In this issue of Blood, Rhee et al1 provide evidence that individual monocytes and their progeny may not be as plastic as previously thought. Instead, their data suggest that the apparent flexibility of monocytes to respond appropriately to diverse stimuli reflects selection of functionally diverse monocyte subtypes that arise from heterogeneous preprogrammed myeloid progenitors (see figure).
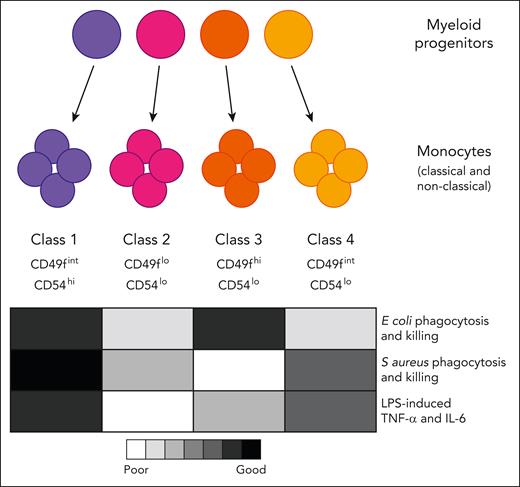
Four functionally specialized subtypes of monocytes arise from distinct myeloid progenitors. Epigenetic preprogramming of the monocytes is evident in the progenitors that produce them. IL-6, interleukin-6; TNF-α, tumor necrosis factor α.
Four functionally specialized subtypes of monocytes arise from distinct myeloid progenitors. Epigenetic preprogramming of the monocytes is evident in the progenitors that produce them. IL-6, interleukin-6; TNF-α, tumor necrosis factor α.
Monocytes and the macrophages they produce have diverse roles and have long been described as functionally plastic, although it is unclear whether this reflects the adaptability of individual cells or a collective property of the whole population. Rhee et al have identified 4 classes of monocytes in mouse and human blood that have specialized functional properties and appear to be independently selected in response to specific challenges.
Using an ER-Hoxb8 fusion construct delivered into mouse bone marrow progenitors, the authors derived monocytes from clones of individual myeloid progenitor cells. Evaluation of the progeny of 25 clones revealed 4 monocyte subtypes with distinct gene expression signatures and functional properties. The monocyte classes differed in their relative capacity for chemotaxis, production of inflammatory mediators following lipopolysaccharide (LPS) stimulation, and phagocytosis and killing of Escherichia coli versus Staphylococcus aureus.
RNA sequencing analysis predicted differentially expressed surface markers that allowed the authors to identify the 4 monocyte classes in both mouse and human blood and to validate their functional differences in primary monocytes in vitro and in vivo. They also showed that upon infection with E coli or S aureus, the monocytes that were best equipped to kill the bacteria accumulated at the infection site.
In vivo differentiation experiments with barcoded progenitors provided additional evidence of the clonal origin of the monocyte subtypes, and in vitro and in vivo analyses demonstrated little or no interconversion between classes during homeostasis or under conditions of inflammatory stress. Instead, comparison of the progenitor clones and their monocyte progeny revealed that the transcriptional signatures of the monocyte subtypes were defined epigenetically in the progenitors they arose from.
Collectively, the data support a model whereby diverse monocyte responses are achieved by selective recruitment, expansion, or activation of functionally specialized monocyte subtypes rather than by functional adaptation of more generic cells responding to different stimuli. Deeper evaluation of the responses of individual monocytes to diverse stimuli will be necessary to define the limits of their preprogramming and the extent to which they may have retained a degree of plasticity, but the insights from this study have broad implications for our understanding of monocyte and macrophage responses to pathogens and other threats.
In light of these findings, it will be important to reexamine other monocyte categorizations to determine how the 4 monocyte classes identified in the current study relate to previously defined subsets. In recent years, it has become apparent that monocyte diversity extends beyond their categorization as classical and nonclassical monocytes,2-4 and notably, Rhee et al detected both classical and nonclassical subsets among all 4 monocyte classes, albeit with differing relative frequencies.
Other recent studies have also suggested that monocyte heterogeneity is, at least in part, defined during their differentiation.2-7 For instance, granulocyte-monocyte progenitors (GMPs) and monocyte-dendritic cell (DC) progenitors (MDPs) independently produce classical monocyte subsets with neutrophil-like and DC-like gene expression signatures, respectively, and monocyte-derived DCs arise from MDPs but not GMPs.7 It will be interesting to determine whether the 4 monocyte subtypes identified in the current study arise via previously defined progenitor pathways (eg, GMP versus MDP) or via as yet unrecognized routes.
The study also casts myeloid progenitors as key players in shaping innate immune responses, which raises the possibility that the balance of myeloid progenitor subsets may contribute to the diverse responses of individuals or the dynamics of monocyte responses over a lifetime. Similarly, understanding the functional heterogeneity of monocytes and the myeloid progenitors that produce them could reveal new candidates for more precise therapeutic targeting to manipulate immune responses.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal