Background: Teclistamab (Tec) is a CD3 x BCMA bispecific antibody granted accelerated FDA approval for treating relapsed/refractory (RR) multiple myeloma (MM) based off the results of the MajesTEC-1 trial (Usmani S, et al. Lancet 2021, Moreau P, et al. N Engl J Med 2022). However, patients with prior exposure to anti-BCMA therapies were excluded from this trial and there is no published data evaluating Tec efficacy in this setting. Additionally, little is known about tumor-intrinsic and patient-specific immunologic predictors of response to Tec.
Methods: We performed an IRB-approved analysis of clinical outcomes for 52 commercially treated Tec patients treated as of 07/28/2023 at our center. Results were correlated to pre-treatment BCMA expression measured by immunohistochemistry (IHC). We also profiled peripheral blood T-cells from pre-treatment peripheral blood mononuclear cell (PBMC) samples from a sub cohort of patients via high-dimensional spectral cytometry using a 37-color panel including lineage, exhaustion, and activation markers.
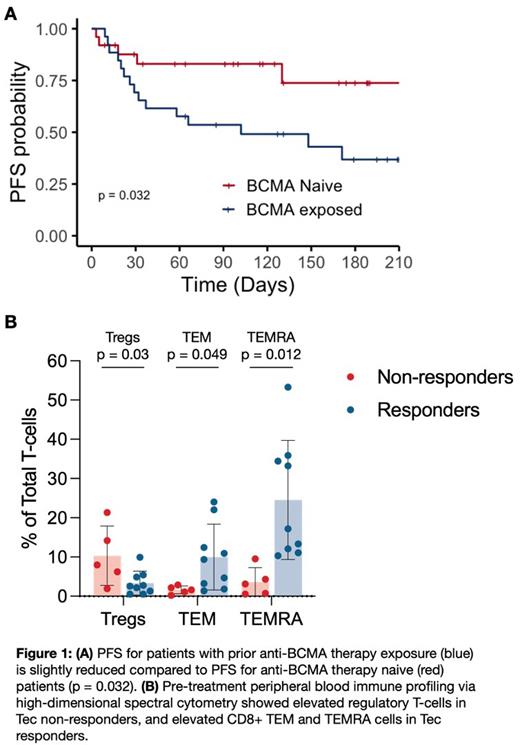
Results: Our commercial cohort was older (median age 70 vs 64), more heavily pretreated (median prior lines of therapy 7 vs 5) and had higher incidence of both high-risk cytogenetic abnormalities (33% vs 26%) and extramedullary disease (35% vs 17%) than the MajesTEC-1 population. Additionally, 52% had prior exposure to anti-BCMA therapies, including belantamab mafodotin (31%), anti-BCMA CAR T-cell therapies (37%), and anti-BCMA bispecific antibody therapies (4%), with some having exposure to multiple prior anti-BCMA therapies (17%). Among response evaluable patients (90%), with a median follow-up time of 100 days, the overall response rate (ORR) to Tec was 64% (36% ≥VGPR). Progression free survival (PFS) in patients without prior BCMA therapy was higher than in the anti-BCMA exposed population (median PFS NR vs 102 days, p = 0.032, Figure Panel A). However, the anti-BCMA therapy exposed population was more heavily pretreated (8 vs 5 median prior lines of therapy) and had more patients with high-risk cytogenetic abnormalities (46% vs 19%) and extramedullary disease (42% vs 27%) than the anti-BCMA therapy naïve cohort. No major differences in BCMA expression were noted between responding and non-responding patients or between anti-BCMA exposed and anti-BCMA naïve patients. However, two patients had absent BCMA expression following prior treatment with other BCMA targeting agents and did not respond to Tec. Cytokine release syndrome (CRS) during step-up dosing was strongly associated with Tec efficacy (median PFS NR vs 27 days, p <0.0001), with a 100% ORR for patients with CRS. Immune profiling experiments (Figure Panel B) revealed that Tec responders had peripheral blood enriched for both CD8 + effector memory T-cells (TEM, >6-fold increase, p = 0.0499) and CD8 + effector memory T-cells re-expressing CD45RA (TEMRA, >5-fold increase, p = 0.012) when compared to Tec non-responders. Tec non-responders had peripheral blood enriched for TIGIT + regulatory T-cells (Tregs, 3-fold increase, p = 0.03) when compared to Tec responders. No differences in PD-1, CTLA-4, LAG-3 or TIM-3 expression were observed in Tec responders vs non-responders. Patients with recent relapse from non-BCMA targeting bispecific antibodies (including products targeting FcRH5 or GPRC5D) had peripheral blood enriched for γδ T-cells with high TIGIT expression.
Conclusion: Tec is an effective therapy in RRMM with a slightly lower efficacy observed in patients with prior anti-BCMA therapy exposure. PFS reductions among these anti-BCMA exposed patients may be partly due to independent disease associated risk factors. A pre-Tec peripheral blood T-cell population enriched with highly cytotoxic effector T-cells was associated with response to therapy, while suppressive TIGIT +Tregs were associated with nonresponse, suggesting a potential therapeutic role for TIGIT blockade or CD25 + cell depletion to enhance the therapeutic efficacy of bispecific antibodies. Clinical and translational data from additional patients will be presented at the meeting.
Disclosures
Shekarkhand:Genentech: Consultancy. Tan:Takeda: Research Funding; Sanofi: Honoraria; Janssen: Current Employment, Honoraria, Research Funding. Hultcrantz:Amgen, Daiichi Sankyo, GlaxoSmithKline: Research Funding; Curio Science LLC, Intellisphere, Bristol Myer Squibb, GlaxoSmithKline: Honoraria. Mailankody:Bristol Myers Squibb: Research Funding; Allogene Therapeutics: Research Funding; Janssen Oncology: Research Funding; OncLive: Honoraria; Physician Education Resource: Honoraria; MJH Life Sciences: Honoraria; Optum Oncology: Consultancy; Janssen Oncology: Consultancy; Legend Biotech: Consultancy; Caribou Therapeutics: Research Funding; Takeda Oncology: Research Funding; Fate Therapeutics: Research Funding. Hassoun:Celgene, Takeda, and Janssen Pharmaceuticals: Research Funding. Shah:C4 Therapeutics: Research Funding; Sabinsa: Research Funding; Plantable: Research Funding; Bristol Myers Squibb: Consultancy, Other: Advisory Board, Research Funding; M and M Labs: Research Funding; Janssen: Consultancy, Other: Advisory Board, Research Funding; Sanofi: Other: Advisory Board. Korde:Amgen, Janssen, Epizyme, AbbVie: Research Funding; Janssen: Other: Advisory Board; CCO, OncLive, Intellisphere, Remedy Health: Consultancy. Landau:Karyopharm, Pfizer, Juno, Prothena, Caelum Biosiences, Legend Biotech, Takeda, Janssen, Nexcella: Honoraria; Alexion Pharmaceuticals, Takeda, Janssen, Prothena, Protego: Research Funding. Scordo:Amgen, Inc.: Research Funding; Omeros Corporation: Consultancy, Research Funding; Medscape, LLC: Honoraria; CancertNetwork (Intellisphere LLC): Honoraria; Angiocrine Bioscience, Inc.: Research Funding. Lahoud:MorphoSys Inc, Kite: Consultancy. Giralt:Amgen, Actinuum, Celgene/BMS, Kite Pharma, Janssen, Jazz Pharmaceuticals, Johnson & Johnson, Novartis, Spectrum Pharma, Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen, Actinuum, Celgene/BMS, Omeros, Johnson & Johnson, Miltenyi, Takeda: Research Funding. Lesokhin:Janssen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Bristol Myers Squibb: Research Funding; ArcellX: Consultancy. Usmani:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding; EdoPharma: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; K36 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Moderna: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; SkylineDX: Membership on an entity's Board of Directors or advisory committees, Research Funding; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Array Biopharma: Research Funding; TeneoBio: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal