KIT D816V confers resistance to TNF via BIRC5 (survivin) and promotes clonal dominance.
High TNF levels are associated with inferior survival in SM.
Visual Abstract
Systemic mastocytosis (SM) is defined by the expansion and accumulation of neoplastic mast cells (MCs) in the bone marrow (BM) and extracutaneous organs. Most patients harbor a somatic KIT D816V mutation, which leads to growth factor–independent KIT activation and accumulation of MC. Tumor necrosis factor α (TNF) is a proapoptotic and inflammatory cytokine that has been implicated in the clonal selection of neoplastic cells. We found that KIT D816V increases the expression and secretion of TNF. TNF expression in neoplastic MCs is reduced by KIT-targeting drugs. Similarly, knockdown of KIT or targeting the downstream signaling cascade of MAPK and NF-κB signaling reduced TNF expression levels. TNF reduces colony formation in human BM cells, whereas KIT D816V+ cells are less susceptible to the cytokine, potentially contributing to clonal selection. In line, knockout of TNF in neoplastic MC prolonged survival and reduced myelosuppression in a murine xenotransplantation model. Mechanistic studies revealed that the relative resistance of KIT D816V+ cells to TNF is mediated by the apoptosis-regulator BIRC5 (survivin). Expression of BIRC5 in neoplastic MC was confirmed by immunohistochemistry of samples from patients with SM. TNF serum levels are significantly elevated in patients with SM and high TNF levels were identified as a biomarker associated with inferior survival. We here characterized TNF as a KIT D816V-dependent cytokine that promotes clonal dominance. We propose TNF and apoptosis-associated proteins as potential therapeutic targets in SM.
Introduction
Systemic mastocytosis (SM) is a clonal hematologic disorder characterized by the expansion and accumulation of neoplastic mast cells (MCs), affecting extracutaneous organs, such as the bone marrow (BM), liver, or spleen.1 SM comprises a heterogenous group of diseases, ranging from indolent variants to aggressive subtypes with a poor survival.2 The WHO classification includes the following subtypes: indolent SM (ISM), bone marrow mastocytosis, smoldering SM (SSM), aggressive SM, SM with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia.2,3 Aggressive SM, SM-AHN, and mast cell leukemia are associated with SM-related organ damage and are collectively summarized as advanced SM (AdvSM).4 The activating KIT D816V mutation is a hallmark of SM and is detected in the neoplastic cells of most patients, irrespective of the SM subtype.5 Tyrosine kinase inhibitors (TKIs) that affect KIT D816V are pivotal for the management of patients with AdvSM,6,7 but the only curative treatment option available to date is allogeneic hematopoietic stem cell (HSC) transplantation.8 Despite novel therapies, only a few patients with AdvSM can be cured, and a better understanding of the pathophysiology of neoplastic MC is crucial for the development of novel therapeutic options.
KIT D816V is a gain-of-function mutation resulting in constitutive activation of KIT, independently of its ligand stem cell factor.9 Nuclear factor κ light-chain enhancer of activated B cells (NF-κB), phosphatidylinositol 3-kinase (PI3K), signal transducer and activator of transcription 5 (STAT5), and mechanistic target of rapamycin (mTOR) are known downstream mediators of KIT D816V.10-13 Upregulation of expression and secretion of various cytokines by KIT D816V has been described, including interleukin-6 (IL-6) and IL-13, oncostatin M, and C-C motif chemokine ligands 2 and 23.14-17 In many instances, upregulated cytokine production and secretion are associated with disease severity and progression. Some of the cytokines have been linked to the development of characteristic BM microenvironment alterations, but little is known about the impact of MC-derived cytokines in the pathogenesis, disease evolution, and progression of SM.
Our previous study on KIT D816V-expressing cell lines suggests that this mutant promotes expression of tumor necrosis factor α (TNF) in neoplastic cells.16 TNF is a proapoptotic and antiproliferative cytokine and a well-defined mediator of inflammation.18 TNF is produced by various cell types, including MCs, macrophages, monocytes, T cells, and eosinophils.19-22 TNF acts via 2 main receptors: TNF receptor superfamily member 1A (TNFRSF1A or TNFR1) and 1B (TNFRSF1B or TNFR2).23 TNFR1 possesses an intracellular death domain, and its signaling primarily mediates inflammation and apoptosis. Instead, activation of TNFR2 regulates proliferation and survival and mediates anti-inflammatory effects.24-26 In general, TNF acts in an autocrine as well as a paracrine manner during inflammation.27 So far, the effects of TNF on neoplastic MC and their microenvironment have not been studied in detail.
A number of previous data implicated TNF in he clonal selection of cancer cells.28,29 As such, TNF has been identified as a key player in the disease evolution and progression of JAK2 V617F+ myeloproliferative neoplasms.30,31 Patients with SM-AHN also show a relevant myeloproliferative component of the disease, with clonal dominance of KIT D816V+ myeloid cells. The mechanisms of disease evolution and clonal selection in SM are still poorly understood. Here, we analyzed the mechanisms of expression and secretion of TNF in KIT D816V+ neoplastic MC and deciphered the contribution of TNF to disease progression in patients with SM.
Material and methods
Patients
The study was conducted in accordance with the Declaration of Helsinki and approved by the local institutional review board (EK#2010/566, EK#2011/404, EK#2014/1018, and EK#2014/1184). Forty patients (discovery cohort) and 142 additional patients (validation cohort) with mastocytosis were examined (Table 1). Mastocytosis was classified according to WHO 2016 criteria.4 For diagnosis and staging, the following analyses were routinely conducted: measurement of serum tryptase levels, histologic and cytologic examination of the BM, flow cytometric analysis of surface expression of CD2 and CD25 on BM MCs, melting point analysis after clamp polymerase chain reaction (PCR) and/or quantitative allele-specific PCR to identify KIT D816V, and sequencing of SRSF2, ASXL1, and RUNX1 (S/A/R).32-34 Sex- and age-matched healthy donors served as control cohort for TNF serum levels. Patients with lymphoma without BM infiltration were used as controls for BM samples. Informed consent was obtained in each case.
Patients’ characteristics of the mastocytosis cohort
| Discovery cohort . | CM/MIS (n = 5) . | ISM/SSM (n = 24) . | AdvSM (n = 11) . | All patients (n = 40) . |
|---|---|---|---|---|
| Age (y; median, range) | 32 (23-55) | 53 (23-82) | 64 (50-74) | 54 (23-82) |
| Sex (number; female/male) | 3/2 | 14/10 | 5/6 | 22/18 |
| KIT D816V mutation (number and % positive) | 4/5 (80.0%) | 21/22 (95.5%) | 9/11 (81.8%)∗ | 34/38 (89.5%)∗ |
| KIT D816V allele burden (%; median, range) | 0.06 (0.034-0.14) | 0.14 (0.009-9.09) | 15.17 (0.002-46.9) | 0.14 (0.002-46.9) |
| TNF† (pg/mL; median, range) | 1.54 (1.05-2.57) | 2.13 (0.48-11.73) | 2.96 (1.09-16.64) | 2.06 (0.48-16.64) |
| Validation cohort | CM/MIS (n = 13) | ISM/SSM (n = 94) | AdvSM (n = 35) | All patients (n = 142) |
| Age (y; median, range) | 45 (20-70) | 47 (20-75) | 63 (25-91) | 50 (20-91) |
| Sex (number; female/male) | 9/4 | 54/40 | 11/24 | 74/68 |
| KIT D816V mutation (number and % positive) | 4/9 (44.4%)‡ | 83/87 (95.4%) | 25/34 (73.5%) | 112/130 (86.2%)‡ |
| KIT D816V allele burden (%; median, range) | 0.27 (0.004-0.54) | 0.15 (0.002-46.6) | 2.05 (0.006-46.7) | 0.255 (0.002-46.7) |
| TNF† (pg/mL; median, range) | 0.67 (0.19-5.41) | 0.81 (0.13-15.65) | 1.3 (0.33-18.89) | 0.86 (0.13-18.89) |
| Discovery cohort . | CM/MIS (n = 5) . | ISM/SSM (n = 24) . | AdvSM (n = 11) . | All patients (n = 40) . |
|---|---|---|---|---|
| Age (y; median, range) | 32 (23-55) | 53 (23-82) | 64 (50-74) | 54 (23-82) |
| Sex (number; female/male) | 3/2 | 14/10 | 5/6 | 22/18 |
| KIT D816V mutation (number and % positive) | 4/5 (80.0%) | 21/22 (95.5%) | 9/11 (81.8%)∗ | 34/38 (89.5%)∗ |
| KIT D816V allele burden (%; median, range) | 0.06 (0.034-0.14) | 0.14 (0.009-9.09) | 15.17 (0.002-46.9) | 0.14 (0.002-46.9) |
| TNF† (pg/mL; median, range) | 1.54 (1.05-2.57) | 2.13 (0.48-11.73) | 2.96 (1.09-16.64) | 2.06 (0.48-16.64) |
| Validation cohort | CM/MIS (n = 13) | ISM/SSM (n = 94) | AdvSM (n = 35) | All patients (n = 142) |
| Age (y; median, range) | 45 (20-70) | 47 (20-75) | 63 (25-91) | 50 (20-91) |
| Sex (number; female/male) | 9/4 | 54/40 | 11/24 | 74/68 |
| KIT D816V mutation (number and % positive) | 4/9 (44.4%)‡ | 83/87 (95.4%) | 25/34 (73.5%) | 112/130 (86.2%)‡ |
| KIT D816V allele burden (%; median, range) | 0.27 (0.004-0.54) | 0.15 (0.002-46.6) | 2.05 (0.006-46.7) | 0.255 (0.002-46.7) |
| TNF† (pg/mL; median, range) | 0.67 (0.19-5.41) | 0.81 (0.13-15.65) | 1.3 (0.33-18.89) | 0.86 (0.13-18.89) |
CM, cutaneous mastocytosis; MIS, mastocytosis in the skin; SSM, smoldering SM.
One additional patient tested positive for KIT D816H.
Different generations of the TNF ELISA had to be applied for the discovery and the validation cohort, showing slightly lower absolute overall levels in the validation cohort but difference well comparable relative to the respective matched healthy controls.
One additional patient was tested positive for KIT V560A.
Reagents, cells, and cell culture
Reagents, including cell culture media, cytokines, and inhibitors, are described in supplemental Material, available on the Blood website. Mo7e cells and the mast cell lines HMC-1 (KIT G560V+ HMC-1.1 clone and KIT G560V+ as well as KIT D816V+ HMC-1.2 clone)35 and ROSA (ROSAKITWT and ROSAKITD816V)36 were used for in vitro and/or in vivo experiments. Cell lines with constitutive expression of KIT D816V were generated as described in the supplemental Material.
Knockdown experiments and CRISPR/Cas9 gene knockout experiments
For doxycycline-inducible gene knockdown of KIT, baculoviral IAP repeat containing 5 (BIRC5), nuclear factor κB subunit 1 (NF-kB1), and MAP2K1 or MEK1, hairpins in a microRNA-E (miR-E) backbone, were cloned into the pRRL-based LT3 GEPIR vector, as described.37 Guide sequences of the hairpins are provided in supplemental Table 1. Production of recombinant lentiviruses and transduction of target cells were performed as reported.38 Cells were selected with puromycin (1 μg/mL), and knockdown was induced by doxycycline (1 μg/mL) and confirmed by immunoblotting (supplemental Figure 1A-B,E). For TNF knockout, the target guide sequence (supplemental Table 1) was cloned into the lentiCRISPRv2 backbone. As control, a nontargeting sequence was used. Lentiviruses were produced as described.38 After transduction, ROSAKITD816V cells were selected with puromycin (1 μg/mL) and single cells diluted to generate a clonal population. Colonies were expanded, and supernatants were harvested. TNF secretion was evaluated by enzyme-linked immunosorbent assay (ELISA) to select knockout clones (supplemental Figure 1D).
Real-time PCR, immunoblotting, flow cytometry, and immunohistochemistry
TNF messenger RNA (mRNA) expression was measured by quantitative real-time PCR. NF-κB1, MAP2K1 (MEK1), BIRC5 (survivin), and Bcl2-like protein 11 (BCL2L11 alias BIM) expression were assessed by immunoblotting. TNFR1 and TNFR2 expression on MCs was measured by flow cytometry. Cell viability/apoptosis was assessed by annexin V/DAPI (4′,6-diamidino-2-phenylindole) or active caspase-3 staining. For assessment of apoptosis-related proteins, ROSAKIT WT and ROSAKIT D816V cells were incubated with 100 ng/mL recombinant human TNF and analyzed for expression of apoptosis-related proteins by the proteome profiler human apoptosis array kit (R&D Systems, Minneapolis, MN). Immunohistochemical staining was performed on human BM sections. Details are described in supplemental Material, primer sequences are provided in supplemental Table 1, and antibodies are specified in supplemental Table 2.
ELISA
An ELISA (R&D Systems) with a lower limit of detection (LLOD) of 15.6 pg/mL was used to measure the levels of TNF protein in cell culture supernatants. Patients’ sera were analyzed by a highly sensitive TNF ELISA (HSTA00D, R&D Systems), exhibiting an LLOD of 0.5 pg/mL and a concentration-dependent interassay coefficient of variation of 7.2%-10.4%. The follow-up product HSTA00E (R&D Systems) with an LLOD of 0.156 pg/mL and a coefficient of variation of 6.2%-6.7% was used for the validation cohort.
Proliferation and clonogenic assay
3H-thymidine incorporation assays were performed using human BM mononuclear cells, ROSAKIT WT, and ROSAKIT D816V cells as well as ROSAKIT D816V cells with BIRC5 knockdown treated with recombinant TNF. A detailed description is provided in supplemental Material.
Human BM mononuclear cells were plated in duplicates or triplicates at a density of 1-3 × 104 cells per mL in a medium with 0.8% methylcellulose (Merck, Darmstadt, Germany), cytokines and supplements (see supplemental Material for details), and different concentrations of recombinant human TNF (0, 1, 10, and 100 ng/mL). The characteristics of patients with SM is provided in supplemental Table 3. Plates were incubated at 37°C and 5% CO2 for 14 days. Granulocyte-monocyte colony-forming unit (CFU-GM)–derived colonies (aggregates of more than 40 cells) were counted under an inverted microscope.
Animal experiments
NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice were injected with ROSAKIT D816V cells with or without TNF knockout. Survival, BM infiltration, and myelopoiesis were evaluated as described in supplemental Material.
Statistical analysis
Data were analyzed using GraphPad Prism software (GraphPad Software, La Jolla, CA) and R (R Foundation, Vienna, Austria). A detailed description can be found in supplemental Material. Significance was defined by a P value <.05.
Results
Neoplastic MC express TNF in a KIT D816V-dependent manner
When examining 40 patients with mastocytosis (Table 1), we realized that TNF serum levels were significantly higher in patients with mastocytosis (median, 2.06 pg/mL; interquartile range [IQR], 1.75 pg/mL) than in age- and sex-matched controls (median, 1.46 pg/mL; IQR, 0.86 pg/mL; P = .0006; Figure 1A). In line, we found that the KIT D816V+ MC lines HMC-1.2 and ROSAKIT D816V expressed and secreted substantial amounts of TNF but not their KIT D816V– counterparts (Figure 1B-C). These data led us to speculate that TNF expression depends on KIT D816V in neoplastic MCs. The KIT-targeting TKI midostaurin and avapritinib reduced TNF expression and secretion in HMC-1.2 cells in a dose-dependent manner (Figure 1D-E; supplemental Figure 2A-B). Furthermore, RNA interference (RNAi)-mediated knockdown of KIT reduced expression and secretion of TNF (Figure 1F; supplemental Figure 2C). In line, expression of KIT D816V significantly increased TNF expression and secretion in Mo7e cells compared with parental and wild-type KIT-overexpressing cells (Figure 1G-H). In summary, we concluded that KIT D816V-dependent oncogenic pathways trigger the expression and secretion of TNF in neoplastic MCs.
KIT D816V induces TNF expression. (A) TNF serum concentrations of patients with mastocytosis (n = 40) and of an age- and sex-matched control cohort were measured by ELISA. Results represent median and IQR. (B-C) TNF expression and secretion levels were measured in the human MC lines HMC-1.1 (KIT D816V–), HMC-1.2 (KIT D816V+), ROSAKIT WT, and ROSAKIT D816V. ROSA cells were cultivated in medium containing 3 ng/mL SCF. Cells were seeded at a density of 0.5 × 106/mL and incubated for 5 hours. TNF mRNA expression of the respective cells was analyzed by quantitative reverse transcription PCR (qRT-PCR) (B), TNF secretion into the supernatants was analyzed by ELISA after 24 hours of cultivation at a density of 1 × 106/mL (C). (D-E) TNF expression levels in HMC-1.2 cells were determined after treatment with different concentrations of the KIT D816V-targeting drugs midostaurin (D) and avapritinib (E) for 5 hours. (F) Expression of TNF was measured in HMC-1.2 cells with doxycycline-inducible RNAi against KIT or a nontargeting control (NTC) after treatment with or without doxycycline (1 μg/mL) for 48 hours. (G-H) Mo7e cells, lentivirally transduced with an empty vector (CO), pWPI KIT WT, or pWPI KIT D816V, were seeded at a density of 1 × 106 cells per mL and cultivated in presence or absence of granulocyte-macrophage colony-stimulating factor (GM-CSF; 2 ng/mL) for 24 hours. TNF mRNA expression levels in the cells were measured by qRT-PCR (G), and TNF secretion into the supernatants was analyzed by ELISA (H). Results represent the mean and standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
KIT D816V induces TNF expression. (A) TNF serum concentrations of patients with mastocytosis (n = 40) and of an age- and sex-matched control cohort were measured by ELISA. Results represent median and IQR. (B-C) TNF expression and secretion levels were measured in the human MC lines HMC-1.1 (KIT D816V–), HMC-1.2 (KIT D816V+), ROSAKIT WT, and ROSAKIT D816V. ROSA cells were cultivated in medium containing 3 ng/mL SCF. Cells were seeded at a density of 0.5 × 106/mL and incubated for 5 hours. TNF mRNA expression of the respective cells was analyzed by quantitative reverse transcription PCR (qRT-PCR) (B), TNF secretion into the supernatants was analyzed by ELISA after 24 hours of cultivation at a density of 1 × 106/mL (C). (D-E) TNF expression levels in HMC-1.2 cells were determined after treatment with different concentrations of the KIT D816V-targeting drugs midostaurin (D) and avapritinib (E) for 5 hours. (F) Expression of TNF was measured in HMC-1.2 cells with doxycycline-inducible RNAi against KIT or a nontargeting control (NTC) after treatment with or without doxycycline (1 μg/mL) for 48 hours. (G-H) Mo7e cells, lentivirally transduced with an empty vector (CO), pWPI KIT WT, or pWPI KIT D816V, were seeded at a density of 1 × 106 cells per mL and cultivated in presence or absence of granulocyte-macrophage colony-stimulating factor (GM-CSF; 2 ng/mL) for 24 hours. TNF mRNA expression levels in the cells were measured by qRT-PCR (G), and TNF secretion into the supernatants was analyzed by ELISA (H). Results represent the mean and standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
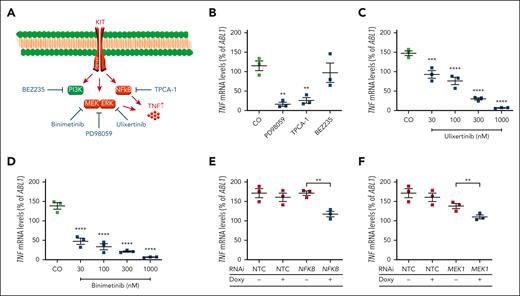
TNF expression in neoplastic MCs depends on MAPK and NF-κB signaling
To study the impact of KIT D816V-dependent downstream signaling on TNF expression, we applied pharmacologic inhibitors of the MAPK (PD98059), NF-κB (TPCA-1) and PI3K/mTOR pathways (BEZ235) (Figure 2A). Although BEZ235 failed to affect TNF expression, TNF expression was significantly reduced by adding TPCA-1 or PD98059, implying a role of MAPK and NF-κB signaling (Figure 2B). This was confirmed by applying the MAPK inhibitors ulixertinib (targeting MAPK3/1 alias extracellular signal-regulated kinase 1/2) and binimetinib (targeting MAP2K1/2 alias MEK1/2). MEK and extracellular signal-regulated kinase inhibition reduced TNF expression in a dose-dependent manner in KIT D816V+ neoplastic MCs (Figure 2C-D). Further validation of the role of MAPK and NF-κB signaling was obtained by RNAi. Knockdown of NF-κB1 and MEK1 reduced TNF expression in KIT D816V+ neoplastic MCs (Figure 2E-F). RNA expression data were confirmed when we studied TNF protein secretion by ELISA (supplemental Figure 2D-H).
Role of NF-κB1 and MEK1 in KIT D816V-dependent expression of TNF. (A) Signaling pathways downstream of KIT D816V and applied pharmacological inhibitors. (B) Effects of different pharmacological inhibitors on TNF expression. HMC-1.2 cells were treated with PD98059 (50 μM), BEZ235 (1 μM), or TPCA-1 (20 μM) for 24 hours and TNF mRNA expression levels were measured by qRT-PCR. (C-D) HMC-1.2 cells were treated with ulixertinib (30-1000 nM) (C) or binimetinib (30-1000 nM) (D) for 5 hours and TNF expression was determined as described above. (E-F) HMC-1.2 cells were transduced with doxycycline-inducible RNAi against NF-kB1 (E), MAP2K1/MEK1 (F), or a nontargeting control (NTC). After induction with doxycycline (1 μg/mL) for 48 hours, TNF mRNA expression levels were measured by qRT-PCR. Results represent the mean and standard error of the mean of 3 independent experiments. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Role of NF-κB1 and MEK1 in KIT D816V-dependent expression of TNF. (A) Signaling pathways downstream of KIT D816V and applied pharmacological inhibitors. (B) Effects of different pharmacological inhibitors on TNF expression. HMC-1.2 cells were treated with PD98059 (50 μM), BEZ235 (1 μM), or TPCA-1 (20 μM) for 24 hours and TNF mRNA expression levels were measured by qRT-PCR. (C-D) HMC-1.2 cells were treated with ulixertinib (30-1000 nM) (C) or binimetinib (30-1000 nM) (D) for 5 hours and TNF expression was determined as described above. (E-F) HMC-1.2 cells were transduced with doxycycline-inducible RNAi against NF-kB1 (E), MAP2K1/MEK1 (F), or a nontargeting control (NTC). After induction with doxycycline (1 μg/mL) for 48 hours, TNF mRNA expression levels were measured by qRT-PCR. Results represent the mean and standard error of the mean of 3 independent experiments. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
TNF promotes expansion of neoplastic MCs via suppression of normal myeloid cells
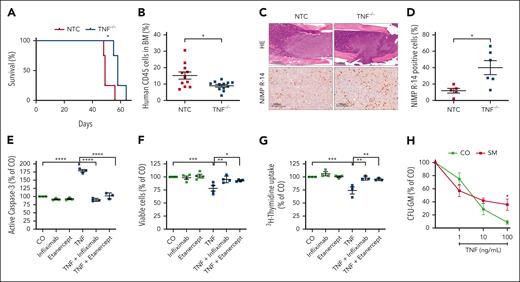
To investigate the role of TNF in neoplastic MCs, CRISPR/Cas9 was used to generate TNF–/– ROSAKIT D816V cells. In these cells, TNF secretion was not detectable, but in vitro growth was not affected (supplemental Figure 1C-D). To test these cells in vivo, we performed xenotransplantation experiments using NSG mice. TNF knockout in ROSAKIT D816V cells significantly prolonged the survival of mice (median, 58 vs 49 days; P < .05; Figure 3A). In an independent experiment, the mice were euthanized after 5 weeks, and a significant reduction of CD45+ human cells was observed in the BM of mice after TNF knockout compared with those of controls (Figure 3B). Immunohistochemical studies on tissue sections indicated no decrease in MC infiltration in the liver, lung, or spleen (supplemental Figure 3). These observations were paralleled by a significantly increased murine myelopoiesis in the BM of mice injected with ROSAKIT D816V cells lacking TNF secretion compared with those injected with ROSAKIT D816V cells producing TNF (Figure 3C-D). These data led us to speculate that TNF suppresses myelopoiesis. In vitro experiments confirmed the effect of TNF on murine and human BM cells (Figure 3E-G). Furthermore, TNF-induced apoptosis and reduced proliferation were counteracted by the TNF-binding antibody infliximab as well as the soluble TNF receptor fusion protein etanercept (Figure 3E-G). TNF was also found to suppress CFU-GM colony formation in human BM cells. This effect was mitigated when we studied TNF in the CFU-GM formation of BM cells derived from patients with KIT D816V+ AdvSM (Figure 3H; supplemental Table 3). Collectively, these data suggest that KIT D816V+ neoplastic MCs generate a TNF-rich BM microenvironment to suppress normal myelopoiesis and thereby contribute to the clonal expansion of neoplastic cells.
Knockout of TNF leads to improved survival, reduced BM infiltration, and increased myelopoiesis in mice. (A) Kaplan-Meier survival plot of NSG mice injected with ROSAKIT D816V cells with CRISPR/Cas9-mediated TNF knockout (blue) compared with a nontargeting control (NTC; red) with retained TNF secretion. (B-D) Workup of NSG mice that were injected with ROSAKIT D816V cells with or without TNF knockout and sacrificed after 5 weeks. Infiltration of ROSAKIT D816V cells was assessed by flow cytometric analysis of flushed BM cells for human CD45 expression (B). BM formalin-fixed paraffin-embedded sections (femur) were stained with hematoxylin and eosin (HE) and antibodies against NIMP R-14 (Ly-6G/-6C) (staining murine myelopoiesis). Representative sections are shown in panel C and the fraction of NIMP R-14–positive cells in the BM in panel D. (E-F) Murine BM cells were preincubated with etanercept or infliximab (10 μg/mL) or remained untreated for 4 hours. Human recombinant TNF (100 ng/mL) was then added and active caspase-3–positive cells were measured after 72 hours by flow cytometry (E). Complementary viable cells were evaluated after 24 hours of incubation using annexin V and 4′,6-diamidino-2-phenylindole staining (F). (G) Human BM mononuclear cells were seeded at a density of 100 000 cells per well and treated with human recombinant TNF (100 ng/mL) and/or etanercept and infliximab, and the proliferation rate was evaluated by 3H-thymidine incorporation assay. (H) CFU-GM formation of BM mononuclear cells obtained from patients with KIT D816V+ SM compared with those from normal controls (n = 3). Results represent the mean and standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Knockout of TNF leads to improved survival, reduced BM infiltration, and increased myelopoiesis in mice. (A) Kaplan-Meier survival plot of NSG mice injected with ROSAKIT D816V cells with CRISPR/Cas9-mediated TNF knockout (blue) compared with a nontargeting control (NTC; red) with retained TNF secretion. (B-D) Workup of NSG mice that were injected with ROSAKIT D816V cells with or without TNF knockout and sacrificed after 5 weeks. Infiltration of ROSAKIT D816V cells was assessed by flow cytometric analysis of flushed BM cells for human CD45 expression (B). BM formalin-fixed paraffin-embedded sections (femur) were stained with hematoxylin and eosin (HE) and antibodies against NIMP R-14 (Ly-6G/-6C) (staining murine myelopoiesis). Representative sections are shown in panel C and the fraction of NIMP R-14–positive cells in the BM in panel D. (E-F) Murine BM cells were preincubated with etanercept or infliximab (10 μg/mL) or remained untreated for 4 hours. Human recombinant TNF (100 ng/mL) was then added and active caspase-3–positive cells were measured after 72 hours by flow cytometry (E). Complementary viable cells were evaluated after 24 hours of incubation using annexin V and 4′,6-diamidino-2-phenylindole staining (F). (G) Human BM mononuclear cells were seeded at a density of 100 000 cells per well and treated with human recombinant TNF (100 ng/mL) and/or etanercept and infliximab, and the proliferation rate was evaluated by 3H-thymidine incorporation assay. (H) CFU-GM formation of BM mononuclear cells obtained from patients with KIT D816V+ SM compared with those from normal controls (n = 3). Results represent the mean and standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
BIRC5 (survivin) mediates resistance of KIT D816V+ MCs to TNF
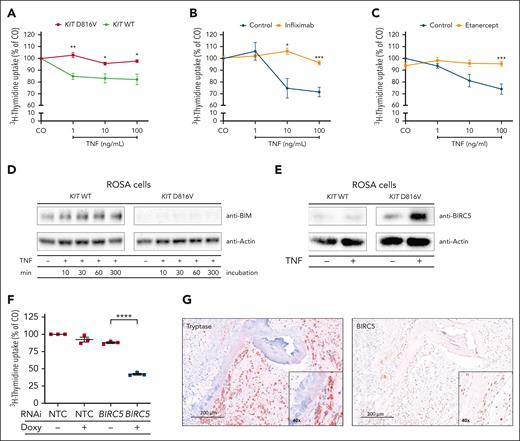
The data so far led us to speculate that KIT D816V-transformed cells are resistant to the growth-inhibitory effects of TNF. Thus, we incubated ROSAKIT WT and ROSAKIT D816V cells with TNF and observed a growth-inhibitory effect of TNF on ROSAKIT WT cells but not on ROSAKIT D816V cells (Figure 4A; supplemental Figure 6A). The effect of TNF was counteracted by the TNF blockers infliximab and etanercept (Figure 4B-C). No substantial differences in surface expression of TNFR1 and TNFR2 in MCs were found as a potential explanation (supplemental Figure 4). Our group has previously identified BCL2L11 alias BIM as a tumor suppressor in mastocytosis.39 TNF led to dose-dependent upregulation of BCL2L11 in ROSAKIT WT but not in ROSAKIT D816V cells, indicating a deregulation of central apoptosis pathways by KIT D816V (Figure 4D). In a screening approach, we found a differential expression of upstream regulatory proteins of the apoptosis cascade between KIT D816V-positive and -negative cells upon TNF treatment. The most pronounced difference was observed for the inhibitor of apoptosis BIRC5 alias survivin (supplemental Figure 5). In ROSAKIT D816V cells, BIRC5 expression was high at baseline and further increased upon TNF treatment, whereas ROSAKIT WT cells expressed lower basal levels that did not increase upon TNF treatment (Figure 4E). Further control experiments showed a moderate increase of BIRC5 after stem cell factor stimulation (supplemental Figure 6B-E) and an inhibitory effect of the TNF blockers infliximab and etanercept (supplemental Figure 6F). To assess the functional role of BIRC5 in KIT D816V-mediated TNF resistance, we used RNAi. In the presence of TNF, BIRC5 knockdown in ROSAKIT D816V cells inhibited cell growth and restored the susceptibility of KIT D816V+ neoplastic MCs to TNF treatment (Figure 4F). To substantiate the impact of BIRC5 in patients with mastocytosis, we analyzed the expression in BM sections of patients with SM by immunohistochemistry. Neoplastic MCs in the BM of patients with SM stained positive for BIRC5 with a particular nuclear expression pattern in MC aggregates (Figure 4G; supplemental Figure 7). The level of BIRC5 expression varied between patients but showed no clear difference between ISM and AdvSM (supplemental Table 4). In summary, we identified BIRC5 as a mediator of resistance to TNF in KIT D816V+ neoplastic MCs.
Resistance of KIT D816V+ MCs to TNF. (A-C) ROSAKIT WT (green) and ROSAKIT D816V (red) cells were treated with different TNF concentrations and cell growth was evaluated by 3H-thymidine incorporation assay. ROSAKITWT cells were additionally incubated with infliximab (10 μg/mL) (B) or etanercept (10 μg/mL) (C). (D) ROSAKIT WT and ROSAKIT D816V cells were incubated with recombinant TNF (100 ng/mL) for up to 300 minutes as indicated. Whole-cell protein extracts were analyzed for BIM expression by immunoblotting. (E) ROSAKIT WT and ROSAKIT D816V cells were incubated with or without recombinant TNF (100 ng/mL) for 6 hours. Whole-cell protein extracts were analyzed for BIRC5 (survivin) protein expression by immunoblotting. Actin served as loading control for immunoblotting. A representative blot is shown in panels D and E. (F) ROSAKIT D816V cells were transduced with doxycycline-inducible RNAi targeting BIRC5 or an NTC. After TNF treatment (100 ng/mL) of doxycycline induced and noninduced cells, cell growth was assessed by 3H-thymidine incorporation assay. Results in panels A-C and F represent mean and standard error of the mean of 3 independent experiments. (G) Serial sections of paraffin-embedded BM from a patient with SM were immunohistochemically stained with an anti-tryptase antibody (left) or an anti-BIRC5 antibody (right). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Resistance of KIT D816V+ MCs to TNF. (A-C) ROSAKIT WT (green) and ROSAKIT D816V (red) cells were treated with different TNF concentrations and cell growth was evaluated by 3H-thymidine incorporation assay. ROSAKITWT cells were additionally incubated with infliximab (10 μg/mL) (B) or etanercept (10 μg/mL) (C). (D) ROSAKIT WT and ROSAKIT D816V cells were incubated with recombinant TNF (100 ng/mL) for up to 300 minutes as indicated. Whole-cell protein extracts were analyzed for BIM expression by immunoblotting. (E) ROSAKIT WT and ROSAKIT D816V cells were incubated with or without recombinant TNF (100 ng/mL) for 6 hours. Whole-cell protein extracts were analyzed for BIRC5 (survivin) protein expression by immunoblotting. Actin served as loading control for immunoblotting. A representative blot is shown in panels D and E. (F) ROSAKIT D816V cells were transduced with doxycycline-inducible RNAi targeting BIRC5 or an NTC. After TNF treatment (100 ng/mL) of doxycycline induced and noninduced cells, cell growth was assessed by 3H-thymidine incorporation assay. Results in panels A-C and F represent mean and standard error of the mean of 3 independent experiments. (G) Serial sections of paraffin-embedded BM from a patient with SM were immunohistochemically stained with an anti-tryptase antibody (left) or an anti-BIRC5 antibody (right). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
High TNF levels are associated with inferior survival in SM
The clinical relevance of TNF serum levels was first evaluated in the initial discovery cohort of patients with mastocytosis (n = 40; Table 1). Although the TNF levels in patients with cutaneous mastocytosis or mastocytosis in the skin40 (median, 1.54 pg/mL) did not differ from the age- and sex-matched control group (median 1.46 pg/mL), significantly higher TNF serum concentrations were observed both in ISM (n = 24; median 2.13; P = .01) and AdvSM (n = 11; median 2.96; P = .006; Figure 5A). For survival analyses, we established a cutoff point for TNF serum levels calculated using the bootstrapped cutpointr function. Patients with mastocytosis with high TNF concentrations (n = 14) had a significantly shorter median overall survival (OS) of 2.3 years, whereas the median OS was not reached in patients with low TNF serum levels (n = 26; P < .001; Figure 5B). A Cox proportional hazard model including age, sex, time from diagnosis, advanced vs nonadvanced disease, alkaline phosphatase, serum tryptase,41 and SRSF2/ASXL1/RUNX1 mutation status indicated TNF as a significant factor for the 5-year survival of patients with mastocytosis in univariable and multivariable analysis (coefficient 5.5; 95% confidence interval, 1.6-19.6; P = .008; Table 2).
TNF is a prognostic marker in mastocytosis. (A) Comparison of TNF serum levels assessed by ELISA between subgroups of mastocytosis (cutaneous mastocytosis [CM], mastocytosis in the skin [MIS], indolent SM [ISM], and AdvSM). Results represent median and IQR. (B-D) Kaplan-Meier survival plots of the discovery cohort (B) and the validation cohort (C-D). Patients were stratified according to TNF serum level (cutoff values: 2.96 pg/mL in the discovery cohort and 1.235 pg/mL in the validation cohort based on different generations of the TNF ELISA) and analyzed for OS (B-C) and PFS (D). Differences in the probability of survival after specimen taking were analyzed. ∗∗P < .01.
TNF is a prognostic marker in mastocytosis. (A) Comparison of TNF serum levels assessed by ELISA between subgroups of mastocytosis (cutaneous mastocytosis [CM], mastocytosis in the skin [MIS], indolent SM [ISM], and AdvSM). Results represent median and IQR. (B-D) Kaplan-Meier survival plots of the discovery cohort (B) and the validation cohort (C-D). Patients were stratified according to TNF serum level (cutoff values: 2.96 pg/mL in the discovery cohort and 1.235 pg/mL in the validation cohort based on different generations of the TNF ELISA) and analyzed for OS (B-C) and PFS (D). Differences in the probability of survival after specimen taking were analyzed. ∗∗P < .01.
Five-year survival model
| . | Survived∗ >5 y . | Died ≤5 y . | Univariable (P value)† . | Multivariable (P value)† . |
|---|---|---|---|---|
| Discovery cohort (n = 38)‡ | ||||
| Age (y) | 53.6 ± 15.2 | 62.1 ± 10.9 | .150 | .422 |
| Time from diagnosis (y)§ | 3.4 ± 4.6 | 5.1 ± 5.2 | .380 | .990 |
| Sex (female/male)|| | 17/10 | 3/8 | .110 | .949 |
| Advanced SM (yes/no)|| | 5/22 | 6/5 | .029 | .078 |
| TNF (high/low)|| | 4/23 | 9/2 | <.001 | .008 |
| AP (high/low)|| | 5/22 | 6/5 | .009 | .186 |
| Serum tryptase (high/low)|| | 3/4 | 6/5 | .010 | .359 |
| S/A/R mutation (yes/no)||,¶ | 1/26 | 1/10 | .690 | .227 |
| Validation cohort (n = 132)# | ||||
| Age (y) | 49.0 ± 14.3 | 65.2 ± 11.6 | <.001 | .097 |
| Time from diagnosis (y)¶ | 3.2 ± 4.1 | 2.4 ± 5.1 | .37 | .017 |
| Sex (female/male)|| | 64/44 | 6/18 | .003 | .329 |
| Advanced SM (yes/no)|| | 14/94 | 20/4 | <.001 | <.001 |
| TNF (high/low)|| | 9/99 | 19/5 | <.001 | .028 |
| AP (high/low)|| | 25/83 | 16/8 | <.001 | .108 |
| Serum tryptase (high/low)|| | 12/96 | 11/13 | <.001 | .832 |
| S/A/R mutation (yes/no)||,¶ | 2/106 | 16/8 | <.001 | .047 |
| . | Survived∗ >5 y . | Died ≤5 y . | Univariable (P value)† . | Multivariable (P value)† . |
|---|---|---|---|---|
| Discovery cohort (n = 38)‡ | ||||
| Age (y) | 53.6 ± 15.2 | 62.1 ± 10.9 | .150 | .422 |
| Time from diagnosis (y)§ | 3.4 ± 4.6 | 5.1 ± 5.2 | .380 | .990 |
| Sex (female/male)|| | 17/10 | 3/8 | .110 | .949 |
| Advanced SM (yes/no)|| | 5/22 | 6/5 | .029 | .078 |
| TNF (high/low)|| | 4/23 | 9/2 | <.001 | .008 |
| AP (high/low)|| | 5/22 | 6/5 | .009 | .186 |
| Serum tryptase (high/low)|| | 3/4 | 6/5 | .010 | .359 |
| S/A/R mutation (yes/no)||,¶ | 1/26 | 1/10 | .690 | .227 |
| Validation cohort (n = 132)# | ||||
| Age (y) | 49.0 ± 14.3 | 65.2 ± 11.6 | <.001 | .097 |
| Time from diagnosis (y)¶ | 3.2 ± 4.1 | 2.4 ± 5.1 | .37 | .017 |
| Sex (female/male)|| | 64/44 | 6/18 | .003 | .329 |
| Advanced SM (yes/no)|| | 14/94 | 20/4 | <.001 | <.001 |
| TNF (high/low)|| | 9/99 | 19/5 | <.001 | .028 |
| AP (high/low)|| | 25/83 | 16/8 | <.001 | .108 |
| Serum tryptase (high/low)|| | 12/96 | 11/13 | <.001 | .832 |
| S/A/R mutation (yes/no)||,¶ | 2/106 | 16/8 | <.001 | .047 |
AP, alkaline phosphatase.
Or censored.
Univariable or multivariable Cox regression.
Exclusion of 2 patients with incomplete data.
Time between diagnosis and sample collection.
Binary variables (cutoff values: TNF 2.96 pg/mL in the discovery cohort and 1.235 pg/mL in the validation cohort based on different generations of the TNF ELISA applied; alkaline phosphatase 100 U/L; and serum tryptase 125 ng/mL).
SRSF2, ASXL1, and RUNX1 mutation status.
Exclusion of 1 statistical outlier and 9 patients with incomplete data.
In the next step, we used an independent validation cohort of 142 patients with mastocytosis (Table 1) and respective controls. Patients with mastocytosis again showed significantly higher TNF levels than the controls (supplemental Figure 8A). Importantly, the association of high TNF levels with inferior OS was confirmed in a Cox proportional hazard model (Table 2). Patients with mastocytosis with high TNF concentrations (n = 28) had a significantly shorter median OS of 2.3 years and median progression-free survival (PFS) of 1.3 years, whereas the median OS and PFS was not reached in patients with low TNF serum levels (n = 104; P < .001; Figure 5C-D). The reason for death was disease related in 75% of cases (supplemental Table 5). TNF serum levels showed no correlation with BM MC burden and a weak correlation with KIT D816V allele burden and serum tryptase level (supplemental Figure 8B-D). Finally, we restricted the analysis to the subcohort of patients with smoldering SM or AdvSM. Also in this subcohort with a higher risk of progression and death, patients with SM with high TNF concentrations (n = 18) had a significantly shorter median OS of 1.5 years, whereas the median OS was not reached in patients with TNF low serum levels (n = 18; P = .002; supplemental Figure 8E). When we analyzed the relatively low-risk patients with ISM and cutaneous mastocytosis/mastocytosis in the skin, high levels of TNF were still prognostic, although only a few events were observed (supplemental Figure 8F). In summary, high TNF levels were associated with inferior survival in SM.
Discussion
Inflammatory cytokines and their effects on the tumor microenvironment are recognized as important factors in the pathophysiology of myeloid neoplasm. In fact, these cytokines have been implicated in the evolution and progression of the disease as well as in various symptoms in patients. We and others have studied cytokines in the context of SM and found that the production of several proinflammatory cytokines and chemokines is triggered by KIT D816V. Our data on TNF presented here add another layer of complexity to the mechanisms underlying tumor-associated inflammation and cytokine secretion. We here show that TNF is expressed in neoplastic MCs in SM in a KIT D816V-dependent manner. Moreover, we found that TNF preferentially blocks the growth of normal BM cells but spares KIT D816V-transformed cells, which provides a growth advantage to neoplastic progenitor cells. Finally, we have identified BIRC5 (survivin) as a mechanism of protection of KIT D816V-transformed cells against TNF effects.
Normal MCs are also known to express and secrete TNF.21,42-44 However, the amounts of TNF produced by normal MCs are low, and we found that KIT D816V+ neoplastic MCs produce and secrete higher levels of TNF than KIT D816V– MCs. Expression of TNF in HMC-1 cells has been described before.45 We combined engineered expression of KIT D816V, TKI treatment, and RNAi to confirm the dependence of TNF expression and secretion on the KIT mutant in various MC models. Perez-Pons et al recently showed that monocytes also contribute to increased serum levels in patients with SM.46 This concept is in line with the importance of TNF in SM shown here. On the one hand, KIT D816V likely also induces production of TNF in the AHN compartment of AdvSM; on the other hand, in vivo activation and functional impairment of circulating blood monocytes could also be a consequence of the underlying MC activation in SM.46 Very recently, Soderlund et al published single cell transcriptomics from 3 patients with ISM.47 When we analyzed these data, expression of TNF was found both in MCs and in monocytes (supplemental Figure 9). Teodosio et al reported no alteration of TNF expression in Affymetrix gene expression array data of enriched BM MCs.48 In summary, although KIT D816V+ MCs are a relevant source of TNF, paracrine effects in the tumor microenvironment also contribute to the increased TNF level observed in patients with SM.
Fleischman et al showed that JAK2 V617F+ neoplastic cells in myeloproliferative neoplasms secrete high levels of TNF but are resistant to TNF. Thus, clonal expansion of mutant cells is promoted within a TNF-rich environment.31 Furthermore, SanMiguel et al observed elevated TNF levels in mice who underwent transplantation with HSCs harboring a clonal hematopoiesis–associated DNMT3A mutation and indicated a selective advantage of mutated HSCs to TNF.49 Our data are consistent with these observations. Furthermore, mice injected with TNF–/– ROSAKIT D816V cells showed reduced BM infiltration and relatively normal myelopoiesis compared to mice injected with ROSAKIT D816V cells expressing TNF. The observed effect may be mitigated by the remaining murine monocyte-derived TNF that is still present in our model.46 Together, the data indicate an inhibiting effect of TNF on the development of normal myeloid cells while indirectly promoting the expansion of KIT D816V+ cells. This effect may be dependent on the specific interaction with the BM microenvironment because we observed no effect of TNF knockout on infiltration of other organs. A similar effect has been described for the expansion of TET2-mutated clonal hematopoiesis.50 Thus, the facilitation of clonal dominance of neoplastic cells via secretion of suppressive cytokines represents a common pathomechanism in chronic myeloid neoplasms. This mechanism may be of particular importance in the context of KIT D816V multilineage involvement and SM-AHN in which the KIT D816V mutation is frequently present in myeloid cells as well as in BM progenitors.51 We thus selected patients with advanced SM with a high KIT D816V mutation burden for the clonogenic assay, as KIT D816V mutations are rare in CFU-GM colonies from patients with ISM. An inflammatory TNF-rich environment generated by KIT D816V+ neoplastic MC infiltration in the BM could contribute to the clonal dominance of KIT D816V+ cells through suppression of nonaffected normal BM (progenitor) cells, thereby preparing the ground for further clonal evolution of the AHN.
When asking for potential mechanisms triggering resistance against TNF, we found that ROSAKIT D816V cells as well as primary neoplastic BM MCs express BIRC5 (survivin), which is a key regulator of cell division and apoptosis.52 Survivin is physiologically expressed by hematopoietic stem and progenitor cells as well as leukemic cells.53,54 Malcles et al showed that overexpression of BIRC5 in HeLa cells prevents apoptosis induced by TNF.55 This is in line with our observation on TNF resistance in neoplastic MCs, in which survivin was identified as a key factor in KIT D816V+ cells. Moreover, we identified BIM as another protein of potential interest in the complex interplay resulting in resistance to TNF-induced growth arrest and apoptosis, in line with the results of Aichberger et al.39 Furthermore, Hagenbuchner et al suggested that BCL2L11 expression was repressed by BIRC5.56 Hence, the deregulation of antiapoptotic and proapoptotic effector proteins likely contributes to evading the apoptotic stimulus of TNF in KIT D816V+ cells.
TNF serum levels in patients with mastocytosis were significantly increased compared to those in age- and sex-matched controls, and elevated TNF levels were associated with impaired OS. Published data on TNF serum levels in SM are inconsistent. An early study by Pardanani et al that used multiplex bead-based Luminex technology for analyzing plasma cytokines did not detect TNF.57 However, the LLOD of the assay used was 6.33 pg/mL and thus clearly above the TNF levels observed in our cohort. Öztop et al reported no differences in TNF serum levels between patients with mastocytosis and healthy controls.58 However, again, the applied assay (Diaclone SAS, Besancon, France) exhibited a sensitivity threshold of 8 pg/mL. Thus, differences in the LLOD of the applied assays most likely explain the observed differences between these studies. In line with our data, Soderlund et al reported increased TNF levels in AdvSM when applying plasma proteomics47 and Perez-Pons et al in SM using a cytometric bead array.46
Recently, a number of studies described prognostic scoring systems for patients with SM.41,59-62 The major factors contributing to these models are age; blood count abnormalities; serum biomarkers such as alkaline phosphatase, β2-microglobulin, and basal serum tryptase; and additional somatic mutations in the S/A/R genes. In addition, the KIT D816V mutation burden has been described as a prognostic factor.60,63 So far, however, cytokine levels have not been described as an independent risk factor in SM. We now found that high TNF levels were associated with inferior OS and PFS in SM, even when corrected for established prognostic variables. This and the large validation cohort are strengths of our study. A limitation of the clinical part of our study is that serum samples for determining TNF levels were not drawn at a defined time point. To address this point, we also included time from diagnosis in our Cox proportional hazard model. However, the fact that the biomarker has been analyzed during follow-up hampers the inclusion of TNF in established prognostic scoring systems.
In summary, we combined in vitro and in vivo studies with clinical data to study the role of TNF in SM. Neoplastic MCs establish an inflammatory microenvironment via KIT D816V-dependent cytokine secretion. TNF plays a pivotal role in SM by facilitating the clonal dominance of KIT D816V+ neoplastic cells. Targeting of TNF or the deregulated network of proapoptotic and antiapoptotic proteins contributing to the relative TNF resistance of KIT D816V-mutated cells could be a strategy to address clonal evolution in SM. Finally, high TNF was identified as a biomarker associated with inferior survival in SM, given the aforementioned limitation.
Acknowledgments
The authors thank Peter Quehenberger, Helmuth Haslacher, and Thomas Perkmann for contributing serum samples for the control cohort and Christoph J. Binder for providing organizational support (all, Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria). They also thank Stefan Grotha (Department of Dermatology, University of Cologne, Cologne, Germany) for performing first experiments on expression of BIRC5 in neoplastic MCs. In addition, they thank Yüksel Filik (Division of Hematology and Hemostaseology, Ludwig Boltzmann Institute for Hematology and Oncology and Department of Internal Medicine I, Medical University of Vienna, Vienna, Austria) and Michaela Prchal-Murphy (Institute of Pharmacology and Toxicology, University of Veterinary Medicine Vienna, Vienna, Austria) for technical assistance and Christoph Kornauth (Munich Leukemia Laboratory, Munich Germany) for helpful discussion on immunhistochemistry.
This study was supported by the Autism Science Foundation project P26079-B13, and SFB projects F4701-B20 and F4704-B20, Scientific Fund of the Major of Vienna.
Authorship
Contribution: N.W., E.J., M.C.G., K.F., K.G.S., and G.G. conducted in vitro laboratory experiments; J.Z. designed hairpins for knockdown experiments, and M.A. contributed ROSA cells; K.K. and V.S. performed and supervised animal experiments; S.T., P.K., L.K., and K.H. performed and analyzed immunohistochemistry; M.J., S.B., and A.R. provided patient samples; G.G., K.V.G., M. Mayerhofer, I.S., I.S.-K., H.E., C.B., W.W., M. Meggendorfer, R.S., T.H., W.R.S., P.V., and G.H. obtained and analyzed clinical data; G.G. and F.R. performed statistical analysis; G.G., N.W., P.V., and G.H. designed the study and wrote the manuscript; and all authors revised and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no conflict of interest related to this study. Outside of this study, G.G. received honoraria form Novartis, Pfizer, Roche, and Thermo Fisher Scientific. M.A. received research grants from Blueprint and honoraria from AB Science, Blueprint, Novartis, and Thermo Fisher Scientific. K.H. has received research funding from Thermo Fisher and consultancy or lecture fees from ALK-Abelló, Allergopharma, Blueprint, Cogent, Leo Pharma, Menarini, Novartis, Pfizer, Sanofi, Takeda, and Thermo Fisher Scientific. W.R.S. received honoraria from AbbVie, Novartis, Bristol Myers Squibb/Celgene, Pfizer, Teva, Stemline, and Blueprint. P.V. received honoraria from Novartis, BMS/Celgene, Pfizer, Incyte, AOP Orphan Pharmaceuticals, Blueprint, Stemline, and Cogent, and a research grant from AOP Orphan Pharmaceuticals. G.H. received honoraria form Novartis, Blueprint, Cogent Biosciences, Incyte, and Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Gregor Hoermann, Munich Leukemia Laboratory, Max-Lebsche-Platz 31, 81377 Munich, Germany; email: gregor.hoermann@mll.com.
References
Author notes
G.G. and N.W. contributed equally to this work.
Individual patient data will not be shared. Other original data are available on request from the corresponding author, Gregor Hoermann (gregor.hoermann@mll.com).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



![TNF is a prognostic marker in mastocytosis. (A) Comparison of TNF serum levels assessed by ELISA between subgroups of mastocytosis (cutaneous mastocytosis [CM], mastocytosis in the skin [MIS], indolent SM [ISM], and AdvSM). Results represent median and IQR. (B-D) Kaplan-Meier survival plots of the discovery cohort (B) and the validation cohort (C-D). Patients were stratified according to TNF serum level (cutoff values: 2.96 pg/mL in the discovery cohort and 1.235 pg/mL in the validation cohort based on different generations of the TNF ELISA) and analyzed for OS (B-C) and PFS (D). Differences in the probability of survival after specimen taking were analyzed. ∗∗P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/11/10.1182_blood.2023020515/2/m_blood_bld-2023-020515-gr5.jpeg?Expires=1769110619&Signature=yLs8ZByB5~fyrpZEiVMXUhBi3DNUPgs3ZszqkzTsIesEggqClegHfbFeMAagXLHmTkQxSzcpC7n-BaX3LUDVcdmFMJd805a3iOr95qwsXOceDXViHLWsaJaICLZxfqZD4FeuJFVs8O5RH9N5ocmZQ7KtHAQwlZfEweNS9PndzP4rwKxrGCIkpeAykvAOFT2XotODMJjj7y-eivXTGmAxJALNTYTOaXAWY1MG8PGnm-AgLyIsOkEURi~480lV3Tt8w1GxZsVJNSc2xNZUD2dY0slacjLVQzZCO8OzPvYtopzwp9U7vhlWUGTDrIKETa3VrE63JU8HYQYHqz6xJNnTEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal