Key Points
Significant disparities exist in cancer outcomes. We examined demographic and geographic representation for pivotal trials in lymphomas.
Abstract
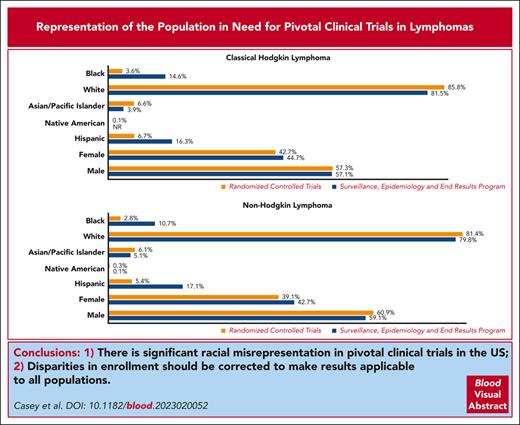
Despite the advances in cancer outcomes, significant health disparities persist. Several new agents have been recently approved for treatment of lymphomas, leading to improved outcomes. Extending the benefits of these new agents starts by adequate enrollment of all affected patient populations. This study aimed to evaluate the extent to which randomized controlled trials (RCTs) match the demographic and geographic diversity of the population affected by lymphoma. Two Food and Drug Administration databases, clinicaltrials.gov, and relevant primary manuscripts were reviewed for drug approval data and demographic representation in RCTs for classical Hodgkin lymphoma (cHL) and non-Hodgkin lymphoma. Maps showing the distribution and frequency of trial participation relative to disease burden, insurance status, and racial representation were created. Black, Hispanic, and female patients were significantly underrepresented in the RCTs for lymphoma compared with that for the disease burden (3.6% [95% confidence interval (CI), 2.8-5.4] vs 14.6% [95% CI, 13.8-15.3]; 6.7% [95% CI, 5.5-7.9] vs 16.3% [95% CI, 15.5-17.1]; and 39.1% [95% CI, 37.3-40.9] vs 42.7% [95% CI, 42.3-43.1], respectively). White and male patients were overrepresented. More counties with higher mortality rates and racial minority representation had low access to the trials, particularly for cHL in the southern region of the United States. There are significant racial misrepresentations in pivotal RCTs in the United States, and geographic distribution of these trials may not provide easy access to all patients in need. Disparities in enrollment should be corrected to make results applicable to all populations.
Introduction
It is estimated that 81 560 individuals were diagnosed with non-Hodgkin lymphoma (NHL) and 8830 with classical Hodgkin lymphoma (cHL) in the United States in 2021.1,2 Despite tremendous strides in improving patient survival and outcomes, not all populations have experienced this positive impact.
Several socioeconomic and demographic factors affect health outcomes and contribute to the significant health care disparities in cancer, particularly in lymphoma.3 Studies have consistently demonstrated lower survival among Black patients than among non-Hispanic, White patients from both NHL and cHL.4-7 These disparate outcomes are observed across sex and age groups and in adult and pediatric populations.8,9 Meanwhile, improvements in survival have been observed in the younger patient population of all races, contrary to older patients who have had slower survival gains over time.10 Some of the improvements in outcomes for the pediatric population (age <15 years) have been attributed to higher enrollment rate of children in clinical trials than that of older children (age 15-19 years) and adults.11
In addition, geographical disparities have become more apparent as clinical trials for new drugs become such a vital part of patient care. We previously showed that availability of clinical trials for leukemias and multiple myeloma is starkly different in rural areas compared with urban areas.12 Structural barriers, such as costs related to obtaining health care services and transportation or travel to cancer centers and study sites, augment the magnitude of the problem.13 Health care insurance coverage is an important determinant of access to care, and it is particularly compromised among rural, underserved populations.13,14 This further influences health-seeking behaviors of rural residents with cancer and contributes to late presentation to the centers, hospitals, or clinics, where screening, treatment, and clinical trial recruitment typically takes place.
Randomized controlled trials (RCTs) have been foundational in the advancement of cancer therapies. The rapid pace of such research has produced many new treatments for all malignancies, including lymphomas. However, the clinical trials in which they are assessed may not be adequately representative of the populations affected by lymphomas, especially considering the potential disparities in outcomes based on demographic factors. The purpose of this study is to evaluate the extent to which the RCTs that led to approval for lymphoma therapies match the demographic and geographic diversity of the population affected by lymphoma. We aim to investigate the racial and ethnic representation in these clinical trials and examine the relationship between representation and disease burden in the US population.
Methods
Study design
We searched 2 Food and Drug Administration (FDA) databases, “Oncology (cancer)/Hematologic Malignancies approval notifications” and “Novel Drug Approvals,” to identify drugs that were approved for lymphoma between 2011 and 2021. We collected information on the clinical trials associated with these approvals, including the National Clinical Trial number and demographics of patients who participated in the trials. Only trials that reported data on race distribution of enrolled patients were included in this analysis. The categorization of race was guided by the availability of data on clinical trial participation for the racial subgroups and included American Indian-Alaskan Native, Asian-Pacific Islander (Asian-PI), Black, White, and other; ethnicity was classified as Hispanic or non-Hispanic. Clinical trials that led to the approval of drugs for pediatric patients were excluded. Trials for chronic lymphocytic leukemia/small lymphocytic lymphoma were excluded from this analysis because of the malignancy being categorized as a leukemia in the general population data; 1 study that had inconsistencies in the total number of patients enrolled and the racial breakdown was also excluded.
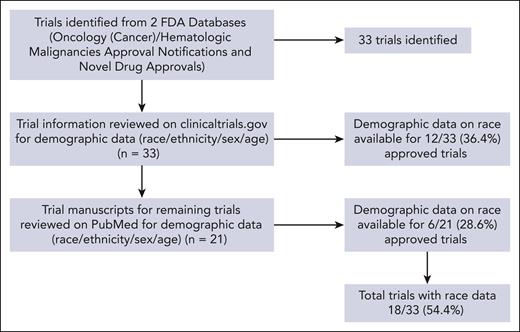
We identified 33 RCTs. The National Clinical Trial numbers and drug names investigated in the studies were used to query clinicaltrials.gov for participant demographic data (race, ethnicity, sex, and age). Location data (city, state, and zip code) of the investigational sites across the 48 contiguous US states were also extracted from clinicaltrials.gov. Data on race were available for 12 (36.4%) of the trials. Another search was conducted of the relevant primary literature through the PubMed database. We found race data for 6 (28.6%) of the remaining 21 trials from the search. In total, 18 (54.5%) of the eligible trials reported data on race (Figure 1), including 12 (66.7%) that reported data on ethnicity. These trials were linked to 18 lymphoma drugs that were approved over the study period, with 3 for cHL and 15 for NHL. The proportions of patients in these trials based on race, ethnicity, and sex were compared for the disease burden estimated using incidence data. We obtained US census population–based data from 2014 to 2018 from the National Cancer Institute Surveillance, Epidemiology, and End Results Program (SEER) Explorer to reflect the overall burden of lymphoma. SEER is composed of 21 registries located in the following locations: Seattle; Idaho; San Francisco; San Jose-Monterey; Los Angeles; California excluding San Francisco, San Jose-Monterey, and Los Angeles; Alaska Native Registry; Hawaii; New Mexico; Louisiana; Illinois; Kentucky; Utah; Atlanta; rural Georgia; New Jersey; Georgia excluding Atlanta and rural Georgia; New York; Connecticut; Iowa; and Massachusetts.
Flow diagram of clinical trials (reporting data on race) identified from the FDA databases,ClinicalTrials.gov, and the PubMed search.
Flow diagram of clinical trials (reporting data on race) identified from the FDA databases,ClinicalTrials.gov, and the PubMed search.
Geographic distribution of clinical trials
We used the location data of investigational sites from clinicaltrials.gov to show the geographic distribution and frequency of clinical trial participation for lymphoma across the nation. Counties with higher racial minority representation and uninsured rates greater than the national average were identified as hot spots of critical need for cancer clinical trials. The US population breakdown based on race used to define areas of high racial minority representation was obtained from the County Health Rankings and Roadmaps,15 whereas the uninsured county-level data were obtained from the Small Area Health Insurance Estimates for 2020 using the American Community Survey.16
Counties with high racial minority representation were defined as those whose racial makeup was greater than the national share for Asian, Native Hawaiian, Other Pacific Islander (5.9%), American Indian/Alaska Native, or persons identifying as multiracial (4%), Black (12.5%), and Hispanic (18.3%).17 Similarly, the uninsured estimates above the national average were calculated for the 48 contiguous states, given that Alaska and Hawaii had no investigational sites that participated in the clinical trials included in this study. This included counties with rates >6.6% in states with Medicaid expansion and >12.7% in states without the expansion as of 2020 (Alabama, Florida, Georgia, Kansas, Mississippi, North Carolina, South Carolina, Tennessee, Texas, Wisconsin, and Wyoming).18,19 We obtained county-level mortality data from the Institute for Health Metrics and Evaluation to show the relationship between frequency of participation in the clinical trials and the disease burden for cHL and NHL.20 We categorized counties as having high mortality if the rates were higher than the respective national average, which were >0.39 and >8.31 deaths per 100 000 patients of the population with cHL and NHL, repectively.
Statistical analysis
We used descriptive analysis to summarize the racial demographic representation in the clinical trials. The subgroup proportions were calculated for lymphoma, in general, and for cHL and NHL subtypes. The mean age for studies that provided medians was calculated using the upper bound, lower bound, and sample size of the RCT.21 Trial data on Asian-PI patients were combined to allow for comparisons using SEER. We also calculated estimates of incident/new cases for each malignancy by multiplying the racial, ethnic, and sex subgroup rates from SEER with the specific US population–based estimates from 2014 to 2018. The relationship between disease burden based on race, ethnicity, and representation in clinical trials was assessed using χ2 test for proportions and Fisher exact test (when appropriate), and it included 95% confidence intervals (CIs). A P value = .05 was considered statistically significant. The SAS version 9.4 (SAS Institute Inc, Cary, NC) software was used for analysis. The ArcGIS 10.7 software was used to geocode the clinical trial location data. The Jenks natural breaks for categorization was used for the mortality rates and racial minority representation data to support the geographical analysis.
Results
Table 1 presents the demographic representation of participants in the RCTs. The participants averaged 57.3 years, with patients with NHL being older, on average, (60.9 years) than those with cHL (38.8 years). Of the participants enrolled in the clinical trials, 4032 (83.2%) were White, 304 (6.3%) were Asian-PI, and 152 (3.2%) were Black. These proportions were comparable for NHL and cHL. Hispanic patient representation was low in the 12 trials that reported data on ethnicity (overall, 6%; NHL, 5.4%; and cHL, 6.7%). More male than female patients were enrolled in the trials (59.5% vs 40.5%); corresponding percentages were 60.9% and 39.1%, respectively, in NHL trials and 57.3% and 42.7%, respectively, in cHL trials.
Demographic representation in pivotal clinical trials for lymphoma therapies approved by the FDA between 2011 and 2021
| Demographic characteristics . | Overall (n = 4849) . | cHL (n = 1957) . | NHL (n = 2892) . |
|---|---|---|---|
| Age (mean) | 57.3 | 38.8 | 60.9 |
| Race (n, %)∗ | |||
| AI-AN | 11 (0.2) | 2 (0.1) | 9 (0.3) |
| ‡Asian-PI | 304 (6.3) | 129 (6.6) | 175 (6.1) |
| Black | 152 (3.1) | 71 (3.6) | 81 (2.8) |
| White | 4032 (83.2) | 1679 (85.8) | 2353 (81.4) |
| Other† | 36 (0.7) | 35 (1.8) | 1 (0.03) |
| Sex (n, %) | |||
| Males | 2884 (59.5) | 1122 (57.3) | 1762 (60.9) |
| Females | 1965 (40.5) | 835 (42.7) | 1130 (39.1) |
| §Ethnicity (n, %) | n = 3605 | n = 1626 | n = 1979 |
| Hispanic | 215 (6) | 109 (6.7) | 106 (5.4) |
| Non-Hispanic | 3044 (84.4) | 1337 (82.2) | 1707 (86.3) |
| U/NR/M | 346 (9.6) | 180 (11.1) | 166 (8.4) |
| Demographic characteristics . | Overall (n = 4849) . | cHL (n = 1957) . | NHL (n = 2892) . |
|---|---|---|---|
| Age (mean) | 57.3 | 38.8 | 60.9 |
| Race (n, %)∗ | |||
| AI-AN | 11 (0.2) | 2 (0.1) | 9 (0.3) |
| ‡Asian-PI | 304 (6.3) | 129 (6.6) | 175 (6.1) |
| Black | 152 (3.1) | 71 (3.6) | 81 (2.8) |
| White | 4032 (83.2) | 1679 (85.8) | 2353 (81.4) |
| Other† | 36 (0.7) | 35 (1.8) | 1 (0.03) |
| Sex (n, %) | |||
| Males | 2884 (59.5) | 1122 (57.3) | 1762 (60.9) |
| Females | 1965 (40.5) | 835 (42.7) | 1130 (39.1) |
| §Ethnicity (n, %) | n = 3605 | n = 1626 | n = 1979 |
| Hispanic | 215 (6) | 109 (6.7) | 106 (5.4) |
| Non-Hispanic | 3044 (84.4) | 1337 (82.2) | 1707 (86.3) |
| U/NR/M | 346 (9.6) | 180 (11.1) | 166 (8.4) |
AI-AN, American Indian-Alaska Native; M, missing; NR, not reported; PI, Pacific Islander; U, unknown.
The unknown or missing values were included in the denominator but not reported.
Includes “other” and “more than 1 race.”
The Asian-PI groups were combined.
Ethnicity results are based on data from 12 of 18 trials.
Table 2 compares clinical trial representation and the disease burden of NHL and cHL in specific demographic subgroups. There were significant differences in representation in the RCTs compared with the disease burden as reported by SEER for female patients and all racial subgroups. Black patients (3.6% [95% CI, 2.8-5.4] vs 14.6 [95% CI, 13.8-15.3] and Hispanic patients, 6.7% [95% CI, 5.5-7.9] vs 16.3% [95% CI, 15.5-17.1]) were the most underrepresented racial groups in the RCTs for cHL, whereas White patients were overrepresented (85.8% [95% CI, 84.3-87.3] vs 81.5% [95% CI, 80.7-82.3]). The results were similar in trials for NHL. Black patients represented 2.8% (95% CI, 2.2-3.4) of patients enrolled in the trials compared with 10.7% (95% CI, 10.4-10.9) of new cases of NHL reported by SEER; corresponding percentages for Hispanic patients were 5.4% (95% CI, 4.4-6.4) vs 17.1% (95% CI, 16.8-17.4), respectively. Compared with the disease burden, female patients were largely underrepresented in clinical trials (39.1% [95% CI, 37.3-40.9] vs 42.7% [95% CI, 42.3-43.1]).
Proportional differences in demographic representation in pivotal clinical trials for lymphoma therapies approved between 2011 and 2021 and the 2018 SEER incidence data
| . | RCTs . | SEER . | ꭕ2 . | P value . |
|---|---|---|---|---|
| % (95% CI) . | % (95% CI) . | |||
| cHL | (n = 1957) | (n = 8179) | ||
| Black | 3.6 (2.8-4.5) | 14.6 (13.8-15.3) | 173.2 | < .0001 |
| White | 85.8 (84.3-87.3) | 81.5 (80.7-82.3) | 19.7 | < .0001 |
| Asian-PI | 6.6 (5.5-7.7) | 3.9 (3.5-4.3) | 28 | < .0001 |
| Native American | 0.1 (0-2.4) | NR 0 (0-0) | 8.4 | .0038 |
| ∗Hispanic | 6.7 (5.5-7.9) | 16.3 (15.5-17.1) | 98.9 | < .0001 |
| Female | 42.7 (40.5-44.9) | 44.7 (43.6-45.8) | 2.6 | .104 |
| Male | 57.3 (55.1-60) | 57.1 (56-58.2) | 0.04 | .8348 |
| NHL | (n = 2892) | (n = 63 470) | ||
| Black | 2.8 (2.2-3.4) | 10.7 (10.4-10.9) | 185.3 | < .0001 |
| White | 81.4 (79.9-82.8) | 79.8 (79.5-80.1) | 4.3 | .0381 |
| Asian-PI | 6.1 (5.2-6.9) | 5.1 (4.8-5.2) | 5.7 | .0168 |
| Native American | 0.3 (0.1-0.5) | 0.1 (0.96-0.11) | 14.8 | .0001 |
| ∗Hispanic | 5.4 (4.4-6.4) | 17.1 (16.8-17.4) | 188.9 | < .0001 |
| Female | 39.1 (37.3-40.9) | 42.7 (42.3-43.1) | 14.7 | .0001 |
| Male | 60.9 (59.2-62.3) | 59.1 (58.7-59.5) | 3.9 | .0495 |
| . | RCTs . | SEER . | ꭕ2 . | P value . |
|---|---|---|---|---|
| % (95% CI) . | % (95% CI) . | |||
| cHL | (n = 1957) | (n = 8179) | ||
| Black | 3.6 (2.8-4.5) | 14.6 (13.8-15.3) | 173.2 | < .0001 |
| White | 85.8 (84.3-87.3) | 81.5 (80.7-82.3) | 19.7 | < .0001 |
| Asian-PI | 6.6 (5.5-7.7) | 3.9 (3.5-4.3) | 28 | < .0001 |
| Native American | 0.1 (0-2.4) | NR 0 (0-0) | 8.4 | .0038 |
| ∗Hispanic | 6.7 (5.5-7.9) | 16.3 (15.5-17.1) | 98.9 | < .0001 |
| Female | 42.7 (40.5-44.9) | 44.7 (43.6-45.8) | 2.6 | .104 |
| Male | 57.3 (55.1-60) | 57.1 (56-58.2) | 0.04 | .8348 |
| NHL | (n = 2892) | (n = 63 470) | ||
| Black | 2.8 (2.2-3.4) | 10.7 (10.4-10.9) | 185.3 | < .0001 |
| White | 81.4 (79.9-82.8) | 79.8 (79.5-80.1) | 4.3 | .0381 |
| Asian-PI | 6.1 (5.2-6.9) | 5.1 (4.8-5.2) | 5.7 | .0168 |
| Native American | 0.3 (0.1-0.5) | 0.1 (0.96-0.11) | 14.8 | .0001 |
| ∗Hispanic | 5.4 (4.4-6.4) | 17.1 (16.8-17.4) | 188.9 | < .0001 |
| Female | 39.1 (37.3-40.9) | 42.7 (42.3-43.1) | 14.7 | .0001 |
| Male | 60.9 (59.2-62.3) | 59.1 (58.7-59.5) | 3.9 | .0495 |
NR, not reported: SEER does not report data if total cases are <16.
Proportions of Hispanic persons in the RCTs were calculated using total participants from trials that reported data on ethnicity.
Geographical distribution of clinical trials
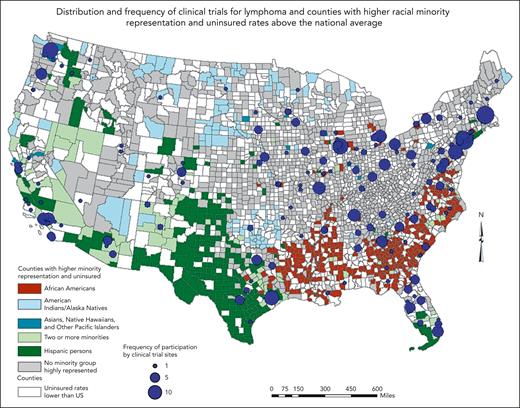
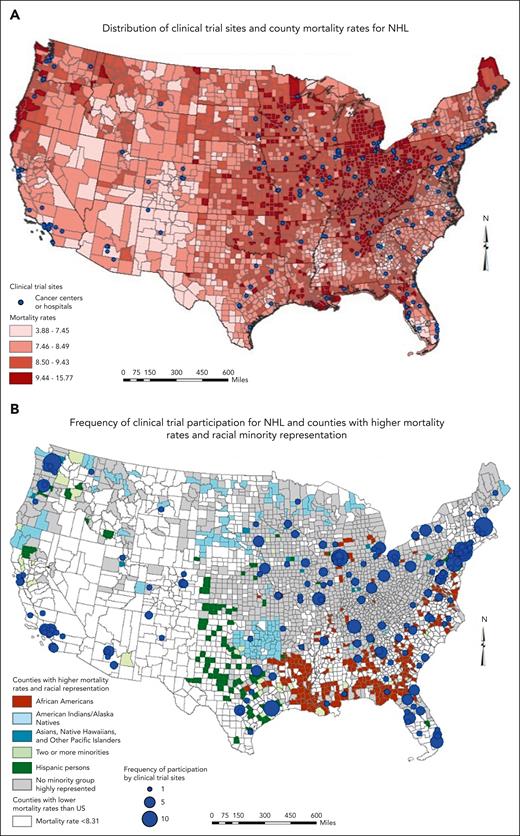
Geographically, RCTs for lymphoma were mostly concentrated in the northeastern (Massachusetts, New York, New Jersey, Pennsylvania, and Illinois) and far western (Seattle and California) regions of the United States, leaving most of the West and Midwest lacking in participation (Figure 2). Southern states such as Maryland, Virginia, Georgia, Florida, and the Carolinas had some representation in the trials. Counties in critical need for trials based on the lack of participation, higher racial minority representation (Black and Hispanic), and uninsured rates were predominantly located in the Southeast and Southwest regions of the United States, particularly in Louisiana, Arkansas, Mississippi, and Texas. However, some major cities in Texas and Tennessee had more trial participation than their southern counterparts. The Appalachian states of Kentucky and West Virginia in the Southeast region had a cluster of counties with higher uninsured rates and no high racial minority representation. Mortality rates for NHL were highest in the Midwest region, particularly in Ohio, Michigan, and Indiana (Figure 3A). The distribution of sites that participated in NHL trials that led to drug approvals and reported data on race followed the general pattern provided in Figure 2. States such as Idaho, Nevada, Wyoming, North Dakota, Kansas, and Mississippi had no study sites in the RCTs, whereas Montana, Oklahoma, and Louisiana had 1 each. Sites with more frequent participation were in the Northeast region (Figure 3B). Most counties (1171 of 3142, 37.2%) with mortality rates lower than the US average (<8.31) were in the western region and parts of the Southwest. The counties with mortality rates higher than the US average and a higher Black population than the national average were clustered in the southern states of Louisiana, Arkansas, and Alabama as well as northern Florida, whereas counties in Texas and in the Kansas-Colorado border area had a higher Hispanic population than the national average. The Northeast region and parts of the Midwest had counties with higher mortality rates, no racial minority group highly represented in the population, and several sites that participated frequently in the NHL RCTs.
Map showing the areas of critical need for cancer trials based on the distribution of clinical trial participation, racial minority representation, and insurance status.
Map showing the areas of critical need for cancer trials based on the distribution of clinical trial participation, racial minority representation, and insurance status.
Maps of distribution and frequency of clinical trials, NHL burden, and the race/ethnicity of the US population.
Maps of distribution and frequency of clinical trials, NHL burden, and the race/ethnicity of the US population.
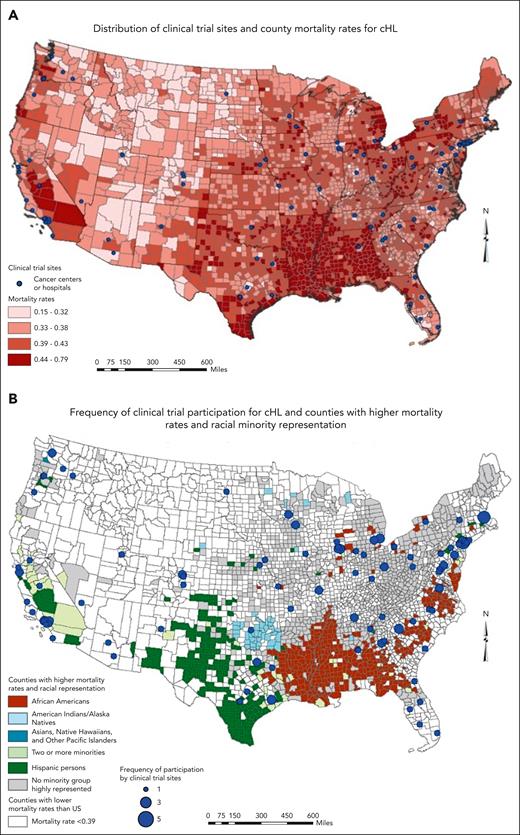
Mortality rates for cHL were highest in the southern United States, particularly in Louisiana, Arkansas, Mississippi, and Alabama, as well as California in the West (Figure 4A). The RCTs for cHL had a relatively similar distribution as NHL; however, the frequency of participation was much lower (Figure 4B). There were no trials in Idaho, Montana, Wyoming, and South Dakota, whereas Oregon, Utah, Nevada, New Mexico, Oklahoma, Kansas, Alabama, and North Dakota each had 1 site participating in the trials. Similar to NHL mortality rates, most counties (1584 of 3142, 50.4%) with cHL mortality rates lower than the US average (<0.39) were in the western region of the United States. A large cluster of counties with higher mortality rates than the US average and higher Black population than the national average were in the South, specifically in Louisiana, Arkansas, Mississippi, Alabama, and southwestern Georgia, with only the last 2 states having sites participating in RCTs for cHL. Similarly, counties with higher Hispanic population than the national average were clustered in West Texas and the multiracial minority group in California.
Maps of distribution and frequency of clinical trials, cHL burden, and the race/ethnicity of the US population.
Maps of distribution and frequency of clinical trials, cHL burden, and the race/ethnicity of the US population.
Discussion
There is growing evidence and awareness of the interaction of socioeconomic and demographic factors with health status and outcomes. In an evaluation of trends over time for adult patients diagnosed with NHL who identify with a specific race, the incidence between 1992 and 2005 was highest among the non-Hispanic White population compared with that among Hispanic White, Asian-PI, and Black populations.3 However, the overall survival across all stages of the 3 NHL subtypes (diffuse large B-cell lymphoma, follicular lymphoma, and small lymphocytic lymphoma) was highest among patients identifying as non-Hispanic White, followed by Hispanic White and Asian-PI, with Black patients having the worst overall survival rates.3 Other studies evaluating diffuse large B-cell lymphoma and mantle cell lymphoma, which is another subtype of NHL, reported lower survival among Black patients than that among White patients.4,5 Our analysis shows that disparities are prevalent across RCTs for lymphoma because White patients were overrepresented, whereas racial and ethnic minorities (Black and Hispanic patients) were underrepresented. Noted here is a positive correlation between representation in clinical trials and survival. The fact that Whites are overrepresented matches the greater improvement in overall survival from lymphomas in this patient population, suggesting that enrollment in those clinical trials may be a contributor to this improvement. Other factors, including social determinants of health, undoubtedly play a role. But the underrepresentation of patients with the greatest need further impedes racial and ethnic minority populations.
In our analysis, Black patients represented only 2.8% of patients in clinical trials for NHL that reported race, whereas SEER reported 10.7% patients were affected by the malignancy between 2014 and 2018, Hispanic patients only had 5.4% of representation, whereas 17.1% were diagnosed with NHL. Similarly, for patients with cHL, survival rates were lower among Black patients than that among non-Hispanic White patients,6,7 with 1 study also reporting lower survival among Hispanic patients than non-Hispanic White patients.6 Interestingly, although not the focus of our study, similar trends are reported in pediatric patients. Of pediatric patients with cHL, Black patients had worse survival than non-Hispanic White and Hispanic White patients,8-10 whereas Hispanic ethnicity was a predictor of inferior disease-specific survival.8 Furthermore, we found that cHL also has significant enrollment misrepresentation, with Black patients only representing 3.6% of participants in clinical trials, whereas they constituted 14.6% of the cHL population. For Hispanic patients, 6.7% were represented in clinical trials compared with 16.3% of new cHL cases reported by SEER data.
Considering age, a recent study analyzing trends in survival among different age groups reported that the 5-year survival is high in the patient group aged from 20 to 49 years diagnosed with cHL, and the survival trend improved steadily over the 5-year periods from 2000 to 2004, 2005 to 2009, and 2010 to 2014.22 The average age across the RCTs included in our study did not represent patients diagnosed at an older age. The average age for diagnosis of cHL is 39 years.2 This implies that the trials included in our analysis, with a median overall age of 38.8 years, adequately matched the age of patients with cHL at diagnosis. However, had pediatric studies been included in our analysis, the average age would have been <38.8 years. Older patients with cHL aged from 75 to 85 years have had a much lower 5-year survival, and improvement in survival rates was not observed until 2005 onward.2 The impact of NHL is more readily observed in the age demographic because the pediatric population is not as heavily affected by this disease, which, as mentioned in “Methods,” was a part of the exclusion criteria. The average age of the study populations included in our analysis was 60.9 years for NHL, with that of new cases in the population reported in SEER being 67 years. Similar to cHL, in NHL, the older population experienced 55% of the deaths for those aged ≥75 years.1 The uneven representation identified in our analysis questions the generalizability of the results in RCTs for lymphomas because the population included in the studies does not fully represent the target population.
In our analysis, misrepresenation is not only shown from a demographic perspective but also from a geographic standpoint. Clinical trials for drugs that are approved by the FDA primarily occur at large cancer centers. Overall, lymphomas were shown to have a predominance of trials in the Northeast, Midwest, East Texas, Florida, Pacific Northwest, and the Los Angeles area. Conversely, there were areas in the Southeast and in western states that lacked availability of these clinical trials. As concluded from evidence in the literature, transportation and distance are barriers to appropriate care. The farther the distance a patient has to travel, the lower the probability that they will be willing or able to seek out care.23 This has been identified as an important barrier to routine primary care visits, and the issue is deemed greater when it comes to accessing speciality care. The distance burden includes time spent traveling and waiting for and receiving care.24 The study found variation in clinic time, with patients of racial/ethnic origin, the less educated, and unemployed had significantly longer wait times. Focusing particularly on racial differences in clinic and travel time, non-Hispanic White patients had an average of 86 and 36 minutes, non-Hispanic Black patients had 99 and 45 minutes, and Hispanic patients 105 and 45 minutes, respectively. It should be noted that the Northeast and parts of the Midwest did have reasonable access to clinical trials but higher mortality. Although access to clinical trials is an important factor in reducing cancer-related mortality, other factors such as the disease stage at diagnosis, comorbidities, and social determinants of health may also affect outcomes, but they are not accounted for in publicly available databases. In addition, patients from racial minority groups tend to have a higher prevalence of comorbidities,25 which can prevent participation in RCTs because of higher risk of mortality. Moreover, patients from racial minority populations, particularly Black patients, mistrust physicians and health researchers, given the evidence of discrimination of present-day health care systems26,27 and the troubling historical events in which Black patients were exploited for the benefit of scientific discoveries.28
Retrospective studies have shown that travel burden in cancer care also leads to worse overall survival in patients.29 One example to note is the toxic air in Louisiana leading to a higher risk of lymphomas.30 Our data show that the only site with availability of these pivotal trials that reported on race in Louisiana was in New Orleans, making it a geographic burden in an area affected by cancer and with a higher than national average population of Black Americans. This is also true for the remainder of the Southeast, with the exception of Florida, where a few locations participated in the trials. Of note, as shown in Figures 3 and 4, the impact on Black Americans because of the lack of clinical trial availability is mostly in the Southeast. The geographic maldistribution is not only a matter of transportation, because travel for many patients to a study site has additional implications related to family, childcare, work, support systems, and others that limit their ability to visit centers located several miles away. Compared with the Northeast, Midwest, and the West, Black Americans in the South fare poorly in comorbidities, whereas Black Americans across the nation fare worse than White Americans.25 Trials should be more available throughout the country to those who are in need. This may require trials to be designed in a way that favor management in local clinics in the communities rather than centralization of care at cancer centers mainly located in major cities and towns.
These data, although striking, did not come without limitations. The RCTs were limited to studies conducted over a 10-year period (2011-2021), whereas SEER data represent a 5-year period (2014-2018) and only a fraction of the general US population. These are global trials, with the United States representing only 33% of locations participating in these RCTs. This may affect the distribution among certain racial groups, perhaps most notably among Asian-PI patients. Patients identifying as Asian-PI have a greater representation in RCTs than in SEER, but this might be confounded by enrollment, sometimes in large proportion, from Eastern Asian countries. However, these trials serve as the basis for FDA approvals despite not representing the US population adequately. Furthermore, Asian-PI patients include individuals who identify as Japanese, Chinese, Indian, Filipino, Vietnamese, and others. There may be important differences within this widely heterogenous populations that might make their representation unequal. The same may apply to ethnicity as Hispanic patients may have a variety of backgrounds to include Caribbean, Central and South American, and Spanish. Similarly, the term Black Americans tends to include all ins who identify as such, yet ethnic, cultural, and socioeconomic differences that influence health outcomes exist within this population.31 Specifically, whether the individual is a Black American born in the United States, an African immigrant, or a Black American born to African immigrant parents residing in the United States can have important implications on study findings. Considering ethnic subgroups within Asian, Hispanic, and Black patients is often overlooked in health research studies and can present challenges in analyzing the smaller samples.
There is underreporting of race and ethnicity that contributes to inaccurate estimates of representation in the trials. This underreporting is in itself an issue, with only 54.5% of trials both on clinicaltrials.gov and primary manuscripts presenting data on demographics. This calls for a review of the current reporting practices and requirements so as to prioritize adequate and complete reporting of demographic characteristics of patients participating in clinical trials. Having a uniform and mandatory system of reporting could encourage the investigators to emphasize recruitment of patients from all racial groups, particularly those experiencing higher disease burden. In addition, funders of clinical trials can play an influential role if they require investigators to recruit patients from all racial and ethnic groups and include them in the reporting. These strategies may improve the representation of racial minorities.
Racial representation in the trials do not adequately match the disease burden attributed to lymphoma. There is significant underrepresentation of African American/Black and Hispanic patients. Underrepresention may lead to results that are not fully translatable to all populations, whereas underreporting of the data can create inaccuracies in the overall results and introduce lack of transparency. Geographic distribution of pivotal trials for lymphoma may not provide easy access in some areas of need. Disparities in enrollment should be corrected to make results applicable to all populations and contribute to achieving equity in health care.
Authorship
Contribution: M.C. designed and performed research, analyzed data, and wrote the paper; L.O. performed research, analyzed data, and wrote the paper; N.A. performed research and wrote the paper; M.S. designed research and wrote the paper; K.M.I. designed research and analyzed data; and J.C. designed research, analyzed data, wrote the paper, and provided administrative support.
Conflict-of-interest disclosure: J.C. received consulting or advisory role fee from Bristol Myers Squibb, Novartis, Pfizer, Bio-Path Holdings Inc, Astellas Pharma, Takeda, Jazz Pharmaceuticals, Menarini, Gilead Sciences, and Centessa Pharmaceuticals; and received research funding from Bristol Myers Squibb (Institution), Novartis (Institution), Pfizer (Institution), Astellas Pharma (Institution), Sun Pharma (Institution), Takeda (Institution), Jazz Pharmaceuticals (Institution), AbbVie/Genentech (Institution), Actuate Therapeutics (Institution), Sellas Life Sciences (Institution), and Bio-Path Holdings Inc (Institution). The remaining authors declare no competing financial interests.
Correspondence: Jorge Cortes, Georgia Cancer Center, 1410 Laney Walker Rd, CN2222, Augusta, GA 30912; e-mail: jorge.cortes@augusta.edu.
References
Author notes
Presented at the 2022 American Society of Oncology annual meeting, Chicago, IL, 6 June 2022.
The data that support the findings of this study are available on request from the corresponding author, Jorge Cortes (jorge.cortes@augusta.edu).
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal