Visual Abstract
The myelodysplastic syndromes (MDSs) constitute a profoundly heterogeneous myeloid malignancy with a common origin in the hemopoietic stem cell compartment. Consequently, patient management and treatment are as heterogeneous. Decision-making includes identifying risk, symptoms, and options for an individual and conducting a risk-benefit analysis. The only potential cure is allogeneic stem cell transplantation, and albeit the fraction of patients with MDS who undergo transplant increase over time because of better management and increased donor availability, a majority are not eligible for this intervention. Current challenges encompass to decrease the relapse risk, the main cause of hematopoietic stem cell transplantation failure. Hypomethylating agents (HMAs) constitute firstline treatment for higher-risk MDSs. Combinations with other drugs as firstline treatment has, to date, not proven more efficacious than monotherapy, although combinations approved for acute myeloid leukemia, including venetoclax, are under evaluation and often used as rescue treatment. The treatment goal for lower-risk MDS is to improve cytopenia, mainly anemia, quality of life, and, possibly, overall survival. Erythropoiesis-stimulating agents (ESAs) constitute firstline treatment for anemia and have better and more durable responses if initiated before the onset of a permanent transfusion need. Treatment in case of ESA failure or ineligibility should be tailored to the main disease mechanism: immunosuppression for hypoplastic MDS without high-risk genetics, lenalidomide for low-risk del(5q) MDS, and luspatercept for MDS with ring sideroblasts. Approved therapeutic options are still scarcer for MDS than for most other hematologic malignancies. Better tools to match disease biology with treatment, that is, applied precision medicines are needed to improve patient outcome.
Patient assessment and goal of treatment
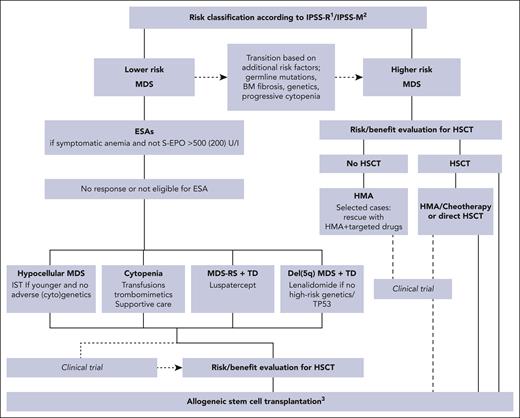
Patients with myelodysplastic syndromes (MDSs) present with a variety of disease manifestations, ranging from indolent to severe, and with a high propensity for progression over time. The most common cytopenia is anemia, usually resulting in long-term transfusion dependency (TD), impaired quality of life, and increased morbidity.1 The genetic background of the myelodysplastic tumor cells as well as their interaction with the germ line genome and the bone marrow microenvironment is profoundly heterogeneous, so understanding biological factors involved in clinical stability and disease progression is key for managing the disease.2-4 The diagnostic procedure involves World Health Organization (WHO) and/or the International Concensus Classification (ICC), both published in 20225,6 and has been discussed in detail by Hasserjian et al.7 In addition, outside the scope of this review are the clinical implications of clonal hemopoiesis of indeterminate potential.8 One difference between the 2 classifications is that ICC defines patients with ≥10% marrow blasts as MDS/acute myeloid leukemia (AML), whereas WHO still uses the term MDS for such patients. With new therapies becoming more targeted and less governed by exact bone marrow blast percentages, the ICC approach could facilitate approval of drugs that have mainly been investigated for patients with AML and with higher-risk MDS by the authorities. The introduction of the prognostically superior International Prognostic Scoring System-Molecular (IPSS-M) risk stratification system, reviewed in this series,9 challenges current treatment recommendations that are usually based on clinical trials using IPSS or International Prognostic Scoring System-Revised (IPSS-R) as inclusion criteria. IPSS-M, with its increased weight of molecular information, usually shifts patients from lower-risk to higher-risk categories, which means that recommendations for azacytidine licensing based on a pivotal study using the IPSS system need to be reconsidered. The challenge to adapt recommendations to the IPSS-M categories will remain for a while because patients included in earlier studies usually were not investigated with sequencing techniques allowing for translation to IPSS-M.4 The consequences of updated risk scores and molecular profiles will also differ between therapies, such as azacytidine, erythropoietin, and hematopoietic stem cell transplantation (HSCT). In other words, the border between lower- and higher-risk MDS has become less stringent with a more personalized risk assessment. To address these issues, management and treatment of MDS should start with a thorough diagnostic and prognostic assessment preferably getting information from several scoring systems. An in-depth analysis of the individual’s symptoms, physical resources, and preferences is also important. It is highly recommended that all categories of patients eligible for any intervention should be discussed at multiprofessional conferences and include as many competence issues as possible (Figure 1).4-6
Treatment algorithm for MDS.1IPSS-R lower-risk: very low and low; higher-risk: intermediate, high, and very high. 2IPSS-M lower-risk: very low, low, and moderate low; higher-risk: moderate high, high, and very high. 3Data from HLA-matched related and unrelated donor, and if not available, from alternative donors such as haploidentical, mismatched unrelated or cord blood after RIC or myeloablative conditioning regimens. BM, bone marrow; IST, immunosuppressive treatment, S-EPO, serum erythropoietin.
Treatment algorithm for MDS.1IPSS-R lower-risk: very low and low; higher-risk: intermediate, high, and very high. 2IPSS-M lower-risk: very low, low, and moderate low; higher-risk: moderate high, high, and very high. 3Data from HLA-matched related and unrelated donor, and if not available, from alternative donors such as haploidentical, mismatched unrelated or cord blood after RIC or myeloablative conditioning regimens. BM, bone marrow; IST, immunosuppressive treatment, S-EPO, serum erythropoietin.
Initial decision-making
MDS is a blood cancer with an overall poor prognosis. Patients with IPSS-M status moderate high to very high show a median survival of ∼1.5 years, whereas patients with moderate low and low risk show an overall survival (OS) of 4.5 and 6 years, respectively. Only those in the very low-risk IPSS-M category have a median survival “not reached” at 10 years. This means that therapeutic approaches aiming for prolonged survival in addition to improved quality of life are important for most patients. All MDS variants share a common origin in the hematopoietic stem and progenitor cell compartment, with the consequence that the only potential cure, currently, is allogeneic HSCT (allo-HSCT). With a median age of close to 75 years, comorbidities and age-related frailty contribute largely to the ability to tolerate various treatment alternatives and most patients will not be eligible for this treatment. However, considering the usually poor prognosis, indications and eligibility for HSCT should always be explored early in the course of disease (Figure 1). The nation-wide Swedish Cancer Registry for MDS includes “intention to HSCT” as part of the diagnostic report; 29% of patients aged between 18 and 74 years planned to undergo allo-HSCT, and two-thirds of these actually underwent transplantation.10 Reasons for failure to reach HSCT were progressive and refractory disease, no donor, comorbidities, and infectious complications. Transplant-eligible patients with a low-risk profile may not be eligible for immediate HSCT at diagnosis but should be subject to a more intense surveillance program in order to identify the disease progression before missing the optimal time point for HSCT.
Patients who are not candidates for immediate HSCT planning will be subject for therapeutic decision-making based on symptoms, risk profile, and disease biology, which will be further discussed later in the article. Table 1 lists therapeutic alternatives for patients with MDS as well as differences between continents.
Therapeutic options for MDS in 2023, licensed or recommended according to guidelines (amended)
| Procedure/treatment option United Sates: formally licensed US EU: formally licensed EU . | Lower-risk MDS . | Higher-risk MDS . | MDS-RS . | Del(5q) MDS . | Comment . |
|---|---|---|---|---|---|
| Transfusion therapy | Yes | Yes | Yes | Yes | Indicated for symptomatic anemia |
| Chelation therapy in TD patients∗ | Yes | If planned HSCT | Yes | Yes | If estimated survival >1-2 y ∗US and ∗EU |
| ESAs | Yes US and EU | Rarely | Yes US and EU | Yes US and EU | Firstline treatment for symptomatic anemia of all lower-risk MDSs. Poor response rate if S-EPO >500 (200) U/L. |
| Trombomimetics | If severe thrombocytopenia | No | No | No | Not licensed for MDS but can be used as in immune-mediated thrombocytopenia |
| Luspatercept | Yes US | No | Yes US, EU | No | Currently licensed only for MDS-RS; recent phase 3 study shows efficacy in other lower-risk MDSs as well |
| Immunosuppressive treatment | Occasionally | No | No | No | Younger patients; severe pancytopenia and hypo/normoplastic BM without high-risk genetics |
| Lenalidomide | No | No | No | Yes | Licensed for TD del(5q) MDS, at ESA failure/ineligibility |
| HMAs | Yes US | Yes US and EU | No | No | Could be used in LR-MDS if aggravating cytopenia or progression |
| Aza | Yes US | Yes US and EU | No | Yes US | Firstline treatment for higher-risk MDS. In United States also approved for lower-risk MDS. |
| Decitabine | Yes (IPSS ≥ INT-1) US | Yes US | No | Yes (IPSS ≥ INT-1) US | Licensed for AML in the EU Licensed for AML and MDS in the US |
| Oral decitabine/cedazuridine | Yes (IPSS ≥ INT-1) US | Yes (IPSS ≥ INT-1) | No | Yes (IPSS ≥ INT-1) US | Not inferior to HMA in randomized studies |
| HMA + venetoclax | No | See comment | No | No | Licensed for AML only. Phase 3 study for MDS is pending. Caution if used as a rescue treatment for MDS: toxicity is usually higher than in AML. |
| Targeted AML drugs (IDH1 + 2 inhibitors) | No | See comment | No | No | Not licensed for MDS. In clinical trials including AML and MDS. For other targeted drugs, see text. |
| Chemotherapy | No | See comment | No | No | Inferior to aza (not tested for CPX). May be relevant for MDS with AML-like genetics and as bridging therapy to allo-HSCT. |
| Allo-HSCT | If adverse risk profile | Yes | Occasionally, if refractory TD | If refractory TD or high-risk genetics | Complex decision making involving MDS risk, comorbidities, and patients’ preferences |
| Procedure/treatment option United Sates: formally licensed US EU: formally licensed EU . | Lower-risk MDS . | Higher-risk MDS . | MDS-RS . | Del(5q) MDS . | Comment . |
|---|---|---|---|---|---|
| Transfusion therapy | Yes | Yes | Yes | Yes | Indicated for symptomatic anemia |
| Chelation therapy in TD patients∗ | Yes | If planned HSCT | Yes | Yes | If estimated survival >1-2 y ∗US and ∗EU |
| ESAs | Yes US and EU | Rarely | Yes US and EU | Yes US and EU | Firstline treatment for symptomatic anemia of all lower-risk MDSs. Poor response rate if S-EPO >500 (200) U/L. |
| Trombomimetics | If severe thrombocytopenia | No | No | No | Not licensed for MDS but can be used as in immune-mediated thrombocytopenia |
| Luspatercept | Yes US | No | Yes US, EU | No | Currently licensed only for MDS-RS; recent phase 3 study shows efficacy in other lower-risk MDSs as well |
| Immunosuppressive treatment | Occasionally | No | No | No | Younger patients; severe pancytopenia and hypo/normoplastic BM without high-risk genetics |
| Lenalidomide | No | No | No | Yes | Licensed for TD del(5q) MDS, at ESA failure/ineligibility |
| HMAs | Yes US | Yes US and EU | No | No | Could be used in LR-MDS if aggravating cytopenia or progression |
| Aza | Yes US | Yes US and EU | No | Yes US | Firstline treatment for higher-risk MDS. In United States also approved for lower-risk MDS. |
| Decitabine | Yes (IPSS ≥ INT-1) US | Yes US | No | Yes (IPSS ≥ INT-1) US | Licensed for AML in the EU Licensed for AML and MDS in the US |
| Oral decitabine/cedazuridine | Yes (IPSS ≥ INT-1) US | Yes (IPSS ≥ INT-1) | No | Yes (IPSS ≥ INT-1) US | Not inferior to HMA in randomized studies |
| HMA + venetoclax | No | See comment | No | No | Licensed for AML only. Phase 3 study for MDS is pending. Caution if used as a rescue treatment for MDS: toxicity is usually higher than in AML. |
| Targeted AML drugs (IDH1 + 2 inhibitors) | No | See comment | No | No | Not licensed for MDS. In clinical trials including AML and MDS. For other targeted drugs, see text. |
| Chemotherapy | No | See comment | No | No | Inferior to aza (not tested for CPX). May be relevant for MDS with AML-like genetics and as bridging therapy to allo-HSCT. |
| Allo-HSCT | If adverse risk profile | Yes | Occasionally, if refractory TD | If refractory TD or high-risk genetics | Complex decision making involving MDS risk, comorbidities, and patients’ preferences |
BM, bone marrow; EU, European Union; INT, intermediate; LR-MDS, low-risk MDS; US, United States.
Licensed for treatment of chronic iron overload, irrespective of diagnosis. Variation in licensing patterns between countries.
Supportive care
Supportive care is a cornerstone in the management of all patients and consists of active disease surveillance, transfusion therapy, and other aspects of dealing with cytopenia. Population-based studies show that ∼50% of newly diagnosed patients require red blood cell (RBC) transfusions and that anemia progresses in the majority of untreated patients.11,12 Both need for RBC transfusion at diagnosis and transfusion intensity over the course of disease are associated with impaired survival, quality of life, and progression-free survival, the latter suggesting an association with more advanced disease biology.1,13,14 Clinical studies indicate that higher transfusion targets are associated with improved quality of life,15 so long-term transfusion therapy should be tailored to individual symptoms and comorbidities and not primarily to hemoglobin (Hb) levels. A preliminary reported large epidemiological study shows that transfusion need may also be a strong time-dependent prognostic marker. Irrespective of IPSS-M risk group, patients without transfusion at landmarks 8 and 12 months after diagnosis, either spontaneously or after routine firstline treatment, had a significantly better survival than those with continuous TD at those landmarks.16 Considering that long-term transfusion therapy may be the only treatment for several years and occasionally decades, only filtered blood products should be administered to reduce the risk of developing immunization.
Approximately 40% of the patients have platelet counts <100 × 109/L, but only 5% require platelet transfusions at diagnosis.11 Severe bleeding symptoms are rare at platelet counts >20 × 100 × 109/L to 30 × 100 × 109/L but with individual differences. However, symptomatic thrombocytopenia becomes more frequent with the course of disease and is common during treatment with chemotherapy and hypomethylating agents (HMAs). Consensus-based guidelines agree that platelet transfusions should be governed by trigger levels during active treatment but mainly be based on bleeding symptoms in case of chronic thrombocytopenia (https://mds-europe.org/management). Other protective measurements, such as granulocyte colony-stimulating factor (G-CSF) and infectious prophylaxis, are not specific to MDS and should be administered as per local and national guidelines.
Iron chelation therapy
It is well-known from the field of hemoglobinopathies that iron overload escalating from childhood impairs organ function and shortens life span. By contrast, the exact degree of iron overload that will lead to morbidity and influence outcome in adult patients with MDS is less well understood. Recent studies indicate that the level of nontransferrin-bound iron is more important for organ damage than the actual body iron storage and that, in particular, hyperplastic ineffective erythropoiesis such as in SF3B1-mutated MDS with ring sideroblasts (MDS-RS) may increase the risk of iron overload–related morbidities.17
Retrospective analyses suggest that iron chelation therapy is associated with improved outcome among patients with lower-risk MDS, with the caveat that the physician’s impression of the expected survival of the patient might have influenced treatment decisions. One prospective randomized phase 2 study compared deferasirox vs placebo, showing improved event-free survival among patients in the deferasirox group, with events defined as death, transformation, and cardiac or hepatic abnormalities. The study was not designed to evaluate the effect on OS.18 Moreover, a propensity score–matched analysis of a large prospective noninterventional registry of patients with lower-risk MDS showed that patients exposed to treatment with iron chelation had a better OS.19 Because iron overload increases the risk of nonrelapse mortality after allogeneic stem cell transplantation, chelation therapy should be started if allogeneic stem cell transplantation is considered in the treatment course; however, this should not postpone the transplantation. If time for a proper chelation is too short, low-dose deferasirox can be administered during conditioning therapy to reduce labile plasma iron20,21 To date, 3 drugs are approved for treating iron overload, with labels varying according to the country. A pragmatic view adopted by the European and National Comprehensive Cancer Nerwork (NCCN) guidelines is that iron chelation is indicated for lower-risk MDS with a permanent transfusion need but with otherwise favorable outcome and for patients eligible for allogeneic stem cell transplantation. The drugs used for iron chelation are currently deferasirox (oral), deferioxamine (IV or subcutaneous via infusion pump), and deferiprone (oral). Most guidelines recommend starting with deferasirox and continuing if there is adequate efficacy and acceptable toxicity. However, in countries where the use of implantable venous-access devices is common, parenteral use of desferioxamine can also provide an effective alternative without any side effects other than the practical aspects of having continuous infusion. Different chelating agents can usually be combined without problems.
Criteria for response to treatment
Uniform criteria for response to interventional treatment have been developed to allow for comparison of results from clinical trials.22-24 Criteria for lower-risk MDS usually require that patients have Hb levels below 10 g/dL to be eligible for a trial aiming for improved Hb levels. Moreover, only erythrocyte transfusions administered at Hb levels below 9 g/dL are recognized as administered per protocol. However, subjects in clinical trials differ from real-world patients in many aspects, and every hematologist knows that symptoms caused by anemia worsen with aging, particularly in case of cardiovascular comorbidity. This knowledge has led to the recognition of symptomatic anemia as a reason to initiate intervention in clinical praxis by both NCCN and MDS Europe guidelines. As for patients with severe hemoglobinopathies, patients with refractory transfusion-dependent MDS should live as well as possible with their disease and not just survive (www.mds-europe.eu; Blum and Malcovati25).
Growth factor treatment
Erythropoiesis-stimulating agents (ESAs) have been used for treating anemia of lower-risk MDS for >3 decades and constitute firstline treatment according to international guidelines. Many phase 2 studies have shown that ESAs improve hemoglobin levels in >50% of treated patients and are, provided supranormal Hb levels are avoided, without side effects. Two randomized placebo-controlled studies including patients at lower risk with no or moderate transfusion need demonstrated significant effects on Hb levels and time to RBC TD by epoetin-α and darbepoetin, respectively.26,27 However, because these studies were not designed to assess OS, the pattern of national reimbursement varies from not reimbursed at all or reimbursed only for transfusion-dependent patients to reimbursement available also for patients with symptomatic anemia. The question whether ESAs affect OS has instead been addressed by other types of studies. A comparison between Nordic and Italian patients with lower-risk MDS, the latter without access to ESAs at the time, showed improved OS for patients with no or low transfusion need treated with ESAs.28 A recent systematic review concludes that ESA treatment, besides increased Hb levels, is associated with improved quality of life and OS and that low or moderate TD and low S-erythropoietin levels predict for a response to treatment.29 The large prospective EU MDS registry enrolled patients with IPSS-R status very low– to intermediate-risk MDS at diagnosis and records treatment given according to clinical routine, depending on whether patients are exposed to ESA treatment based on national legislation and therapeutic tradition. An analysis based on 1696 patients from 17 countries showed that ESA treatment significantly prolonged the time to first transfusion when initiated before or early after the onset of permanent TD.30 Importantly, propensity score–matched analysis comparing patients exposed and unexposed to ESA, irrespective of the response or duration of treatment, showed a tendency of improved survival in the ESA-exposed group (P = .07).30 A preliminary report update of this analysis based on 2448 patients (ESA untreated, n = 1265 and ESA treated, n = 1183) showed that the median OS from reaching the eligibility criteria in the ESA-exposed vs -unexposed groups were 44.9 and 34.8 months, respectively, giving a clear survival advantage to the ESA group (P < .003).31 The biological reasons for this survival advantage are probably multifactorial and may encompass better cardiopulmonary and physical function and less exposure to the negative effects of transfusion therapy. Subsequent treatment failure in patients with an initial response to ESAs is usually not associated with disease progression but rather with an increasing clonal dominance at the hematopoietic stem cell level.32
Several randomized phase 2 studies have shown that the addition of low-dose G-CSF to erythropoietin may improve or restore the response to ESA.33-35 The synergistic effect is particularly seen in MDS-RS and related to the antiapoptotic effects of G-CSF on mitochondria-mediated erythroid apoptosis.36 G-CSF is not formally approved for the anemia of MDS in any country, and the possibility, hence tradition, to assess the combination for patients who are refractory to erythropoietin depends on the local reimbursement system.
To conclude, ESA constitute firstline treatment for those with anemia of lower-risk MDS and should preferably be initiated before the onset of a permanent transfusion need. High S-EPO levels, ranging between 200 and 500 U/L in various studies, are associated with clearly reduced response rates and may explain other therapeutic choices. IPSS-M has not been evaluated in the context of ESA, but it is likely that molecular profiles indicating a high risk of progressive disease speak against treatment. Table 2 shows a practical recommendation for how to use ESA with or without G-CSF for those with anemia of lower-risk MDS.
Practical use of erythropoietin-stimulating agents in lower-risk MDS
| Indication | IPSS-R very low, low, or intermediate risk MDS |
| Reasons to consider other treatment | IPSS-Mol ≥ moderate high risk |
| Primary eligible for curative treatment (HSCT) | |
| Serum erythropoietin levels >200 to 500 U/L | |
| When to initiate treatment | At onset of symptomatic anemia (patient-reported symptoms) |
| Transfusion-dependent anemia at diagnosis | |
| Prerequisites for response | Adequate iron reserves |
| Initial dosing∗ (1 dose per week) | Epoetin: 30 000 U/wk and darbepoetin: 150-240 μg/wk |
| Reason for lower starting dose | Small body weight. Subnormal renal function (individual dosing) |
| Maximal dose | Epoetin: 60 000 to 80 000 U/wk (2 doses) Darbepoetin: 300 μg/wk |
| Duration before decision about response | 16 wk at the highest dose |
| Action if no response† | If very low/low-risk disease, consider addition of G-CSF for up to 12 wk |
| If intermediate-risk disease or signs of progression, new diagnostic evaluation | |
| Action if supranormal hemoglobin levels‡ | If Hb levels are greater than upper normal level, stop treatment and restart at lower Hb levels |
| If complete erythroid response, less frequent dosing (1 dose per 2-3 weeks) | |
| Administration | Depends on national legal and reimbursement rules. Self-administration is common. |
| Indication | IPSS-R very low, low, or intermediate risk MDS |
| Reasons to consider other treatment | IPSS-Mol ≥ moderate high risk |
| Primary eligible for curative treatment (HSCT) | |
| Serum erythropoietin levels >200 to 500 U/L | |
| When to initiate treatment | At onset of symptomatic anemia (patient-reported symptoms) |
| Transfusion-dependent anemia at diagnosis | |
| Prerequisites for response | Adequate iron reserves |
| Initial dosing∗ (1 dose per week) | Epoetin: 30 000 U/wk and darbepoetin: 150-240 μg/wk |
| Reason for lower starting dose | Small body weight. Subnormal renal function (individual dosing) |
| Maximal dose | Epoetin: 60 000 to 80 000 U/wk (2 doses) Darbepoetin: 300 μg/wk |
| Duration before decision about response | 16 wk at the highest dose |
| Action if no response† | If very low/low-risk disease, consider addition of G-CSF for up to 12 wk |
| If intermediate-risk disease or signs of progression, new diagnostic evaluation | |
| Action if supranormal hemoglobin levels‡ | If Hb levels are greater than upper normal level, stop treatment and restart at lower Hb levels |
| If complete erythroid response, less frequent dosing (1 dose per 2-3 weeks) | |
| Administration | Depends on national legal and reimbursement rules. Self-administration is common. |
Considerable variation in dosing between studies, no fixed recommendation can be given.
Important to explain to the patient that treatment will be stopped in case no response has been confirmed.
Overdosing of darbepoetin leading to supranormal Hb levels may require venesectio for patients at risk of thromboembolic events.
The oral thrombomimetic compound eltrombopag is licensed for severe chronic immune-mediated thrombocytopenia and has been assessed in clinical trials enrolling patients with MDS.37-39 Randomized studies of eltrombopag for higher-risk MDS, alone or in combination with azacytidine, have failed; in fact, the response rate to azacytidine was lower in patients receiving azacitidine + eltrombopag. By contrast, smaller phase 2 trials have shown that patients with thrombocytopenic lower-risk MDS may respond with increased platelet counts. Eltrombopag has not shown a survival advantage in any clinical trial and is not approved for MDS; nevertheless, clinical experience indicates that it can be used to elevate platelet counts and reduce bleeding symptoms in case of severe chronic thrombocytopenia in patients at lower-risk. Thrombopoietin (romiplostim) was also assessed for lower-risk MDS. In comparison with eltrombopag, these studies failed to reach significant efficacy, but extension studies have not given rise to safety concerns. Considering that romiplostim is administered subcutaneously and that MDS hemopoietic stem cells express the thrombopoietin receptor, it is probably a less attractive alternative than eltrombopag in compassionate use situations.40 G-CSF is not licensed for low neutrophil counts associated with MDS but is frequently used as supportive care in case of neutropenia caused by HMA treatment, especially after recurrent infectious events.
Luspatercept
ACE-536 (luspatercept), a transforming growth factor β superfamily ligand trap, corrects anemia by diminishing Smad2/3 signaling, thereby enhancing late-stage erythropoiesis.41 Luspatercept was initially shown to cause hypererythrocytosis in patients without bone marrow failure. A phase 2 trial showed significant effects on anemia of lower-risk MDS, particularly in patients with MDS-RS and SFB1 mutation.42,43 A subsequent double-blind, placebo-controlled phase 3 trial with primary end point transfusion independence (TI) for ≥8 weeks enrolled only patients with MDS-RS, the majority carrying SF3B1 mutations, and confirmed a significant effect.44 The response rate among patients with a prestudy transfusion need between 4 and <6 units per 8 weeks was 37% to 38%, with TI reaching a maximum duration of 30 weeks, whereas response rates among patients with higher-intensity transfusion were lower. In this study, S-erythropoietin was not associated with response to luspatercept, indicating that the mechanism of action may differ from that of erythropoietin. However, similar to ESAs, luspatercept did not seem to inhibit the mutated clone.45 A recently published interim report of a 1:1 randomized phase 3 trial evaluating luspatercept vs erythropoietin for those with lower-risk MDS who had received ≥2 RBC units for a Hb level less than 9 g/dL showed a significantly higher rate of 12 to 24 weeks TI in the luspatercept arm.46 Interestingly, the efficacy for erythropoietin was higher in patients with lower mutational burden, whereas a similar association was not observed in the luspatercept arm. Moreover, the response rate to luspatercept was significantly better than that to erythropoietin for patients with RS and in those carrying SF3B1 mutations but not in other subgroups. These studies further underline that luspatercept provides a novel mechanism of action in lower-risk MDS, particularly in SF3B1-mutated MDS-RS. Since 2020, luspatercept has been FDA and EMA approved for ESA-refractory MDS-RS, and recently FDA approved the drug for lower-risk MDS with TD. At least in Europe, wide access to the drug is pending in many countries.
Immunosuppressive treatment
Hypoplastic MDS defines a subgroup with bone marrow cellularity <30%, often deep pancytopenia but without genetic high-risk factors or evidence of a germ line condition. Treatment with immunosuppressive agents, such as antithymocyte globulin and cyclosporin A may improve the cytopenia in a small proportion of younger patients with such hypoplastic and occasionally normoplastic lower-risk MDS. There are few large prospective studies, most of them with short follow-up time periods, and each study used different inclusion criteria and immunosuppressive treatment regimens. In a large meta-analysis of 570 patients with a median age of 62 years, 80% of the patients had IPSS scores low or intermediate-1, the complete remission and RBC TI rates were 12.5% and 33%, respectively, and the progression rate to AML was 8.6% per patient year. Immunosuppressive therapy has not been confidently evaluated in relation to mutational profiles. Both European and NCCN guidelines identify a group of younger patients with lower-risk MDS with hypoplastic or normo-plastic bone marrow and normal karyotype, except for trisomy 8, who may respond to immunosuppressive therapy.47-49
Lenalidomide treatment
Lenalidomide is licensed for transfusion-dependent lower-risk MDS with del(5q) based on a large phase 2 and a randomized placebo-controlled phase 3 study.50,51 The latter showed RBC TI in more than 50% of the patients, with the duration not reached and with 3-year OS rate and AML risk of 56% and 25%, respectively. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q)-containing cells, leading to a strong inhibitory effect on the malignant progenitor cells, and is, in fact, the only drug with this action approved for lower-risk MDS.52 Lenalidomide is not a curative treatment, the hematopoietic stem cells are spared, and the median response duration is ∼2 years with a clear risk of molecular and disease progression in relapsing patients.53 Approximately 20% of patients with lower-risk del(5q) MDS carry TP53-mutated subclones (variant allele frequency [VAF], 1%-54% in the original article), and because TP53-mutated cells are insensitive to lenalidomide, treatment can lead to clonal selection of mutated clones.54 Recently, it was shown show that lenalidomide also can promote development of TP53 mutations in an experimental setting.55 A recent real-world registry analysis confirmed a high response rate in lower-risk MDS with isolated del(5q), with significantly better outcome if initiated before the onset of a permanent transfusion need.56 An earlier study based on the Spanish MDS registry failed to show significant differences in OS and AML transformation in lenalidomide-exposed and -nonexposed patients, respectively. AML transformation rate in the lenalidomide group was 31% after a median follow-up of 22 months from the start of treatment.57 Studies evaluating paused treatment among patients with a molecular response are ongoing. NCCN and MDS Europe guidelines recommend lenalidomide treatment for patients with transfusion-dependent del(5q) MDS who are refractory to or ineligible for ESAs and lack TP53 mutations, as analyzed by conventional next-generation sequencing analysis with a minimum sensitivity of VAF = 2% or with positive p53 staining by immunohistochemistry, which, however, can be more difficult to interpret. In addition, lenalidomide was assessed in numerous studies of non(del5q) lower-risk MDS and in combination with azacytidine for higher-risk MDS but failed to reach expected endpoints.
In summary, lenalidomide provides a unique anticlonal mechanism of action in MDS with del(5q) and can induce complete erythroid response with a duration of several years. Eligible patients should undergo next-generation sequencing analysis, and treatment is not recommended in case of TP53 mutations. The experience is that transformation to AML in case of TP53-driven disease progression can be very fast, leaving few opportunities for effective intervention. In younger patients for whom HSCT could be an option, lenalidomide treatment, if administered, should be accompanied by frequent molecular follow-up. For patients who are not HSCT candidates, treatment should also be accompanied by molecular surveillance. Offering lenalidomide to patients with severely transfusion-dependent TP53-mutated del(5q) not eligible for HSCT should be a decision preceded by detailed information and joint decision-making.
Imetelstat and other novel targeted drugs for lower-risk MDS
As the scope of this review is to describe treatment options that are clinically available, this paragraph is limited. A plethora of novel compounds target hematopoiesis through vastly different mechanisms and have been evaluated in phase 1 to 2 clinical trials. Few have proven successful in subsequent randomized phase 3 trials, with 1 exception. Imetelstat is a first-in-class telomerase inhibitor evaluated in transfusion-dependent patients with lower-risk MDS in a phase 2 study followed by a 2:1 randomized placebo-controlled phase 3 trial. The phase 2 study encompassing 57 patients reported 8- and 24-week RBC TI rates of 37% and 23%, respectively, with a median TI duration of 65 weeks.58 Platzbecker et al met the expected end point and report a meaningful increase of TI among imetelstat- vs placebo-treated patients. Interestingly, these studies report a decrease in VAF among responding patients. If confirmed, it would be the second agent for lower-risk MDS with a potential tumor-inhibiting effect.59
Hypomethylating agents
HMAs have become firstline standard treatment for higher-risk MDS. Decitabine, 5-azacitidine (5-aza), and oral decitabine/cedazuridine are approved in the United States for all MDS subsets; the latter 2 for IPSS ≥ intermediate-1, whereas, in Europe, only 5-aza is approved for MDS and only for IPSS ≥ intermediate-2. Although no survival benefit could be shown for decitabine in randomized trials,60,61 the first randomized study of 5-aza for higher-risk MDS showed significant improvement with respect to leukemic transformation or death (21 vs 12 months) for 5-aza compared with that for best supportive care (BSC; CALGB 9221 study).62 The subsequent international randomized AZA-001 trial showed significantly improved median OS for 5-aza (24.5 vs 15 months) compared with standard care options consisting of physician's choice of AML-like chemotherapy, low-dose cytosine arabinoside (ara-C) or BSC.63 The survival benefit was seen in all subgroups, including older patients. Patients treated with 5-aza also showed delayed AML progression, less RBC transfusion, and a lower rate of infectious complications.
The hematologic response rate to HMAs is ∼40%, but treatment rarely leads to complete remission (8%), and most patients eventually relapse. In the AZA-001 study, the overall response rate (ORR) was higher for azacytidine than for BSC and low-dose ara-C but not in comparison with that for intensive chemotherapy. Duration of hematologic response was longer for 5-aza (13.6 vs 5.2 months in the control group). A majority of the patients responded within the first 6 cycles, which is the recommended number of cycles before efficacy assessment, unless allo-HSCT is planned.64 Despite the survival benefit of 5-aza in this trial, real-world data have not been able to confirm a solid survival benefit of this treatment.65
CC-486, an oral form of 5-aza, has been shown to improve survival in AML as postconsolidation therapy.66 A randomized phase-3 evaluating CC-486, mainly for lower-risk MDS, showed a significant reduction of TD and better response duration compared with placebo but did not result in improved survival.67 The approved 5-aza dose is that used in the AZA-001 trial: 75 mg/m2 × 7 days. However, alternative doses of HMAs have been investigated for lower-risk MDS, and data support a lower dose of both decitabine (3 instead of 5 days) and 5-aza (5-day instead of a 7- or 10-day schedule).68,69 In addition, some centers use a 5 + 2 + 2 schedule or administer the entire dose over 5 days to avoid treatment during weekends. A recent large analysis based on 449 Japanese and Swedish patients with MDS showed that complete remission (CR) and marrow CR are often associated with a clonal reduction (VAF%) and that such a reduction predicts a long-term outcome. The data are particularly strong for patients carrying TP53 mutations, in fact all patients with TP53 mutations with long-term survival after subsequent HSCT, showed a marked clonal reduction after 5-aza.70
HMA in combination with novel agents and molecularly targeted drugs
Over the last decades several new drugs have been tested in combination with 5-aza to further improve patient outcome but with limited success. The combination of 5-aza plus lenalidomide or 5-aza plus vorinostat showed similar ORRs and survival as azacitidine alone for higher-risk MDS in a prospective randomized trial.71 Likewise, pevonedistat, a selective inhibition of NEDD-8 activating enzyme, in combination with 5-aza failed to improve event-free survival in comparison with 5-aza alone for higher-risk MDS.72 After encouraging results of 5-aza in combination with an anti-CD47 antibody, magrolimab, in an early phase 1b study73 the ongoing phase 3 study was discontinued because of lack of efficacy.74 The recent approval of the BCL-2 inhibitor venetoclax in combination with 5-aza for AML has prompted investigation of this combinations for MDS as well. The combination induces 75% ORR in HMA-naïve patients and 44% ORR in patients after HMA failure.75 Despite these high response rates reported in early clinical studies, valid results from a recently completed randomized trial of 5-aza ± venetoclax are pending.73,76,77 Novel combinations with high remission rate may become suitable alternatives to chemotherapy for reducing the number of blasts before allogeneic stem cell transplantation. Hence, to date, there is no solid evidence to support the use of 5-aza plus venetoclax as a firstline treatment for MDS, but the combination has developed to a relatively common secondline alternative. It should, however, be used with care and competence because deep cytopenia and complications are more common than in standard-risk AML. Moreover, data on different molecular MDS subtypes are pending.
IDH1 or -2 mutations occur in 5% to 15% of patients with MDS and enasidenib has shown response in patients with IDH2-mutated MDS.78 Administering 5-aza in combination with APR-246 for patients with p53-mutated MDS induced 50% complete response,79 but a subsequent phase 3 trial did not show a difference in outcome. It may be mentioned that the new ICC classification that classifies previous WHO 2016 MDS with ≥10% blasts as MDS/AML potentially would allow for the use of AML-approved drugs for higher-risk MDS as well.6 To conclude, HMAs are currently the firstline treatment for higher risk MDS but will probably be replaced by 5-aza combinations in the future, at least for younger patients.
Chemotherapy
AML-like chemotherapy protocols including anthracycline–ara-C combinations have been used for patients with MDS with high blast counts but with lower response rates and shorter CR duration than for de novo AML. In addition, AML-like chemotherapy for older patients or patients with unfavorable karyotype and/or TP53 mutations resulted in even lower response rates with no clear benefit in comparison with 5-aza treatment.80-82 Less toxicity can be achieved using CPX-351, a liposomal anthracycline, plus ara-C–based chemotherapy with 52% CR rate in patients with high-risk MDS.83 Currently AML-like chemotherapy is mainly used as induction therapy before allogeneic stem cell transplantation and here recommended only for younger patients with MDS without unfavorable karyotype or biallelic TP53 mutations.
Stem cell transplantation
Allogeneic stem cell transplantation is currently the only curative treatment, but its inherent therapy-related morbidity and mortality limit its use for patients with MDS. Because optimal time for allogeneic stem cell transplantation in MDS has not been addressed in prospective clinical trials, comparisons between patients with MDS who underwent transplant and those who did not have been performed by using sophisticated statistical multistate models. In these models immediate allogeneic HSCT was favorable for patients at IPSS intermediate-2 or high risk, whereas patients at IPSS intermediate-1 or low risk benefit more from nontransplant approaches.84,85 In 2 prospective donor vs no-donor comparisons, patients at IPSS intermediate-2 and high risk as well as IPSS-R intermediate with high-risk cytogenetics with available donor had a significantly improved OS compared with those without donors.86,87 In the BMT-CTN study, the adjusted donor arm had an OS rate of 47.9% compared with 26.6% in the no-donor arm.87 A similar prospective study (VidazaAllo) compared 5-aza with allo-HSCT after 5-aza induction, according to donor availability, among patients with higher-risk MDS aged 55 to 70 years and reported event-free survival rates at 3 years of 34% after HSCT and 0% after 5-aza,88 which additionally support the use of allogeneic stem cell transplant in higher-risk MDS, even in older patients (Table 3).89
Prospective comparison of allogeneic stem cell transplantation vs conventional therapy
| Authors . | MDS risk . | Age, median (range), y . | Donor (matched) vs no donor . | LFS . | OS . |
|---|---|---|---|---|---|
| Robin et al84 | IPSS: intermediate-2 or high risk with poor cytogenetics or platelet TD or CMML | 50-70 | n = 112 vs n = 50 | na | At 4 y: 37% vs 15%; P = .02 |
| Nakamura et al (BMT-CTN 1102)85 | IPSS: intermediate-2 and high risk | 50-75 | n = 260 vs n = 124 | At 3 y: 35.8% vs 20.6%; P = .03 | At 3 y: 47.9% vs 26.6%; P = .001 |
| Kröger et al (VidazaAllo-Study)86 | IPSS-R: intermediate-2 or high risk or intermediate-2 with poor cytogenetics | 50-70 | n = 81 vs n = 27 [no donor: 5-aza treated] | At 3 y: 34% vs 0%; P < .0001 | At 3 y: 50% vs 32%; P = .12 |
| Yu et al88 | IPSS: low, intermediate-1 + 2 | 19-63 | randomization allo: n = 91 no allo: n = 91 | At 3 y: 74.7% vs 53.9%; P < .05 | At 3 y: 79% vs 56%; P < .05 |
| Authors . | MDS risk . | Age, median (range), y . | Donor (matched) vs no donor . | LFS . | OS . |
|---|---|---|---|---|---|
| Robin et al84 | IPSS: intermediate-2 or high risk with poor cytogenetics or platelet TD or CMML | 50-70 | n = 112 vs n = 50 | na | At 4 y: 37% vs 15%; P = .02 |
| Nakamura et al (BMT-CTN 1102)85 | IPSS: intermediate-2 and high risk | 50-75 | n = 260 vs n = 124 | At 3 y: 35.8% vs 20.6%; P = .03 | At 3 y: 47.9% vs 26.6%; P = .001 |
| Kröger et al (VidazaAllo-Study)86 | IPSS-R: intermediate-2 or high risk or intermediate-2 with poor cytogenetics | 50-70 | n = 81 vs n = 27 [no donor: 5-aza treated] | At 3 y: 34% vs 0%; P < .0001 | At 3 y: 50% vs 32%; P = .12 |
| Yu et al88 | IPSS: low, intermediate-1 + 2 | 19-63 | randomization allo: n = 91 no allo: n = 91 | At 3 y: 74.7% vs 53.9%; P < .05 | At 3 y: 79% vs 56%; P < .05 |
CMML, chronic myelomonocytic anemia; na, not assessed.
In a prospective study from China including patients with low and intermediate-1 and -2 MDS allo-HSCT resulted in an improved OS rate (79% vs 56%) in comparison with conventional therapy.90 A large retrospective EBMT study for patients with low and intermediate-1 risk MDS reported a 58% OS and 16% relapse incidence after allo-HSCT.91 Even if the risk of relapse after allo-HSCT is lower in lower-risk than in higher-risk MDS the multistate comparisons do not support allo-HSCT for patients at lower risk. However, patients failing HMA treatment have a generally poor outcome, and allo-HSCT should be considered even for lower-risk cases.92 Current recommendation for allo-HSCT in lower-risk MDS includes patients with severe cytopenia or progressive disease and those who have failed conventional treatment. HSCT might also be indicated if additional unfavorable factors exist in addition to IPSS-R, such as bone marrow fibrosis or unfavorable molecular genetics.93
Outcome after HSCT is strongly dependent on cytogenetics and molecular genetic features. Unfavorable cytogenetics such as complex karyotype or monosomal karyotype are associated with a higher risk of relapse and mortality.94,95 The increasing importance of molecular genetics is reflected by the recently introduced IPSS-M.4 ASXL1 and RUNX96 and p5396,97 as well as RAS pathway mutations are independent risk factors for higher relapse and mortality after HSCT and a combination of molecular genetics and cytogenetics may even better predict outcome after HSCT.98 The IPSS-M usually shifts patients to the higher-risk category, and 2 retrospective studies reported a modest advantage in prognostication of MDS using IPSS-M before allo-HSCT. Patients with MDS classified in the low-risk category according to IPSS-R may be classified to the higher-risk group according to IPSS-M and, therefore, become candidates for allogeneic transplantation. The role of IPSS-M in HSCT decision, however, is currently under evaluation.99,100 Despite worse outcome after stem cell transplantation for patients harboring p53 mutations, a posthoc analysis from BMT-CTN 1102 trial showed improved survival for patients with TP53single and TP53multihit and patients at IPSS-M high risk without TP53 mutation after stem cell transplantation in comparison with that after nontransplant approaches.101 Molecular testing before HSCT is recommended to exclude inherited bone marrow failure symptoms and germ line mutations such as GATA2, SAMD9/SAMD9L, RUNX1, ETV6, and DDX41, which have implications on conditioning regimens and donor selection.102 After adjustment of other risk factors, age, per se, is not associated with worse OS,89 but poor performance status, comorbidity index, and Karnofsky index are associated with higher nonrelapse mortality (NRM),89,103 even if not all comorbidities result in similar risk of NRM.104 Even if blast reduction before transplant had positive impact on the outcome and pretransplant therapy in MDS with ≥10% blasts is recommended by international expert teams,105 no prospective study comparing pretransplant therapy vs no therapy exists, and most of the retrospective studies that investigated HMA or chemotherapy did not show a significant impact on OS after transplantation,106 presumably because the outcome of patients who were nonresponding worsened after HSCT.88
All patients with higher-risk MDS with good performance status, and no severe comorbidities should be considered for curative allo-HSCT. Age should not be considered as a contraindication, per se. Patients with lower-risk MDS may also benefit from allo-HSCT if conventional therapy fail and high-risk molecular genetics are present. In any case, balancing the risk of HSCT with life expectancy with and without transplantation is needed, taking the patient’s perspective into account as well.
Retrospective studies of conditioning regimen intensity showed higher NRM but less relapse for myeloablative conditioning regimens.107 One prospective EBMT study (RICMAC) showed no difference in the outcome between myeloablative conditioning and reduced intensity conditioning (RIC) regimen.108 The BMT-CTN study for AML and MDS showed higher risk of relapse after RIC, but the number of included patients with MDS was limited.109 Better outcome was seen for treosulfan in comparison with that fir busulfan/fludarabine-based RIC regimen110 and less relapse for melphalan/fludarabine-based vs busulfan/fludarabine-based RIC regimen.111 The broader availability of unrelated donors and the introduction of posttransplant cyclophosphamide as graft-versus-host disease prophylaxis in haploidentical stem cell transplantation has further increased the donor pool and the number of allo-HSCTs for MDS, and registry studies suggest a similar outcome of haploidentical HSCT with posttransplant cyclophosphamide in comparison with that of matched unrelated donors.112 Because relapse is the most frequent treatment failure after HSCT in MDS, reducing the risk of relapse by introducing posttransplant maintenance strategies is a hot topic and currently under intensive clinical investigation. Posttransplant monitoring with chimerism or molecular markers with sensitive sequencing methods are helpful in guiding posttransplant interventions.113,114 However, the largest study so far with 5-aza maintenance for patients with AML and MDS failed to show a reduction of relapse after HSCT.115
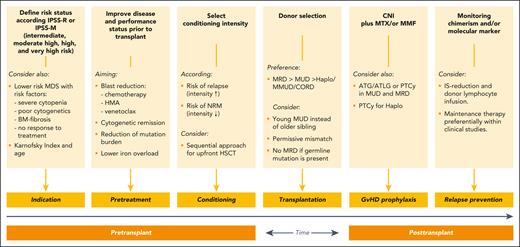
Novel approaches to prevent or delay relapse are eprenetapopt plus 5-aza for p53-mutated MDS, enasidinib for IDH2-mutated MDS, or measurable residual disease–triggered preemptive 5-aza.116-118 Donor lymphocyte infusions alone or in combination are indicated for relapsed patients, but available data as prophylactic treatment are not conclusive.119,120 Results from ongoing prospective studies for preemptive donor lymphocyte infusions as well as maintenance with oral 5-aza or 5-aza–venetoclax combinations are pending; thus, currently no valid recommendation can be given. Figure 2 summarizes the different steps and interventions during the transplant course of patients with MDS.
Summary
MDS is a profoundly heterogeneous disease characterized by a multitude of cellular and molecular mechanisms, with disease outcomes varying from normal life expectancy and no symptoms to severe pancytopenia, rapid disease progression, and very poor survival. The fact that MDS originates in the hematopoietic stem cells limits therapeutic options, and fewer novel drugs have been licensed for MDS than for any other blood cancer. On the other hand, the disease often develops over several years, and lessons from the clonal hematopoiesis field may render new tools to influence clonal competition in a way favoring healthy stem cells over their mutated counterparts. Several new pathways are currently explored to improve erythropoiesis and outcome of the only curative regimen, stem cell transplantation, is steadily improving.
Authorship
Contribution: E.S.H.-L. and N.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eva S. Hellström-Lindberg, Department of Medicine, Karolinska Institutet, Center for Hematology and Regenerative Medicine, Karolinska University Hospital Huddinge, 141 86 Stockholm, Sweden; email: eva.hellstrom-lindberg@ki.se.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal