In this real-world evaluation of tafasitamab-lenalidomide (TL) in relapsed or refractory LBCL, patients receiving TL had higher rates of comorbidities and high-risk disease characteristics, and substantially lower progression-free survival and overall survival, compared with the L-MIND registration clinical trial for TL.
TO THE EDITOR:
Combined tafasitamab and lenalidomide (TL) is a standard-of-care therapy for relapsed or refractory large B-cell lymphoma (R/R LBCL) in patients who are not eligible for transplant.1,2 Approval was based on the phase II registration trial of tafasitamab and lenalidomide in diffuse large B-cell lymphoma (L-MIND) (NCT02399085), in which TL therapy resulted in a complete response rate (CRR) of 43% and median progression-free survival (PFS) of 12.1 months.3,4 However, this trial excluded patients with ≥4 prior lines of therapy for LBCL, primary refractory disease, high-risk cytogenetics, prior CD19-directed therapy, and Eastern Cooperative Oncology Group performance status (ECOG PS) ≥ 3. Evaluation of outcomes with TL therapy in real-world populations was needed to assess the generalizability of these findings.
We performed a retrospective study evaluating patients with R/R LBCL treated consecutively with TL at 11 institutions from August 1, 2020, to August 1, 2022. Patients must have had a pathologic diagnosis of LBCL and have received at least 1 dose of TL as standard-of-care therapy. Baseline patient characteristics were collected, and L-MIND trial eligibility was determined for all patients. Because patients with laboratory-based abnormalities suggestive of renal, hepatic, or hematologic dysfunction were still eligible for L-MIND if the abnormalities were due to underlying lymphoma, which could not be reliably determined on review, L-MIND eligibility was defined using 2 sets of criteria, 1 including laboratory values and 1 omitting laboratory values. Primary refractoriness as a risk factor and exclusion criterion was defined as per the L-MIND study (supplemental appendix, available on the Blood website). This study was approved by the institutional review boards of all 11 participating institutions.
The primary outcome of interest was PFS, defined as the time from the first dose of tafasitamab to the earliest documented disease progression or death. Other outcomes of interest after receiving TL included overall response rate (ORR), CRR, and overall survival (OS). Responses were determined using imaging modalities and schedules per local institutional practices, as determined by radiology reports and oncologist notes; evaluation per Lugano criteria was the standard practice.5 Data on drug administration and clinically significant adverse events (AEs) were collected, defined as events resulting in delay, discontinuation, or dose reduction of TL, hospitalization, or death. Biostatistical analysis is summarized in the supplemental appendix.
A total of 178 patients received TL for R/R LBCL and were included. Baseline patient characteristics were compared with those in the L-MIND trial (Table 1). Of 149 patients evaluable for eligibility, most did not meet L-MIND eligibility criteria; with laboratory values included, 131 (89%) were ineligible, and 116 (78%) were still ineligible if laboratory values were not considered.
Characteristics of patients in the present series and those treated in the L-MIND study for reference
| Characteristic . | Real world . | L-MIND . |
|---|---|---|
| Number of patients | 178 | 81 |
| Female sex | 87 (49) | 37 (46) |
| Age (y), median (range) | 75 (26-94) | 72 (41-86) |
| Race | ||
| White, all ethnicity | 161 (90) | 72 (89) |
| Asian | 9 (5) | 2 (2) |
| Other or unknown | 8 (4) | 1 (1) |
| Diagnosis | ||
| DLBCL-NOS | 96 (54) | 72 (89) |
| Transformed indolent lymphoma | 59 (33) | 7 (9) |
| HGBCL (nontransformed) | 19 (11) | 2 (2) |
| Other∗ | 4 (2) | 0 (0) |
| Cell of origin by immunohistochemistry | ||
| GCB | 100 (56) | 38 (47) |
| Non-GCB | 62 (35) | 21 (26) |
| Unknown | 16 (9) | 22 (27) |
| Risk (IPI) | ||
| 0-2 | 43 (27) | 40 (49) |
| 3-5 | 114 (73) | 41 (51) |
| Ann Arbor stage | ||
| I-II | 20 (12) | 20 (25) |
| III-IV | 149 (88) | 61 (75) |
| Prior lines of therapy for DLBCL | ||
| Median (range) | 2 (0-11) | 2 (1-4) |
| 0† | 12 (7) | 0 (0) |
| 1 | 49 (28) | 40 (49) |
| 2 | 49 (28) | 35 (43) |
| 3 | 27 (15) | 5 (6) |
| 4 | 13 (7) | 1 (1) |
| ≥5 | 28 (16) | 0 (0) |
| Primary refractory (progression within 6 months of first-line therapy) | 87 (49) | 15 (18) |
| Refractory to last therapy | 118 (67) | 36 (44) |
| Prior SCT | 23 (13) | 9 (11) |
| Prior CAR-T | 52 (30) | 0 |
| L-MIND eligible (not considering laboratory values) | 33 (22) | - |
| L-MIND eligible (including laboratory values) | 16 (11) | - |
| Disease features for L-MIND exclusion | ||
| Primary refractory (progression within 3 months of first-line therapy) | 63 (37) | - |
| Prior CAR-T | 52 (30) | - |
| 4 or more prior lines of therapy for DLBCL | 41 (23) | - |
| Prior lenalidomide or IMiD | 22 (13) | - |
| HGBCL with double or triple hit cytogenetic | 28 (17) | - |
| History of CNS disease | 16 (9) | - |
| ECOG PS > 2 | 21 (13) | - |
| Other characteristics for L-MIND exclusion | ||
| Liver function test or blood count abnormalities | 53 (31) | - |
| Inadequate renal function (EGFR < 60) | 51 (30) | - |
| Other malignancy within 5 y | 29 (16) | - |
| Hepatitis B | 3 (2) | - |
| Hepatitis C | 2 (1) | - |
| HIV | 1 (1) | - |
| Characteristic . | Real world . | L-MIND . |
|---|---|---|
| Number of patients | 178 | 81 |
| Female sex | 87 (49) | 37 (46) |
| Age (y), median (range) | 75 (26-94) | 72 (41-86) |
| Race | ||
| White, all ethnicity | 161 (90) | 72 (89) |
| Asian | 9 (5) | 2 (2) |
| Other or unknown | 8 (4) | 1 (1) |
| Diagnosis | ||
| DLBCL-NOS | 96 (54) | 72 (89) |
| Transformed indolent lymphoma | 59 (33) | 7 (9) |
| HGBCL (nontransformed) | 19 (11) | 2 (2) |
| Other∗ | 4 (2) | 0 (0) |
| Cell of origin by immunohistochemistry | ||
| GCB | 100 (56) | 38 (47) |
| Non-GCB | 62 (35) | 21 (26) |
| Unknown | 16 (9) | 22 (27) |
| Risk (IPI) | ||
| 0-2 | 43 (27) | 40 (49) |
| 3-5 | 114 (73) | 41 (51) |
| Ann Arbor stage | ||
| I-II | 20 (12) | 20 (25) |
| III-IV | 149 (88) | 61 (75) |
| Prior lines of therapy for DLBCL | ||
| Median (range) | 2 (0-11) | 2 (1-4) |
| 0† | 12 (7) | 0 (0) |
| 1 | 49 (28) | 40 (49) |
| 2 | 49 (28) | 35 (43) |
| 3 | 27 (15) | 5 (6) |
| 4 | 13 (7) | 1 (1) |
| ≥5 | 28 (16) | 0 (0) |
| Primary refractory (progression within 6 months of first-line therapy) | 87 (49) | 15 (18) |
| Refractory to last therapy | 118 (67) | 36 (44) |
| Prior SCT | 23 (13) | 9 (11) |
| Prior CAR-T | 52 (30) | 0 |
| L-MIND eligible (not considering laboratory values) | 33 (22) | - |
| L-MIND eligible (including laboratory values) | 16 (11) | - |
| Disease features for L-MIND exclusion | ||
| Primary refractory (progression within 3 months of first-line therapy) | 63 (37) | - |
| Prior CAR-T | 52 (30) | - |
| 4 or more prior lines of therapy for DLBCL | 41 (23) | - |
| Prior lenalidomide or IMiD | 22 (13) | - |
| HGBCL with double or triple hit cytogenetic | 28 (17) | - |
| History of CNS disease | 16 (9) | - |
| ECOG PS > 2 | 21 (13) | - |
| Other characteristics for L-MIND exclusion | ||
| Liver function test or blood count abnormalities | 53 (31) | - |
| Inadequate renal function (EGFR < 60) | 51 (30) | - |
| Other malignancy within 5 y | 29 (16) | - |
| Hepatitis B | 3 (2) | - |
| Hepatitis C | 2 (1) | - |
| HIV | 1 (1) | - |
Values are represented as number (%) unless otherwise stated.
CNS, central nervous system; EGFR, estimated glomerular filtration rate; GCB, germinal center B-cell; HGBCL, high-grade B cell lymphoma; IMiD, immunomodulatory drug; IPI, international prognostic index; NOS, not otherwise specified; SCT, stem cell transplantation.
Other diagnoses: T-cell histiocyte-rich B-cell lymphoma (2), primary mediastinal B-cell lymphoma (2), posttransplant lymphoproliferative disorder with DLBCL morphology (1).
Twelve patients with transformed indolent lymphoma had received prior therapy for indolent disease but not for aggressive LBCL.
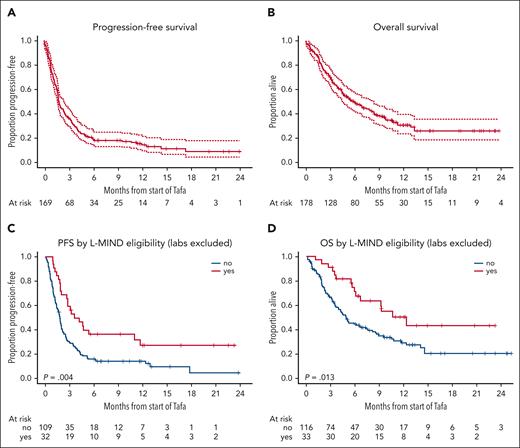
At a median time to censoring of 12 months,6 the primary end point, median PFS, was 1.9 months, with a 95% confidence interval (CI) of 1.7 to 2.6 months (Figure 1). Median OS was 6.5 months (95% CI 5.0-9.1). Best ORR was 31% (95% CI 24-39), with CRR of 19% (95% CI 13-26). With laboratory criteria omitted, L-MIND trial-eligible patients (n = 33) had a significantly improved PFS (hazard ratio [HR] 0.51, 95% CI 0.32-0.81) and OS (HR 0.50, 95% CI 0.29-0.87).
Survival after TL therapy. PFS (A) and OS (B) after TL initiation for all patients receiving TL off-trial. Progression-free (C) and overall survival (D) stratified by L-MIND eligibility, excluding laboratory-based markers of renal, hepatic, and hematologic function. Tafa, tafasitamab.
Survival after TL therapy. PFS (A) and OS (B) after TL initiation for all patients receiving TL off-trial. Progression-free (C) and overall survival (D) stratified by L-MIND eligibility, excluding laboratory-based markers of renal, hepatic, and hematologic function. Tafa, tafasitamab.
Subgroup analysis of PFS (see forest plot in supplemental Figure 1) demonstrated a higher risk of progression in patients with age < 70, worse ECOG PS, lactate dehydrogenase elevation, higher international prognostic index, more than 2 prior lines of therapy for LBCL, prior chimeric antigen receptor T-cell therapy (CAR-T), primary refractory disease, and refractory disease to last therapy. On multivariate analysis (supplemental Table 1), factors significantly associated with inferior PFS included higher ECOG PS (PS 2 vs 0-1, HR 1.76, P = .014; PS 3-4 vs 0-1, HR 2.79, P < .001), lactate dehydrogenase elevation (HR 1.58, P = .034), and refractoriness to last therapy, defined as less than CR or relapse within 6 months (HR 1.78, P = .017). Patient age ≥ 70 was independently associated with lower risk of progression (HR 0.56, P = .019). Prior CAR-T exposure was associated with increased risk for progression on univariate analysis (HR 1.46, P = .043) but not multivariate analysis (HR 1.04, P = .89).
Lenalidomide initiation delays occurred in 48% of patients; in patients with delays, median time between first tafasitamab and first lenalidomide was 7 days (range 1-84). Lenalidomide delays were not associated with inferior PFS (HR 1.05, P = .78). Lenalidomide dose reductions at initiation occurred in 107 of 169 (63%) patients and were not associated with inferior PFS (HR 1.09, P = .63). In patients with dose reductions, median starting dose was 15 mg/d (range, 2.5-20). Reasons for initial dose reduction included poor performance status (38%), renal dysfunction (30%), and cytopenias (6%).
Of all patients, 156 had safety data available, of whom 54% had at least 1 clinically significant AE during treatment (supplemental Table 2). The most common clinically significant AEs were hematologic in nature, including neutropenia (26%), thrombocytopenia (15%), and anemia (14%). Febrile neutropenia occurred in 7% of patients. Discontinuation of TL for toxicity occurred in 14%.
At follow-up, 162 of 178 patients had discontinued therapy, most commonly for progression of disease (75%), toxicity (14%), and death (3%). A total of 108 deaths were reported; lymphoma progression was the most common cause of death (80%) followed by unknown (10%) and unrelated causes (10%).
In this real-world study of TL in R/R LBCL, key clinical outcomes including ORR, CRR, PFS, and OS were markedly lower than observed in the L-MIND registration trial. These differences may be related to the restrictive selection criteria of the L-MIND clinical trial, with lower-risk disease features and fewer comorbidities than those observed in real-world patients. Other real-world studies of TL have now been presented with similar findings, with a reported ORR of 33% to 41% and median OS 3.0 to 6.3 months.7-9
Rates of significant toxicities did not appear to differ significantly from those reported in L-MIND, despite more comorbidities and high-risk features. This may be due to dose reductions of lenalidomide at initiation (seen in 63%), earlier discontinuation of TL for disease progression, or underreporting due to retrospective collection of toxicity data, with certain AE characteristics, such as transfusion requirement, unable to be collected.
An unusual finding was a significant association of older age with prolonged PFS. This may reflect unmeasured factors of disease severity in younger patients or favorable characteristics in elderly patients. Patients over 70 were more likely to meet all non-laboratory-based L-MIND criteria than those under 70 (25% vs 13%, respectively). Alternatively, there may be biological differences in LBCL in elderly patients that increase sensitivity to tafasitamab- or lenalidomide-based therapy.10,11
This study is limited by its retrospective and multicenter design, with heterogeneity of therapies and response assessments. Participating institutions were tertiary centers, which may bias the patient population. Additionally, patients who did not receive lenalidomide were excluded, given the limited activity of tafasitamab monotherapy in DLBCL,12 which may result in immortal time bias for patients receiving delayed lenalidomide.
In conclusion, this real-world study demonstrates lower PFS and OS after TL therapy than observed in the pivotal L-MIND clinical trial, with more high-risk disease features and comorbidities observed in this population. These data suggest that TL is reasonable for patients meeting eligibility criteria for the L-MIND clinical trial; however, patients not meeting eligibility criteria, and particularly those with high-risk features, such as refractory disease to initial therapy or last therapy, are poor candidates for TL, and alternatives should be considered. This study highlights the risks associated with extrapolating results from nonrandomized studies with restrictive eligibility criteria, in which the generalizability of efficacy data may be limited. Greater emphasis should also be made to develop phase 2 studies in lymphoma with more inclusive criteria that reflect the population of interest.
Acknowledgments
D.A.Q. has received training support and funding from the Lymphoma Research Foundation.
This research was funded in part by the National Institutes of Health/National Cancer Institute cancer center support grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center.
Authorship
Contribution: D.A.Q. wrote the manuscript; D.A.Q., M.J.B., C.L.B., and G.S. conceived and designed the study; D.A.Q., N.L., P.F.C., M.M., P.P., R.C.G., D.A.B., G.T.W., J.R., B.A., L.L., L.J.N., J.L.C., J.S.A., A.K., G.S.N., K.M., S.C.R., and B.K. performed chart review and data collection; M.O. built data collection forms; V.S. performed data analysis; and all authors provided important feedback and reviewed the final manuscript.
Conflict-of-interest disclosure: P.F.C. has received research funding from ADC Therapeutics, Genentech, and AbbVie and honoraria for participation in advisory boards/consulting from ADC Therapeutics, Beigene, BMS, Genentech, Kite, Lilly, MEI Pharma, Novartis, and Takeda. D.A.B. has received research funding from Incyte/Morphosys, Novartis, and Nurix Therapeutics and honoraria for consulting from SeaGen, Kite/Gilead, Nurix Therapeutics, and Novartis. L.L. has received honoraria for consulting and/or participation in speaker bureau from Kite/Gilead, Beigene, Pharmacyclics, AbbVie, Genmab, SeaGen, Janssen, AstraZeneca, Eli Lilly, Epizyme, TG Therapeutics, Merck, and ADC Therapeutics. L.J.N. has received honoraria for participation in advisory boards/consulting from AbbVie, ADC Therapeutics, Atara Biotherapeutics, BMS/Celgene, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech/Roche, Genmab, Janssen, Incyte, Morphosys, Novartis, and Takeda and research support from BMS/Celgene, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech/Roche, Genmab, Gilead/Kite, IGM Biosciences, Janssen, Novartis, and Takeda. J.L.C. has received research support (to institution) from Genentech, Merck, Bayer, and AbbVie and honoraria for consulting from ADC Therapeutics, Kite Pharma, Genmab, and Incyte/MorphoSys. J.S.A. has received honoraria for consulting from AbbVie, AstraZeneca, BeiGene, BMS, Caribou Biosciences, Cellectar, Century Therapeutics, Epizyme, Genentech, Genmab, Incyte, Interius, Janssen, Kite Pharma, Kymera, Lilly, MorphoSys, Mustang Bio, Ono Pharma, Regeneron, and Takeda and research support (to institution) from BMS, Cellectis, Merck, Mustang Bio, and Seattle Genetics. G.S.N. has received honoraria for consultancy from AbbVie, ADC Therapeutics, Bantam Pharmaceutical LLC, Blueprint Medicines, Bristol Myers Squibb, Celgene Corporation, Curis, Debiopharm, F. Hoffmann-La Roche Limited, Fate Therapeutics, Genentech, Incyte, Karyopharm Therapeutics, Kite Pharma, Kymera Therapeutics, MEI Pharma, MorphoSys AG, Ryvu Therapeutics, Seagen, Selvita Inc, TG Therapeutics, and Zai Lab Limited and research funding from Bristol Myers Squibb and MorphoSys and is a member on a board of directors or advisory committee for Karyopharm Therapeutics, Ryvu Therapeutics, and Fate Therapeutics. K.M. has received research funding from Pharmacyclics, Novartis, Merck, BMS, and Celgene and honoraria for consulting from AbbVie, ADC Therapeutics, AstraZeneca, BMS, Celgene, Gilead/Kite, GenMab, Genentech, Incyte, Janssen, Merck, Morphosys, Lilly, and Pharmacyclics. S.C.R. has received research funding from Constellation, Karyopharm, and Genentech/Roche and honoraria for consulting from ADC, Genmab, Karyopharm, Kite, and Seagen. B.K. has received honoraria for consulting from AbbVie, AstraZeneca, ADCT, BeiGene, BMS, Genentech, Genmab, Gilead, Janssen, and Lilly and research funding from Genentech, ADCT, AstraZeneca, and BeiGene. C.L.B. is currently employed at Roche/Genentech; participation in the study occurred prior to employment at Roche/Genentech. G.S. has received, in the last 12 months, financial compensation for participating in advisory boards or consulting from AbbVie, ATB Therapeutics, Beigene, BMS/Celgene, Debiopharm, Genentech/Roche, Genmab, Incyte, Ipsen, Janssen, Kite/Gilead, Loxo/Lilly, Merck, Molecular Partners, Nordic Nanovector, Novartis, Nurix, and Orna; is shareholder of Owkin; and has received research support managed by his institution from Genentech, Janssen and Ipsen. The remaining authors declare no competing financial interests.
The current affiliation for C.L.B. is Roche/Genentech, San Francisco, CA.
Correspondence: Gilles Salles, Memorial Sloan Kettering Cancer Center, 1275 York St, New York, NY 10065; email: sallesg@mskcc.org.
References
Author notes
Data are available on request from the corresponding author, Gilles Salles (sallesg@mskcc.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal