Efforts to understand chronic lymphocytic leukemia (CLL) subgroups have focused on multi-omics profiling of patient cohorts (in steady-state) and linking biomarkers to responses. Here, we aim to combine ex-vivo drug screening and gene expression profiling (GEP) in primary CLL samples to dissect disease biology based on the observed phenotype as a qualitative and quantitative function of steady-state features and drug perturbation effects.
As most differential gene expression changes in CLL are retrieved from highly expressed genes, we hypothesized that a relevant set of genes could still be reliably captured by reduced sequencing coverage. We profiled five samples with traditional whole transcript TruSeq and 3'-end QuantSeq (Moll et al., Nat Methods, 2014) library preparation with deep and shallow depth sequencing, respectively. We obtained 43.48-72.68 million mappable reads with TruSeq and 2.24-3.78 million with QuantSeq. Although the read coverage was reduced, QuantSeq was able to recover 71.17 ± 0.87% of all genes that were detected by TruSeq with reproducible quantification ( R > 0.85, P < 0.001 for all samples). Shallow sequencing of CLL samples reliably profiled intermediate to highly abundant genes with reduced sensitivity only for lowly expressed genes.
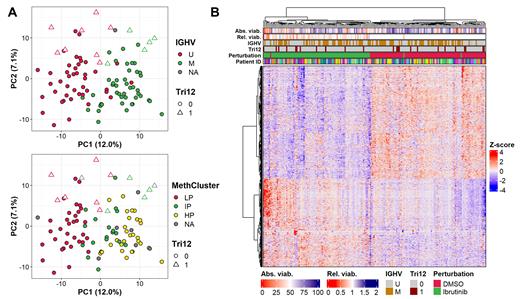
We then selected 105 representative CLL patient samples from a well-characterized cohort, treated them ex-vivo with 100 nM ibrutinib for 48h and performed GEP using shallow depth QuantSeq. Sample viability was measured by flow cytometry before and after perturbation. Library preparation and sequencing was successful for 91% of samples. With a median coverage of 2.19 million mappable reads per sample, the principal components (PC) of transcriptional variation in DMSO-treated samples included IGHV (12.0%), methylation subgroup and trisomy 12 status (7.1%). These data show that the screening method reliably captured the transcriptional features of CLL at steady-state and thus support the use of shallow depth RNA sequencing.
We were interested in the landscape of transcriptional change after perturbation of the B-cell receptor (BCR) pathway. Samples from the same patients clustered together and spread along a gradient of sample viability in the first PC. After correlation of gene counts from DMSO-treated samples with sample viability and subsequent gene set enrichment analysis, we found activation of the canonical apoptosis pathway via NFκB-mediated TNF-α signaling and cell stress to be associated with this signature of cell death. BTKi-treated samples showed the same signature of spontaneous apoptosis and additionally strong suppression of MYC and OXPHOS activity.
To identify the ibrutinib-regulated transcriptional signature, we performed differential gene expression analysis between ibrutinib and DMSO. BTKi treatment induced 904 differentially expressed genes (DEGs) ( FDR < 0.005). These genes were negatively enriched in the hallmarks allograft rejection, MYC targets, TNF-α signaling via NFκB and KEGG pathways associated with BCR signaling, and immune cell response. Top DEGs included e. g. FCRL5, PTPN6, FILIP1L, LRMP and LAPTM5 ( FDR < 0.001) which were also significantly downregulated after in-vivo ibrutinib exposure (Rendeiro et al. Nat Commun, 2020).
Inhibition of the BCR induces apoptosis in CLL in an IGHV status-dependent manner. Samples with unmutated IGHV (UM-CLL) were more sensitive to BTKi compared to samples with mutated IGHV (M-CLL) (83.1 vs. 92.3% relative viability, P < 0.001). On gene expression level, we observed more DEGs within UM-CLL (387) compared to M-CLL (182) ( FDR < 0.005). Response to BTKi was largely similar although the effect size in M-CLL tended to be smaller. To identify BCR-dependent genes that were significantly modulated by IGHV, we tested for the interaction between BTKi treatment and IGHV status in the differential expression models. We found the effects on the expression of CA12, FIG RPL5 and P2RY11 to be different upon BTK inhibition between UM- and M-CLL ( FDR < 0.1).
In conclusion, we establish shallow depth sequencing in CLL and show it can be used to reliably capture disease biology and drug effects. By treating patient samples with ibrutinib, we show that abrogating BTK strongly suppresses BCR-dependent processes which are largely independent of IGHV status. By adding more pathway perturbations, this approach could be used for further functional dissection of CLL biology.
Disclosures
Zenz:Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal