In this issue of Blood, Takahashi et al1 show that higher abatacept exposure decreases the occurrence of acute graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT) from unrelated donors (URDs), without increasing the risk of relapse.
GVHD prevention is still a central issue of allogeneic HSCT, and tremendous efforts to improve GVHD prophylaxis have been made in recent years. Despite the evidence of efficacy of several drugs (ie, posttransplant cyclophosphamide, antithymocyte globulin [ATG], or anti-T-lymphocyte globulin [ATLG], m-TOR inhibitors),2-4 abatacept is the first drug approved by the Food and Drug Administration for the prevention of GVHD5 when given in association with calcineurin inhibitor and methotrexate. Abatacept is a selective costimulation inhibitor that interrupts the T-cell costimulatory signal through the CD28-CD80/CD86 pathway. It is a recombinant protein obtained by fusion of the extracellular domain of the lymphocyte-associated antigen 4 (CTLA-4) with the Fc portion of human immunoglobulin that has been modified to prevent complement fixation and antibody-dependent cellular cytotoxicity. The resulting decrease of CD28-mediated T-cell activation accounts for its prevention of acute GVHD. Accordingly, transcriptomic analysis of the CD4+ compartment showed a deep reprograming of T-cell activation by abatacept that correlates with acute GVHD occurrence and severity.6
The ABA2 study (NCT01743131), a phase 2 double-blind, randomized trial, has already demonstrated a significant reduction of severe acute GVHD and an increase of acute GVHD-free survival in a placebo-controlled study of 8/8-HLA-matched URD transplants comparing calcineurin inhibitor/methotrexate plus abatacept to calcineurin inhibitor/methotrexate plus placebo and a single-arm study (7/8-HLA-mismatched URDs) comparing calcineurin inhibitor/methotrexate plus abatacept versus calcineurin inhibitor/methotrexate controls from the Center for International Blood and Marrow Transplant Research.6 The effect was particularly striking for the 7/8 cohort, which is a high-risk population for GVHD development. The doses in the ABA2 trial used the following schedule: 10 mg/kg on days −1, +5, +14, and +28. This dosing schedule compressed the administration of the doses given in the first 30 days compared with the schedule used for rheumatoid arthritis. This more intensive dosing schedule was also targeted to achieve a higher concentration at the first dose, given on day −1 (Ctrough_1) of >10 μg/mL. However, both in the ABA1 study (NCT01012492)7 and in the ABA2 study, the authors found increased abatacept exposure (ie, higher trough levels) after HSCT compared with patients treated for rheumatoid diseases, raising the question of the optimal dose of abatacept for GVHD prophylaxis, where optimal means maximizing the efficacy and minimizing toxicity.
To answer this question, the hypothesis of a dose-response relationship between abatacept exposure and acute GVHD was studied. This was the goal of the study from Takahashi et al, in which the authors performed a detailed pharmacokinetic (PK) analysis on the 184 patients of the ABA2 study. The results showed a positive exposure-response correlation between abatacept exposure and acute grade 2 to 4 GVHD (with a threshold of Ctrough_1 ≥ 39 μg/mL). The authors also found the lack of association between abatacept exposure with relapse and viral infections, despite the limitation of the trial not being specifically powered to address these questions. Patients receiving abatacept with a Ctrough_1 < 39 μg/mL showed a rate of acute GVHD comparable to those receiving placebo and, concordantly, the immunological reconstitution followed the same slope.
Why do patients receiving abatacept for HSCT achieved higher plasma drug concentrations than patients with rheumatologic diseases? The current belief is that it is related to the expansion of the antigen-presenting cells in the latter group and their relative depletion during the early posttransplant phases in the former.6 However, in the context of the transplant setting, the antigen-presenting cells and B-cell numbers early after abatacept administration were not correlated with PK parameters.
Abatacept also reduced steroid-refractory acute GVHD occurrence, thus limiting the use of secondary immunosuppressive agents. Detailed data on this issue are not available, and further analyses are needed to investigate the impact of abatacept on the exposure to other immunosuppressants. Likewise, why the 7/8 cohort showed higher exposure to abatacept in comparison with 8/8 group is currently not understood. However, it does explain the unexpectedly favorable outcome of the mismatched cohort in this trial.
The relationship between exposure to abatacept and acute GVHD was so cogent and persuasive that the readers cannot help but wonder whether it is already legitimate to escalate the dose in those patients with lower abatacept exposure, with the goal of providing a PK-driven approach for tailored GVHD prophylaxis. My answer is concordant with the authors’ proposal, discouraging dose adjustments in the routine clinical practice but testing this hypothesis in a dedicated trial.
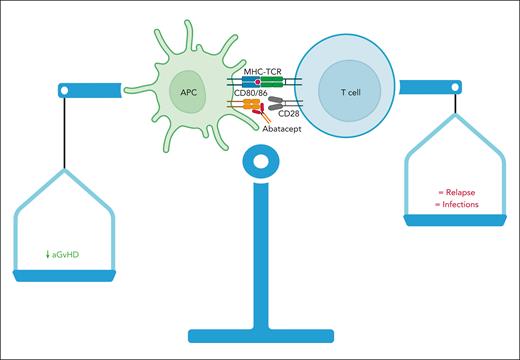
Finally, the article from Takahashi et al does not find any correlation between PK parameters and chronic GVHD, which was not reduced by abatacept in the ABA2 trial. This may be due to the schedule used in the transplantation setting, with intensive but brief administration course, mainly targeted to prevent acute GVHD. GVHD prevention is at the heart of a very delicate balance between graft versus leukemia and GVHD itself. Intensifying GVHD prevention can affect infections, immune reconstitution, and, more importantly, relapse. With the caution due to the limited power of the study, infections and relapse did not increase after abatacept administration, and, even more intriguingly, Takahashi et al found that they were not affected by increased exposure to abatacept. Thus, higher exposure to abatacept minimized the risk of acute GVHD without increasing the undesirable “collateral damage,” such as relapse and viral infections (see figure). Using a drug at inappropriate dosage may increase the risk of missing its great potential. It has already occurred, at least in part, in the history of GVHD prevention (ie, ATLG/ATG) in which optimal dosing is still an open issue.8,9
Abatacept reduces acute GVHD (aGVHD) without increasing relapse and infections. The article from Takahashi et al demonstrates a strong correlation between efficacy (aGVHD reduction) and abatacept exposure but not with relapse and infections. APC, antigen-presenting cell; MHC-TCR, major histocompatibility complex-T-cell receptor.
Abatacept reduces acute GVHD (aGVHD) without increasing relapse and infections. The article from Takahashi et al demonstrates a strong correlation between efficacy (aGVHD reduction) and abatacept exposure but not with relapse and infections. APC, antigen-presenting cell; MHC-TCR, major histocompatibility complex-T-cell receptor.
An ancient aphorism from Paracelsus, a Swiss physician, alchemist and astrologer (1493-1541), says that “alchemy serves to separate the true from the false”: PK analysis and therapeutic drug monitoring should be part of routine transplant care. It is time to do it.
Conflict-of-interest disclosure: F.B. participated in advisory boards and received speaker fees from Neovii, Sanofi, Takeda, Novartis, Kite-Gilead, Celgene, Janssen, Amgen, MSD, Pfizer, and Jazz Pharmaceuticals.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal