In this issue of Blood, Musallam, Forni, and colleagues show that survival in transfusion dependent β-thalassemia (TDT) is measurably better in patients whose pretransfusion hemoglobin levels are maintained >10.5 g/dL, at the upper end of the currently recommended treatment range.1 In this landmark study, the 10-year survival of a group of 779 patients with TDT of median age 33.1 years (range 18.1-61 years) increased monotonically from 91% to 100% in 5 categories marked by median pretransfusion hemoglobin levels that increased from <9.0 to ≥10.5 g/dL in 0.5-g/dL increments. Of note, 88% of the thalassemia-related deaths were in groups with a median hemoglobin <10 g/dL and 70% were from cardiovascular disease. When the data were stratified by ferritin, the association with hemoglobin level groups was only significant for ferritin <1000 ng/mL, consistent with an effect of anemia on survival separate from that of iron overload.
This 9% increase in survival may not seem like a huge improvement, but it teaches us several things about long term anemia, especially considering data in non-transfusion-dependent thalassemia (NTDT),2-5 the role of ineffective erythropoiesis (IE),6 and data from acute-care settings leading physicians to withhold transfusions for hemoglobin levels over 7 or 8 g/dL.7 First, these data support a target hemoglobin level in the 9.5- to 10.5-g/dL range if not >10.5 g/dL for patients with TDT. This is a big departure from practice at many centers and emphasizes a second very important lesson: the consequences of anemia depend on the cause and duration of the anemia. It appears that a seemingly small increase in average hemoglobin from 9.0 to 10.5 g/dL in an IE-associated disorder like thalassemia5,6 integrated over 10 years improves survival. As a corollary, anemia in a high oxygen demand tissue like the heart has significant consequences.
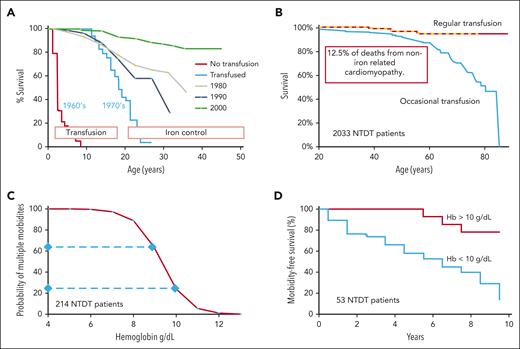
Before regular transfusions, half of patients with thalassemia major died before 10 years of age mainly from anemia-related heart failure (see figure panel A).8 Median survival increased significantly to about 18 years with implementation of regular transfusions, and then death was due to iron overload. Survival improved dramatically over 30 years with the ability to normalize iron levels (see figure panel A).5,8,9 Now, individuals with optimally managed TDT can have excellent quality of life into their seventh decade. However, at least in non-resource-limited countries, the specter of morbidity and mortality from iron overload that has been the hallmark of thalassemia greatly overshadows the consequence of anemia, which is in fact the actual main problem. With near-normal survival and iron mostly controlled, the current work puts a much finer point on the effects of small changes in hemoglobin in the 9.5- to 10.5-g/dL range and, importantly, the effects of anemia on the heart as well as on overall survival emerge again.
Relation of anemia to outcome in thalassemia. (A) Regular transfusions increased the median survival in thalassemia major from less than 10 years in the 1960s to about 18 years in the 1970s with subsequent further significant improvement due to advances in management of iron overload.8,9 (B) Survival in patients with NTDT placed on regular transfusion is much better than patients with NTDT only occasionally transfused, with 12.5% of deaths from non-iron-related cardiomyopathy.3 (C) Number of morbidities in NTDT over 10 years is directly related to hemoglobin (Hb) level with the probability of multiple morbidities about 3 times higher at 9 g/dL than at 10 g/dL.4 (D) Morbidity-free survival is significantly better if the Hb is >10 g/dL.2
Relation of anemia to outcome in thalassemia. (A) Regular transfusions increased the median survival in thalassemia major from less than 10 years in the 1960s to about 18 years in the 1970s with subsequent further significant improvement due to advances in management of iron overload.8,9 (B) Survival in patients with NTDT placed on regular transfusion is much better than patients with NTDT only occasionally transfused, with 12.5% of deaths from non-iron-related cardiomyopathy.3 (C) Number of morbidities in NTDT over 10 years is directly related to hemoglobin (Hb) level with the probability of multiple morbidities about 3 times higher at 9 g/dL than at 10 g/dL.4 (D) Morbidity-free survival is significantly better if the Hb is >10 g/dL.2
When we think of thalassemia, iron-overloaded patients with TDT come to mind first and dominate. However, patients with thalassemia intermedia who can maintain a survivable hemoglobin over 6.5 g/dL have the most common yet underappreciated type of thalassemia. These individuals with NTDT have IE-related chronic anemia resulting in significant morbidities such as pulmonary hypertension, osteoporosis, bony malformations, extramedullary hematopoiesis, severe splenomegaly, stroke, and premature death.5,6 The median survival was 46.3 years (interquartile range: 28.3-61.9) in 2033 patients with NTDT. However, 95% of the 254 patients with NTDT eventually placed on regular transfusion were still alive at 75 years compared with 62.2% in the nontransfused group. Transfusion resulted in an 80% reduction in mortality overall and an 80% reduction in mortality from cardiac disease (see figure panel B).3 The probability of having multiple anemia-related morbidities during a subsequent 10-year period in NTDT roughly tripled from 20% to 60% at a hemoglobin in the 9-g/dL range compared with 10 g/dL (see figure panel C),4 and the morbidity-free survival was significantly better if the hemoglobin level was greater than 10 g/dL (see figure panel D).2 It seems hemoglobin level matters a lot in NTDT.
Myelodysplastic syndrome (MDS) is a prototype for nonthalassemic chronic IE-related anemia. These patients initially have hypercellular marrow and IE not dissimilar from what is seen in thalassemia.6 Interestingly, the risk of nonleukemia death in MDS is roughly 3 times higher at 10 years for those with a hemoglobin <9 g/dL (male) or 8 g/dL (female) with optimal 10-year survival of >80% at a hemoglobin greater than 11 g/dL. Cardiac failure accounted for 63% of nonleukemic deaths, and cardiac deaths in general were significantly more common in patients with low hemoglobin levels.10 It seems that patients with MDS are following the thalassemia playbook with hemoglobin levels in the 10- to 11-g/dL range having better outcome and cardiac disease being associated with low hemoglobin.
So there are some very important common messages here. Hemoglobin levels >10 g/dL are associated with significantly better outcomes for chronic IE-associated anemia in all the settings discussed. At least with thalassemia, transfusing to levels >10 g/dL seems to eliminate or at least greatly reduce morbidities and improve survival. It also seems clear that cardiac disease is related to degree of anemia, and heart failure related to anemia independent of iron overload is not uncommon. This does not seem surprising as the heart has an extremely high oxygen demand, and at a hemoglobin level of 8.5 g/dL, the heart is basically running on fumes with inadequate oxygen supply. Even a drop in average hemoglobin from 10.5 to 9 g/dL matters over time to the heart. All these fancy data notwithstanding, a teenager with TDT previously kept at 8.5 g/dL and now at 11.5 g/dL after moving to our center clearly feels a significant improvement while participating on the high school tennis team. This optimal range of 9.5 to 10.5 g/dL or greater is much higher than what is recommended by transfusion medicine based on evidence from intensive care studies.7 It seems that data from the acute, relatively short-term experience in the intensive care unit7 does not apply to anemia from IE that is persistent over decades. The old recommendations for transfusion in thalassemia were adequate, but now we know >10.5 g/dL is measurably better.
Conflict-of-interest disclosure: T.D.C. declares no competing financial interest.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal