments identified CCL22, CD70, ICOSL, and transforming growth factor β overexpression by tumor B cells as drivers of T-cell reprogramming toward exhausted and regulatory phenotypes.29,38,48-50 More generally, MHC II silencing in FL B cells, frequently found in patients with genetic alterations in CREBBP or EZH2,17 is associated with a reduced tumor infiltration by exhausted cytotoxic CD8 and CD4 T cells, suggesting that T-cell exhaustion in FL relies on persistent MHC II expression by tumor cells.20 Interestingly, a recent evaluation of the T-cell receptor repertoire coupled with single-cell RNA sequencing (RNA-seq) further argues in favor of a contribution of tumor antigen–driven clonal expansion of cytotoxic CD8 T cells and Tfh/Treg subsets in FL.22 This study also reveals that a substantial proportion of clonotypes are shared between FL Tfr and FL Tfh, identifying a FL Tfh–to–FL Tfr developmental trajectory under the continuous contact with malignant FL B cells. A similar process has recently been described in reactive lymphoid tissues for Tfh-descendant Tfr with dual T-cell suppressive/B-cell helper function,51 suggesting that FL coopts an existing differentiation pathway to trigger the amplification of tumor-supportive T cells. Regarding other cytotoxic effectors, FL B cells were proposed to engage the inhibitory receptor BTLA on infiltrating γδ T cells in patients with wild-type HVEM, thereby blocking their proliferation.52 Similarly, overexpression of lectin-like transcript 1 by FL tumor cells dampens in vitro the function of CD161+ NK cells.53 In conclusion, the FL T-cell compartment displays heterogeneous phenotypes and functions, collectively forming a protumoral immune niche dependent on a bidirectional cross talk with malignant B cells and providing the basis for immunotherapeutic interventions.
Stromal cells as organizers of the FL TME
FL CAFs develop at the expense of normal LSCs within infiltrated LNs and display quantitative and qualitative alterations supporting LN remodeling and tumor growth (Figure 3). Data on FL stromal cells initially result from the analysis of in vitro expanded cells and in situ low-throughput phenotypic characterization. However, the recent transcriptomic profiling of native FL FRCs and FL FDCs at bulk and single-cell levels has paved the way to a better understanding of their heterogeneity, plasticity, mechanisms of polarization, and tumor-supportive function. First, both FL FRCs and FL FDCs overexpress CXCL12,31,54 directly affecting tumor B-cell growth. In addition, heparinase, which increases the bioavailability of heparin-binding factors such as CXCL12, is strongly expressed in ∼50% of FL.55 Within normal GCs, CXCL12 expression is restricted to CD21− DZ FDCs (also called CXCL12-expressing reticular cells), whereas CD21+ LZ FDCs are CXCL12−.8 Within FL follicles, CXCL12 upregulation parallels the loss of LZ FDC markers, including CD21, CD23, and CD35, related to a lower expression of LT by malignant FL B cells than in normal CCs.31,56,57 Interestingly, CD23/IL-4 receptor α expression on LZ FDCs was recently shown in mice to restrict IL-4 availability for normal LZ CCs.58 Together with the expansion of IL-4hi FL Tfh, the loss of CD23+ FDCs in FL could, thus, contribute to the increase of IL-4 bioavailability and function. All FL LSC subsets also overexpress the chemokines CCL19/CCL21, which could contribute to the dissemination of CCR7+ FL B cells but also to the recruitment of the immune TME.54 Transcriptomic analyses identified a huge deregulation of genes involved in cell adhesion and ECM composition and organization in FL LSCs.54,59 Recently, elegant in vitro 3D models, integrating stromal cells and/or functionalized hydrogels, have been developed in order to capture the role of the ECM and biomechanical forces in B-cell lymphomas. These pioneering works have proposed a specific role for the α4β1 integrin signaling and shear stress in stroma-dependent lymphoma B-cell growth60 and drug resistance. A 3D alginate spheroid model coencapsulating LSCs with lymphoma B cells and integrating a layer of ECM lining the inner wall of the capsules has been proven efficient in sustaining primary FL B-cell survival in vitro.61 Importantly, ECM remodeling could also impair the accessibility of tumor B cells to cytotoxic T cells, as described for solid cancer CAFs. In addition, FL CAFs express the TIGIT ligands CD122 and CD155 as well as PGE2,35,62 which could participate in the defective immune response in FL niches. Beyond T cells, FL CAFs affect tumor-infiltrating myeloid cells. In particular, FL CAFs overexpress CCL2, involved in the recruitment of monocytes that could then be converted into proangiogenic and anti-inflammatory TAMs, as demonstrated by in vitro cocultures.63 As discussed earlier, FL TAMs contribute to FL B-cell activation, in particular, by triggering weak but long-lasting BCR signaling through the crosslinking of the oligomannosylated FL BCR by DC-SIGN.27,64 Furthermore, even if the phagocytosis of tumor B cells by TAMs is involved in the clinical activity of anti-CD20 antibodies, FL B cells express huge amounts of the “don't eat me” receptor CD47,65 inhibiting phagocytic and inflammatory functions of macrophages expressing signal regulatory protein α, found to display T-cell suppressive activity in FL.66 Finally, FL TAMs express classical immune checkpoint inhibitors, such as programmed cell death ligand 1 (PD-L1)67 and the immunosuppressive enzymes indoleamine-2,3 dioxygenase68 and IL-4–induced gene 1,69 involved in the catabolism of tryptophan and phenylalanine, respectively. However, the impact of immunosuppressive myeloid cells on immune escape and drug resistance is less clear in FL than in diffuse large B-cell lymphoma.
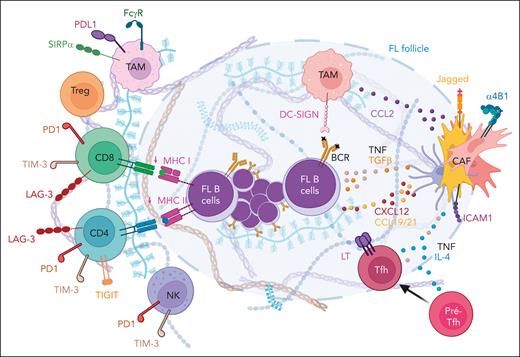
Role of stromal cells within the FL microenvironment. FL CAFs are central organizers of the FL TME. They overexpress CXCL12, CCL19, and CCL21, contributing to tumor B-cell recruitment and activation, and CCL2, involved in TAM recruitment. FL CAFs also show a deregulated expression of adhesion molecules and extracellular matrix components affecting B-cell and immune cell migration. Finally, they contribute to the polarization of TAMs and pre-Tfhs. Figure created with BioRender.com.
Role of stromal cells within the FL microenvironment. FL CAFs are central organizers of the FL TME. They overexpress CXCL12, CCL19, and CCL21, contributing to tumor B-cell recruitment and activation, and CCL2, involved in TAM recruitment. FL CAFs also show a deregulated expression of adhesion molecules and extracellular matrix components affecting B-cell and immune cell migration. Finally, they contribute to the polarization of TAMs and pre-Tfhs. Figure created with BioRender.com.
Malignant FL B cells play a central role in driving the reprogramming of FL stromal cells (Figure 3). In particular, B-cell–derived TNF and LT have been involved in the upregulation of CCL2,63 and TNF/LT synergize with transforming growth factor β to induce CCL19 and CCL21 production by FL CAFs.54 Gain-of-function mutations of the methyltransferase EZH2, found in 20% to 30% of patients with FL, are associated with an early overexpression of LT and TNF by LZ CCs in genetically-engineered murine models, resulting in a preferential interaction with FDCs.70 In agreement, the FDC network was found more expanded in patients with EZH2-mutated FL. Thus, both HVEM and EZH2 mutations result in FL stromal cell activation but by different mechanisms, including the overexpression of TNF/LT by Tfh cells vs FL B cells. Tfh also contribute to the polarization of FL CAFs, in particular by producing high amounts of IL-4, TNF, and LT.31,42 Interestingly, FL Tfh overexpress CD40L and interferon-γ, both of them being able to activate stromal cells and regulate their production of inflammatory chemokines and immunosuppressive factors.71,72 In turn, FL CAFs contribute to the polarization of FL Tfh by favoring the early commitment of pre-Tfh into IL-4hi–producer cells, in a Notch- and ICAM1/LFA1-dependent manner.73 As a whole, FL CAFs are engaged in a bidirectional cross talk with tumor B cells and the other TME components and display pleiotropic activities.
Spatial and kinetic heterogeneity of the FL TME
An important open question is to understand how the heterogeneity of FL B cells, Tfh cells, and CAFs translates into the organization of distinct tumor niches reflecting coordinated spatial and kinetic heterogeneity and with different capacities to sustain disease progression and relapse. Spatial heterogeneity has emerged as an important determinant of disease heterogeneity in FL. First, the comparison of synchronous distant nodal sites reveals a unique correlation between site-to-site tumor cell subclonal, transcriptomic, and phenotypic dissimilarity and site-to-site variability in FL Tfh abundance, confirming Tfh as key players in FL lymphomagenesis.34 Furthermore, analysis of paired LN and BM tumor lesions identifies specific features of BM FL B cells, including lower cytological grade; reduced proliferation; altered phenotypic, metabolic, and transcriptomic profiles; and divergent subclonal evolution compared with LN B cells.74-76 Regarding stromal cells, ectopic FRC-like and FDC-like cells are detectable within BM follicle-like structures, confirming the dependence of FL B cells on LSCs.7,77 BM and LN FL CAFs share other common features, including upregulation of CCL2, CXCL12, and secreted protein acidic and rich in cysteine (SPARC), a matricellular protein involved in collagen deposition and organization.31,63,78 Although they produce less LT than normal CCs, the large number of GC-like B cells found in invaded FL BM leads to a local increase in TNF and LT levels, which may contribute to the induction of LSC commitment and CCL2 expression in BM stromal cells. In addition, as described in LNs, IL-4 and CXCL12 are correlated in FL BM, suggesting a contribution of the FL immune TME to BM FL CAF activation.31 Although the CD4:CD8 T-cell ratio is increased in FL-invaded BM, it remains lower than in FL LNs77,79 and the presence of IL-4–producing Tfh-like cells in FL BM remains to be firmly established. Interestingly, BM stromal cells obtained from noninvaded FL BM show an intermediate gene expression profile between healthy donor BM stromal cells and FL-invaded BM stromal cells, suggesting a priming of BM stromal cells in the absence of direct contact with tumor B cells and expanded CD4 T cells.76 This phenotype may be related to the production of extracellular vesicles by FL B cells that are internalized by BM stromal cells, increasing their capacity to support BM FL B-cell survival and quiescence, at least in part, through the upregulation of CXCL12.76 This study further suggests that differences in LN vs BM stromal cell features support specific patterns of molecular interactions with malignant B cells and contribute to tumor spatial heterogeneity. To date, to our knowledge, no study has been designed to evaluate whether, at the intratissular level, the dynamic continuum of transcriptional states that characterize FL B cells20,80 is related to the integration of various signals from their surrounding TME, eventually corresponding to microanatomical areas supporting an additional layer of spatial heterogeneity. Collectively, these studies underscore the difficulty of capturing FL disease via a single biopsy.
In addition to spatial heterogeneity, the issue of kinetic heterogeneity is another key element with potential therapeutic implications. A primary question pertains to the niche of FL-committed precursor cells, which can be detected years before FL diagnosis, exhibit early driver genetic events, can be traced between paired prediagnostic blood and tumor biopsies, and are responsible for seeding FL relapses and transformation.17 These cells are virtually impossible to directly visualize or isolate because of their scarcity and lack of phenotypic markers, but their specific localization and capability to integrate the local microenvironment cues are an object of intense interest. In healthy individuals, FL precursor cells accumulate within the GC as nonproliferative CCs81 and can also be detected in the BM.82 However, the niches in which residual committed precursor cells remain after treatment are completely unknown. Besides, data are missing regarding the impact of FL treatment on the TME. Histologic transformation occurs in 8% to 15% of patients with FL, usually into the GC B-cell subtype of diffuse large B-cell lymphoma,83,84 in association with an increased proportion of large cells diffusely infiltrating LNs and acquiring specific genetic features, an effacement of the follicular architecture, and a loss of FDC meshwork and Tfh infiltration, whereas TAMs and CD8+ T cells increase.85 Several studies based on limited patient cohorts and/or targeted approaches have proposed variable TME-related markers predicting the transformation risk.86,87 Recently, a large whole-exome sequencing study has proposed a classifier predicting transformation from genetic features present at diagnosis.88 Moreover, a thorough characterization of B-cell/TME features at diagnosis identified a subgroup of FL patients with a B-cell profile related to memory B cells and an increased Tfh content as being more prone to transformation,89 suggesting that B-cell–TME interplay may play a central role in FL evolution. Further studies are necessary to confirm the biological and clinical significance of these findings.
TME and nonconventional FL
Few studies have explored the composition and spatial organization of nonconventional FL. Duodenal-type FL, which share their genetic alteration profile and nodular pattern with conventional FL, displays a specific proinflammatory TME, with increased abundance of Th17 and activated T cells, and a reduced FDC network, CCL21 expression, and Treg infiltration.90-92 Interestingly, B cells from duodenal-type FL exhibit memory B-cell features, including CCR6 expression, supporting the idea of an impact of TME features on FL B-cell plasticity.91,93 Diffuse t(14;18)−CD23+ FCL show a gene expression profile enriched in T-cell, NK, and dendritic cell signatures, whereas tumor B cells produce very low amounts of LT in association with a lack of mature FDCs.16,57 This specific TME composition may reflect how FCL B cells cope with the absence of the GC niche that characterize typical FL. Finally, when compared with t(14;18)+ FL cases, t(14;18)− conventional FL cases (after excluding FCL and pediatric-type FL) show an enrichment of inflammatory signatures, including overexpression of NF-κB–target genes and cytotoxic T-cell markers.94 Importantly, N-glysosylation sites were detected less frequently in the Ig-variable regions of the t(14;18)− FLs that display a bias for IGHV4-34 usage.95 These features may indicate a responsiveness to autoantigens, unlike to DC-SIGN–dependent signaling, in this FL subgroup. Even if these nontypical FL entities are rare and heterogeneous, the demonstration that they have specific TME characteristics further underscores the role of tumor-TME bidirectional cross talk in FL pathogenesis.
Clinical impact of B-cell–TME cross talk
Since the pioneering study published in 2004,96 most prognostic biomarkers in FL reflect the immune TME composition and organization rather than the tumor genetic landscape. However, these studies have given contradictory conclusions, highlighting the significant influence of treatment regimens on the predictive value of individual biomarkers and the lack of validated tools to assess the composition, organization, and function of the FL TME. Nevertheless, they suggest that heavy infiltration by T cells in the presence of tumor cells retaining antigen-presenting capacities provides a good prognosis, even when associated with an immunosuppressive context.14,20,97-99 The role of local T-cell diversity, as could be studied directly or inferred from bulk RNA-seq using appropriate bioinformatic tools,100,101 should be better evaluated in clinically annotated patient series to conclude on its impact on patient outcome. Some cell types, such as Tfh/Tfr cells, stromal cells, or myeloid cell subsets, are challenging to explore using bulk transcriptomic data or conventional immunohistochemistry/immunohistofluorescence, the main technical approaches suitable for analyzing reproducibly large patient cohorts using formalin-fixed, paraffin-embedded samples. Conversely, advanced multiparametric strategies including single-cell RNA-seq approaches or flow cytometry/mass cytometry on a limited number of samples have been instrumental in identifying TME complexity and plasticity and remain the only way to fully explore TME dynamics based on iterative tumor samples. Of note, the analysis of myeloid and stromal cell properties requires tissue enzymatic dissociation and is less advanced than the analysis of tumor-infiltrating lymphocytes. Finally, high-parameter spatial proteomic studies, either on tissue microarrays (ie, transferable to clinical application) or whole tissue sections (ie, integrating intratumoral heterogeneity) are of utmost significance but require substantial work of standardization before moving to clinical practice, being currently better suited for expert analysis of clinical trials. The different spatial tools are detailed in another review in this series by Radtke and Roschewski.102 As a whole, innovative technologies are essentially low throughput and difficult to standardize at the preanalytical, analytical, and data integration steps but could produce new relevant cell signatures, currently essentially derived from peripheral blood or solid cancers, to develop better deconvolution strategies and transfer this knowledge to bulk RNA-seq or targeted RNA/protein quantification, routinely proposed to study tumor heterogeneity. For example, we recently demonstrated that the frequency of exhausted cytotoxic T cells is associated with clinical outcome using a deconvolution approach of bulk RNA-seq data.22 Importantly, given the interplay between tumor genetics and the TME, the mutational landscape should be analyzed when evaluating FL TME impact on clinical response, as reported for cysteine protease cathepsin S mutations and the response to standard immunochemotherapy.44 Regarding stromal cells, FL CAF–related ECM genes were recently proposed as markers of poor prognosis,59 but this study used transcriptomic data from patients treated with chemotherapy alone and should be reevaluated for patients treated with classical immunochemotherapy regimens. It is unclear to what extent peripheral blood can be used to identify soluble or cellular TME–related predictive biomarkers in FL. Nevertheless, it enables convenient patient follow-up, looking for the impact of therapeutic strategies on (re)circulating immune cells, in particular T cells.103
The combination of the immunomodulatory agent lenalidomide with immunochemotherapy was proven effective in FL104 based on the capacity of lenalidomide to induce early T-cell activation and reprogramming and restore long-term immune synapse formation in vivo in patients with FL.103 Conversely, minimal responses to anti-PD1/anti–PD-L1 antibodies have been obtained in FL. This may be related to the low expression of PD-L1 in FL, whereas PD-1 is expressed by various T-cell subsets with different functions, including not only exhausted T cells but also fully functional Tfh and Treg/Tfr cells, requiring the targeting of other checkpoint inhibitors, including TIGIT or LAG-3, and/or of other suppressive mechanisms to achieve efficient antitumor T-cell activation. Recently, new immunotherapy strategies, such as tumor-targeting chimeric antigen receptor (CAR) T cells and bispecific antibodies redirecting endogenous T cells to the tumor, have been introduced for the treatment of FL.105 Despite the success of these novel treatments, clinical responses are heterogeneous, and identifying biomarkers for response prediction requires new strategies, probably involving functional approaches to evaluate the capacity of tumor-infiltrating T cells to recognize and kill tumor B cells within the tumor-supportive TME. As an example, ex vivo resistance to bispecific antibodies has been recently correlated to the frequency of Helios-positive Tregs, data that should be prospectively validated in clinical cohorts.106 Similarly, resistance to lenalidomide and anti-CD20 association was found associated with a high percentage of preexisting Tregs.103 These data argue for considering Treg depletion and/or functional blockade in FL, as recently described in solid cancers.107
In addition to the direct activation of immune effectors by immunotherapy approaches, other therapeutic options initially designed to target B cells can reprogram B-cell–TME cross talk. EZH2 mutations found in 25% of patients with FL and associated with a loss of MHC I and II expression108 have been associated with a better outcome after cyclophosphamide, doxorubicine, vincristine, and prednisone–based, unlike bendamustine-based, immunochemotherapy regimens in the GALLIUM clinical trial (NCT01332968).109 The differential impact of the EZH2 mutational status is not due to a difference in the sensitivity of tumor B cells to chemotherapy. In contrast, unlike bendamustine, doxorubicine restores MHC I/II expression on EZH2-mutated tumor B cells, potentially promoting cytotoxic T-cell response.110 How EZH2 inhibitors currently evaluated in FL modulate immune and stromal TME polarization and functional interplay with tumor B cells remain to be explored but may be involved in the clinical response of patients with EZH2-mutated and wild-type disease. To date, only limited attempts have been made to design therapies targeting FL tumor-supportive niches. IL-4, which plays pleiotropic roles in FL B-cell growth and is associated with ibrutinib resistance in chronic lymphocytic leukemia,111 is a putative target. Moreover, interfering with BCR–DC-SIGN interaction112-114 would also be relevant to block TME-dependent BCR activation. Finally, Tfh-derived signals could be abrogated by CAR T cells targeting CXCR5, expressed on both B and Tfh cells,115 or by CD19-targeting CAR T cells delivering soluble HVEM inside the tumor bed.30 Besides their direct tumor-supportive activities, CAFs have been demonstrated in solid cancers to counteract the activity of immunotherapies, notably by maintaining antitumor T cells at the periphery of the tumor, by recruiting and activating Tregs, and by inhibiting CD8 T-cell and NK cell activity.116 Targeting the CAFs or CAF-derived ECM in FL may be an appealing strategy in the context of the development of novel immunotherapy approaches in this disease. Conversely, the huge expression of CCL21/19 by FL CAFs could contribute to the recruitment of antitumor immune effectors. The clinical impact of B-cell–TME cross talk in FL deserves further evaluation through well-designed trials including thorough ancillary studies repositioning tumor cell functional heterogeneity within their various ecosystems.
Acknowledgments
C.L. is supported by the LABEX TOUCAN. K.T. is supported by research grants from the Institut National Du Cancer (INCA AAP PLBIO-21-197 and INCA AAP PNP19-009) and the Ligue Nationale Contre le Cancer (Equipe Labellisée). C.L., S.D., and K.T. are funded in a TRANSCAN-3 Joint Transnational Call for Proposals-2021 European program (BIALYMP program).
Authorship
Contribution: All authors contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karin Tarte, Faculté de Médecine, INSERM, UMR U1236, MOBIDIC, 2 Ave du Pr Léon Bernard, F-35043 Rennes, France; email: karin.tarte@univ-rennes1.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal