Introduction: Myeloproliferative Neoplasms (MPN) are clonal blood diseases caused by hyperactive JAK/STAT signaling and overproduction of myeloid lineages. MPN patients are at increased risk for transformation to myelofibrosis (MF) and acute myeloid leukemia (AML), both resulting in poor clinical outcomes. Importantly, targetable mechanisms to prevent progression remain elusive. We discovered that HMGA1 chromatin regulators are required for leukemogenesis and progression to MF in JAK2 V617Fmice (Li et al, Blood 2022). Emerging evidence suggests that immune escape is a fundamental pathway required for progression in diverse tumors. We therefore sought to: 1) determine whether HMGA1 drives MPN progression by dysregulating gene networks involved in immune evasion, and, 2) identify mediators of immune escape that could be modulated in therapy.
Methods: To identify pathways regulated by HMGA1 in JAK2 V617F MPN, we performed multi-omics sequencing (seq), including RNAseq, chromatin immunoprecipitation (ChIPseq), and assays of chromatin accessibility (ATACseq) in JAK2 V617Fmutant AML cell lines (DAMI, SET-2, UKE-1) + HMGA1 depletion. HMGA1 was inactivated via CRISPR or short hairpin RNA (shRNA). We used gene set enrichment analysis (GSEA) to dissect molecular mechanisms underlying HMGA1. Transcriptional networks were validated (mRNA, protein) via quantitative(q)-PCR, immunoblots, and flow cytometry. ChIP-PCR was used to assess chromatin binding of HMGA1, repressive histones, and the CCCTC-binding factor (CTCF). Human T cells were isolated from healthy donor peripheral blood mononuclear cells (PBMCs) for co-culture assays. To identify pathways relevant in patients, we assessed gene expression in MPN cohorts.
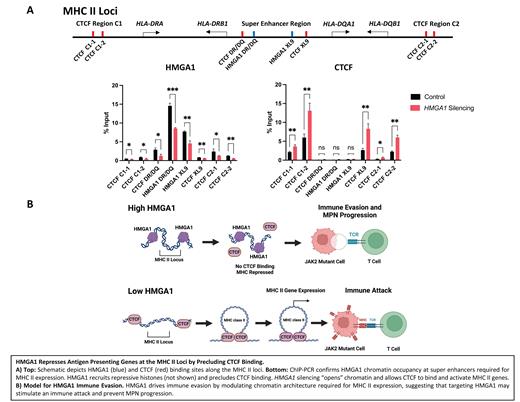
Results: To elucidate transcriptional networks governed by HMGA1 in MPN, we integrated multiomics approaches (RNA/ChIP/ATACseq) in JAK2 V617F mutant AML cells, which revealed that HMGA1 represses genes involved in interferon γ (IFNγ) signaling and antigen presentation, including genes encoding major histocompatibility complex (MHC) class I and II proteins. HMGA1 also represses the CD74 gene which encodes a chaperone protein that assembles MHC Class II at the cell surface. Silencing HMGA1 via CRISPR or shRNA results in up-regulation of CD74 and MHC genes, with greatest induction of MHC Class II genes ( HLA-DRA, DRB1, DPA1, DPB1). Similarly, HMGA1 depletion increases protein levels of MHC Class II and CD74 by flow cytometry and/or immunoblots. To determine how HMGA1 represses MHC Class II genes, we examined genome architecture modulated by HMGA1 at the MHC Class II loci. By ChIP-PCR, HMGA1 recruits repressive histone marks to promoter regions for MHC Class II genes. Strikingly, HMGA1 depletion enhances chromatin accessibility at critical enhancers (super enhancers) involved in MHC Class II gene expression which overlap with binding sites for CTCF, a chromatin regulator involved in genome organization. Further analyses revealed that HMGA1 chromatin occupancy precludes binding by CTCF, whereas HMGA1 silencing allows CTCF binding to sites within the MHC Class II loci required for MHC Class II gene expression. Because the histone deacetylase inhibitor, entinostat, induces MHC Class II genes in solid tumors, we assessed MHC Class II gene expression with entinostat and HMGA1 depletion. HMGA1 silencing enhances up-regulation of MHC Class II genes by entinostat. Finally, to test the functional significance of this pathway in immune evasion, we performed co-culture experiments with T cells and JAK2 V617F AML cells which showed that HMGA1 silencing in AML cells results in T cell activation and cytotoxic T cell killing of JAK2 V617F AML cells. To examine the relevance of this pathway in patients, we interrogated RNAseq from PBMCs and found that HMGA1 is up-regulated and associated with repression in MHC Class II genes in 30% of MPN patients after progression to AML.
Conclusions: We discovered a novel epigenetic program whereby HMGA1 drives immune evasion during MPN progression by repressing MHC Class II genes. We also define a new mechanism of gene repression by HMGA1 through chromatin compaction and repressive histone marks that preclude CTCF binding to the MHC Class II loci. Both HMGA1 depletion and entinostat up-regulate MHC Class II genes, thus unveiling HMGA1 as a therapeutic target to stimulate an immune attack and prevent MPN progression.
Disclosures
Rampal:Morphosys/Constellation: Consultancy; Servier: Consultancy; CTI BioPharma Corp: Consultancy; GSK-Sierra: Consultancy; Pharmaessentia: Consultancy; Ryvu: Research Funding; Constellation: Research Funding; Stemline: Research Funding; Zentalis: Research Funding; Kartos: Consultancy; Zentalis: Consultancy; Galecto: Consultancy; Karyopharm: Consultancy; Incyte: Consultancy; Sumitomo: Consultancy; Dainippon: Consultancy; Incyte: Research Funding; Celgene-BMS: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal