Background: B-cell malignancies, a diverse and complex spectrum of lymphoproliferative disorders, are marked by a gradient of severity, ranging from indolent non-Hodgkin lymphomas (NHLs) such as follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL), to more aggressive variants of NHL like diffuse large B-cell lymphoma (DLBCL). This wide array of disorders brings unique challenges in treatment, particularly for relapsed or refractory (r/r) cases where there is a significant dearth of effective therapeutic options. The PI3K pathway, a vital regulator of cellular functions such as cell growth, survival, and differentiation, has increasingly been recognized as a promising therapeutic target in the context of B-cell lymphomas. Accumulating evidence points towards its role in the pathogenesis of these disorders, underlining the potential of PI3K inhibitors in their treatment. Linperlisib, an orally administered PI3Kδ inhibitor, has emerged as a potential candidate for the management of r/r B-cell malignancies, with a previous phase I study demonstrating its safety and suggesting preliminary clinical activity. In this light, our phase Ib study aimed to further assess the safety and efficacy of linperlisib in a broader cohort of patients with r/r B-cell malignancies.
Methods: Patients with histologically or cytologically confirmed B-cell malignancies, relapsed, refractory, or lacking standard-of-care systemic therapies, were enrolled. The study encompassed several subtypes of the disease, including DLBCL, mantle cell lymphoma (MCL), FL, marginal zone lymphomas (MZL), and CLL/small-cell lymphocytic leukemia (SLL). Linperlisib was administered orally at a dose of 80 mg daily in a 28-day cycle until disease progression, onset of unacceptable toxicity, or patient withdrawal. The primary endpoint of the study was the objective response rate (ORR), as per the 2007 International Working Group Criteria for lymphomas. Secondary endpoints included safety, disease control rate (DCR), duration of response (DOR), progression-free survival (PFS), and overall survival (OS).
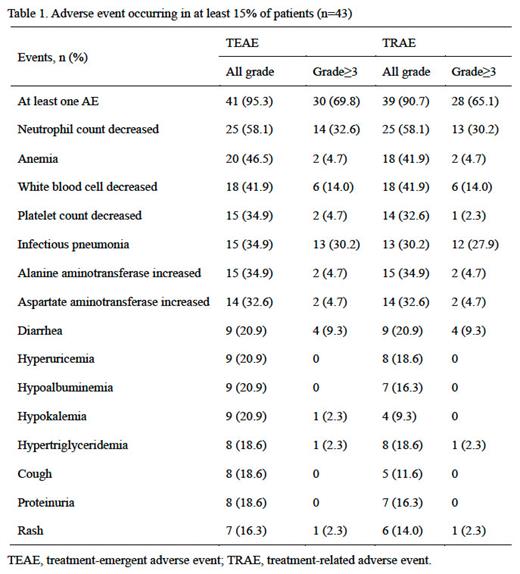
Results: A total of 43 patients with r/r B-cell malignancies were enrolled between Jun 15, 2020, and Apr 02, 2021, and subsequently received linperlisib. At the data cutoff point of Jan 10, 2023, the median follow-up was 24.7 (range: 0.8, 30.6) months. The ORR was recorded at 46.5% (95%CI, 31.2% to 62.3%), and the DCR at 72.1% (95%CI, 56.3% to 84.7%) across all patients. More specifically, the ORR for individual diseases were as follows: 30.8% (4/13) for GCB DLBCL, 27.3% (3/11) for non-GCB DLBCL, 55.6% (5/9) for MCL, 100.0% (4/4) for FL, 66.7% (2/3) for CLL/SLL, and 66.7% (2/3) for MZL. The median DOR for the entire patient cohort was 9.3 months (95% CI: 5.4 to not reached), and the median PFS was 8.3 months (95% CI: 3.7 to not reached). The median OS was not reached, with 1-year and 2-year OS rates of 79.1% (95% CI: 63.6% to 88.5%) and 76.7% (95% CI: 61.1% to 86.8%), respectively. In terms of safety, the most common adverse events of grade 3 or higher were decreased neutrophil count, infectious pneumonia, and decreased white blood cell count (Table 1).
Conclusion: In summary, our study underscores the significant potential of linperlisib in providing broad anti-tumor activity across a range of r/r B-cell malignancies, including DLBCL, MCL, MZL, FL, and SLL/CLL. These encouraging results justify further investigation of linperlisib, both as a monotherapy and in combination with other therapeutic agents. It is hoped that these findings will help evolve and enhance our treatment strategies for B-cell malignancies and ultimately improve patient outcomes.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Linperlisib is a novel oral inhibitor with high selectivity for PI3Kδ, which has been demonstrated the anti-tumor activity in r/r B-cell malignancies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal