Visual Abstract
Risk stratification and prognostication are crucial for the appropriate management of patients with myelodysplastic syndromes (MDSs) or myelodysplastic neoplasms, for whom the expected survival can vary from a few months to >10 years. For the past 5 decades, patients with MDS have been classified into higher-risk vs lower-risk disease phenotypes using sequentially developed clinical prognostic scoring systems. Factors such as morphologic dysplasia, clinical hematologic parameters, cytogenetics, and, more recently, mutational information have been captured in prognostic scoring systems that refine risk stratification and guide therapeutic management in patients with MDS. This review describes the progressive evolution and improvement of these systems which has led to the current Molecular International Prognostic Scoring System.
Introduction
Myelodysplastic syndromes (also termed as myelodysplastic neoplasms, both abbreviated MDS) are a heterogeneous group of hematologic neoplasms characterized by cytopenia and bone marrow (BM) dysplasia caused by underlying clonal hematopoiesis.1 Because the natural history and prognosis of MDS varies from mild isolated cytopenia to BM failure and a high risk of evolution to acute myeloid leukemia (AML),2 prognostication and risk stratification are critical for identifying subsets of patients with MDS who may benefit from disparate management strategies. Given this range of clinical outcomes, some patients may be able to undergo surveillance with peripheral blood tests for years without any therapeutic intervention, whereas others may require aggressive monitoring, dense transfusional support, chemotherapy, and potentially curative hematopoietic stem cell transplantation. Efforts to assess disease risk and predict outcomes have evolved over decades for this spectrum of heterogenous diseases. Here, we review the field’s path to current clinical- and molecular-based prognostic approaches for such analyses.
Early prognostic tools
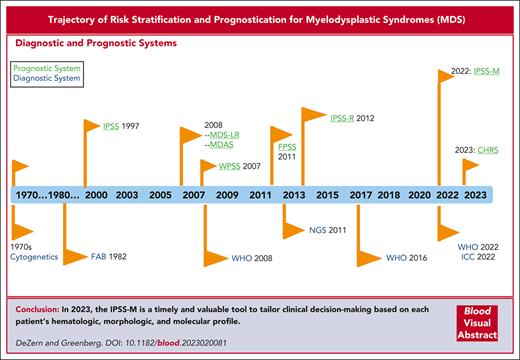
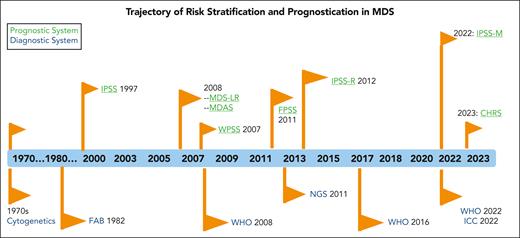
Given that the initial descriptions of MDS were retrospective and required distinction as well as comparison to overt AML, controversy persists regarding the ability to classify such patients prospectively.3-8 These early patients had refractory cytopenia and subtle but characteristic qualitative BM morphologic abnormalities, initially termed hemopoietic dysplasia.8 The French-American-British (FAB) cooperative group was the first to generate an analysis of these non-AML myeloid disorders, displaying a wide range of morphological appearances in the blood and BM and, thus, developed their proposal for the diagnosis of MDSs.9 These patients had a variation in the risk of transformation to a blastic phase, which correlated with specific histologic features. These features particularly related to the degree of myeloid maturation, which were identified as 5 morphologic subgroups: refractory anemia (RA), RA with ring sideroblasts, RA with an excess of blasts, chronic myelomonocytic leukemia, and RA with an excess of blasts in transformation.9 Over time, clear integration occurred with diagnostic and prognostic scoring systems for patients with MDS (Figure 1).
Trajectory of risk stratification and prognostication in MDS. The chronology of the prognostic scoring systems is shown in parallel with that of the diagnostic systems. CHRS, Clonal Hematopoiesis Risk Score; ICC, International Consensus Classification; FPSS, French Prognostic Scoring System; MDAS, MD Anderson Risk Model Score; NGS, next generation sequencing.
Trajectory of risk stratification and prognostication in MDS. The chronology of the prognostic scoring systems is shown in parallel with that of the diagnostic systems. CHRS, Clonal Hematopoiesis Risk Score; ICC, International Consensus Classification; FPSS, French Prognostic Scoring System; MDAS, MD Anderson Risk Model Score; NGS, next generation sequencing.
Prior prognostic studies of in vitro biologic features of hematopoietic precursor cell growth in patients with preleukemia had been analyzed regarding patient survival and their potential to remain indolent vs undergoing transformation to AML.10 Abnormal myeloid progenitor cell (colony-forming unit granulocyte-macrophage) growth in MDS was similar to that in AML, suggesting that MDS BM consisted of either a potentially leukemic clone with an increased capacity for differentiation in vivo or a leukemic clone held in abeyance. Subsequently, these in vitro studies yielded prognostic value, with the most abnormal granulocyte-macrophage colony-forming unit growth being associated with poor patient survival.10-12 These in vitro myeloid culture studies were useful adjuncts to marrow morphology and clinical features for the diagnostic and prognostic characterization of patients with this syndrome.
The next steps en route to MDS outcome prognostication included at least 6 additional risk classification system proposals regarding the prognosis of MDS and their potential for survival and evolution to AML13-18 These classification methods variably included clinical parameters, such as BM myeloblast percentage, specific cytopenia, age, lactate dehydrogenase level, and/or BM cytogenetic patterns. Additionally, several other cytogenetic classification methods had been suggested to categorize patients with MDS.19-22 However, imprecision associated with varying degrees of sensitivity and specificity persisted with these classification systems regarding the clinical outcomes.
International efforts for prognostic scoring system
An International MDS Risk Analysis Workshop was convened to improve the clinical and prognostic use of these systems and develop a consensus prognostic risk-based analysis system. In this workshop, cytogenetic, morphological, and clinical data were combined and collated from patients with primary MDS from 7 previously reported studies using independent risk-based prognostic systems.13-18 A global analysis of the combined data obtained from these patients was analyzed centrally. Critical prognostic variables were then re-evaluated using the data set from this combined patient cohort to generate a prognostic system called the International Prognostic Scoring System (IPSS).23 This study, obtained from a large representative group of well-defined untreated primary patients with MDS with a prolonged follow-up period, refined the BM cytogenetic subgroups and combined them with statistically defined major clinical parameters (number of cytopenias and percentage of marrow blasts) to evaluate clinical outcomes and generate statistically weighted prognostic risk categories (Table 1). This IPSS provided an improved method for evaluating prognosis in MDS and proved useful for the more precise design and analysis of therapeutic trials for this disease.
International MDS risk stratification systems
| Features . | IPSS23 . | WPSS25 . | IPSS-R45 . | CCF/ MLL62 . | EuroMDS65 . | IPSS-M68 . |
|---|---|---|---|---|---|---|
| Patients (n) | 816 | 426 | 7012 | 1471 | 2043 | 2701 |
| Hemoglobin | + | + | + | + | + | + |
| Platelets | + | + | + | + | + | + |
| Absolute neutrophil count | + | + | + | |||
| Transfusion dependence | + | |||||
| BM blast percentage/morphology | + | + | + | + | + | + |
| Time dependent | + | |||||
| Cytogenetics | + | + | + | + | + | + |
| Categories/abnormalities | 3/9 | 3/9 | 5/17 | 5/17 | 5/17 | 5/17 |
| Gene mutations (n) | + (24) | + (63) | + (31) | |||
| Survival, (y, median) | ||||||
| Risk categories | Low, 5.7 | Very low, 8.5 | Very low, 8.8 | 0, 5.9 | 0, 6.5 | Very low, 10.6 |
| Int 1, 3.5 | Low, 6 | Low, 5.3 | 1-2, 4.9 | 1-2, 6.4 | Low, 6.0 | |
| Int 2, 1.1 | Int, 3.3 | Int, 3.0 | 3-4, 3.0 | 3-4, 4.0 | Mod low, 4.6 | |
| High, 0.4 | High, 1.8 | High, 1.6 | ≥5, 1.7 | 5-6, 2.1 | Mod high, 2.8 | |
| Very high, 1.0 | Very high, 0.8 | ≥7, 1.6 | High, 1.7 | |||
| Very high, 1.0 |
| Features . | IPSS23 . | WPSS25 . | IPSS-R45 . | CCF/ MLL62 . | EuroMDS65 . | IPSS-M68 . |
|---|---|---|---|---|---|---|
| Patients (n) | 816 | 426 | 7012 | 1471 | 2043 | 2701 |
| Hemoglobin | + | + | + | + | + | + |
| Platelets | + | + | + | + | + | + |
| Absolute neutrophil count | + | + | + | |||
| Transfusion dependence | + | |||||
| BM blast percentage/morphology | + | + | + | + | + | + |
| Time dependent | + | |||||
| Cytogenetics | + | + | + | + | + | + |
| Categories/abnormalities | 3/9 | 3/9 | 5/17 | 5/17 | 5/17 | 5/17 |
| Gene mutations (n) | + (24) | + (63) | + (31) | |||
| Survival, (y, median) | ||||||
| Risk categories | Low, 5.7 | Very low, 8.5 | Very low, 8.8 | 0, 5.9 | 0, 6.5 | Very low, 10.6 |
| Int 1, 3.5 | Low, 6 | Low, 5.3 | 1-2, 4.9 | 1-2, 6.4 | Low, 6.0 | |
| Int 2, 1.1 | Int, 3.3 | Int, 3.0 | 3-4, 3.0 | 3-4, 4.0 | Mod low, 4.6 | |
| High, 0.4 | High, 1.8 | High, 1.6 | ≥5, 1.7 | 5-6, 2.1 | Mod high, 2.8 | |
| Very high, 1.0 | Very high, 0.8 | ≥7, 1.6 | High, 1.7 | |||
| Very high, 1.0 |
CCF, Cleveland Clinic Foundation; int1, intermediate 1; int2, intermediate 2; MLL, Munich Leukemia Laboratory; mod, moderate.
Addition of further variables to MDS prognostic systems
After the publication of the IPSS in 1997,23 modifications of existing parameters and additional prognostic systems were suggested to provide meaningful differences in patients’ clinical outcomes.24-27 These further iterations (Table 1) carried the expectation that additional clinical variables could refine the models to gain improved prognostication and possibly be used dynamically over the MDS disease course. There were also concerns that IPSS had relatively limited cytogenetic analysis. This system included marrow blasts up to 30% and lacked application at the time of hypomethylating agent (HMA) failure.
The World Health Organization (WHO) has added morphologic refinement to the FAB classification.28 The 2007 WHO Prognostic Scoring System (WPSS25) provided new insights into prognostic variables, adding red blood cell transfusion dependence along with IPSS cytogenetic classification and WHO dysplastic categories.29 This cohort was derived from independent learning and validation cohorts of nearly 1200 patients from a combination of an Italian hospital and the Düsseldorf MDS registry. This model used WHO subgroups (a key feature of this model), karyotypes, and transfusion requirements to stratify patients to the 5 risk categories. As compared with the 4 groups defined by the IPSS, WPSS (with its 5 categories) showed relevant improvement in prognostic ability among patients with MDS without excess blasts. This was attributable to the strong impact of lineage involvement and transfusion dependency. This is an important feature because transfusion dependency is known to be an independent prognostic factor, given that it remains a consistent indicator of the severity of MDS.30
In 2007, the MD Anderson group sought to develop an alternative tool to identify patients with lower-risk MDS and poor prognosis, who may benefit from earlier therapeutic intervention. The resultant MDA-LR (MD Anderson Lower-Risk MDS Prognostic Scoring System)31 was based on 856 patients with low- and intermediate-1-risk MDS per the IPSS23 referred to their center. The median follow-up of this cohort was relatively short, at <1 year, and only 50% of the cohort was untreated. Even with this heterogeneity, the study showed that advancing age, male sex, poor performance status, cytopenias, unfavorable risk cytogenetics, increased marrow blasts, higher creatinine, lower albumin, and infection at referral were all significantly associated with worse survival. This score highlighted searching proactively for a patient population at lower risk but with higher-risk disease biology to be considered for earlier treatment.
In 2011, Groupe Francophone des Myelodysplasies reviewed the prognostic factors for response and survival in patients with higher-risk MDS treated with azacitidine.32 The definitive trial, and report,33 leading to the approval of azacitidine as the standard of care for patients at higher risk had been performed over the prior decade; thus, there was justified interest in defining the prognosis for azacitidine-treated patients. The authors reviewed nearly 300 patients with high- or intermediate-2 risk MDS (per IPSS23) who had received azacitidine in a compassionate use program. The authors reviewed features before and after treatment, which affected survival, and generated their prognostic score (FPSS), which discriminated the 3 risk groups with good granularity. Thereafter, they validated their prognostic scores in an independent set of patients who received azacitidine in the AZA-001 trial.33 The authors demonstrated that the IPSS cytogenetic classification retained prognostic significance of the overall survival (OS) rate in patients treated with azacitidine.
In further studies, additional variables suggested to provide prognostic information in MDS included serum lactate dehydrogenase34,35 ferritin,36 and B2-microglobulin levels37,38 as well as marrow fibrosis,39-41 patient comorbidities, and performance status.7,27,39-43 Importantly, newer cytogenetic groupings were reported to be prognostically valuable and have refined the features used in IPSS.44
Beyond the IPSS
Thus, to examine the prognostic impact of these new clinical and cytogenetic variables and refine the IPSS, the coordination of investigators and coalescence of MDS databases from multiple international institutions provided a much larger combined database of patients by the International Working Group for Prognosis in MDS project. This 2012 study refined the IPSS and generated the revised IPSS (IPSS-R).45 This accomplishment involved reassessing the prior major predictive features, determining the impact of the suggested newer clinical features on prognostic power, incorporating larger and more differentiated cytogenetic subgroups, and re-evaluating their prognostic impact. Statistically weighted clinical features were used to generate a prognostic categorization model. This larger combined database permitted better analyses of the specific impact of marrow blast percentage, depth of cytopenia, patient age, and less-frequent clinical features (ie, performance status serum ferritin and lactate dehydrogenase levels), particularly further evaluating the cytogenetic subgroups (Table 1). As indicated in Table 1, the more refined cytogenetic categorization provided by Schanz et al was quite valuable.44 The IPSS-R had 5 risk categories and upstaged 27% and downstaged 18% patients compared with the IPSS. Further evaluation of the IPSS-R with a focus on the intermediate category indicated that the cutoff score of 3.5 discriminated well between patients at higher and lower risk.46 The IPSS-R schema for patients with MDS has been validated extensively for assessing prognosis in patients with MDS46-49 and developing risk-based protocols for the design and evaluation of clinical trials.
Although the IPSS-R has been useful prognostically for evaluating patients from the time of diagnosis, important information regarding dynamic changes over time from diagnosis was subsequently developed to describe changes in risk over time and their potential clinical implications.46 Constituent individual predictors for major prognostic risk scoring systems (including the IPSS, IPSS-R, WPSS, and LR-PSS) were analyzed in 7212 primary untreated patients with MDS from the International Working Group for Prognosis in MDS database.46 Analysis of changes from diagnosis demonstrated that for patients with higher-risk MDS, hazards regarding the risks of mortality and AML transformation diminished over time from diagnosis, whereas they remained stable in patients with lower-risk MDS. The inclusion of age resulted in an increased initial prognostic power for survival and less attenuation of hazards. This disparate evolution of prognosis between the initial risk groups requires ongoing consideration in clinical decision-making.
In the evaluation of treatment-related MDS, the overall IPSS-R scores separated these patients into 5 prognostic risk groups, with each category showing statistical differences in OS and AML progression probability, indicating that BM blast count, cytopenia(s), and cytogenetic data remain powerful predictors in this setting.50,51 However, compared with de novo MDS, the median survival for each of the IPSS-R–risk groups was shorter in patients with treatment-related MDS. These data likely reflect the finding that prior cytotoxic exposures often cause high-risk cytogenetic abnormalities as well as other genetic and epigenetic alterations in hematopoietic stem cells.51 Of note, after HMA-treatment failure, the IPSS-R and other early models were shown to lack predictive ability,52 being indicative of the prognostic but not predictive value of these systems. Additional models have been proposed to assess this point.52
In the generation of the IPSS-R prognostic system, performance status was found to have a minor additive impact on the survival and AML evolution relative to the major effects of the patients’ clinical and cytogenetic features.45 However, specifically focused studies have provided additional data regarding the association of patient frailty, performance status, and quality of life (QoL; health care–related QoL) with clinical outcomes in MDS. Treatment tolerance and life expectancy in the setting of medical comorbidities have not been formally standardized across the field but are available to address increased vulnerability, separate from the current prognostic scoring systems. Some studies have investigated both comorbid medical conditions and frailty to demonstrate infection-related mortality with therapy.53 An MDS-specific frailty index (FI) was developed using the Canadian prospective MDS registry, which assessed its ability to add prognostic power to conventional prognostic scores.54 Ultimately, FI included performance, comorbidities, laboratory values, instrumental activities of daily living, QoL, and performance status among others. The median FI score correlated with age and IPSS/IPSS-R–risk scores and discriminated OS, leading to a prognostic scoring system with 5 risk groups and distinct survivals.55
Other studies have considered specific QoL issues beyond prognostic scoring systems that affect patients with MDS, including time in the health care setting and anemia, specifically.55-57 A widely used and validated health care–related QoL instrument in this patient population is the MDS-specific 38-item Quality of Life in Myelodysplasia Scale,58 which demonstrates important physical burden, benefit-finding, and emotional burden components. Additional validation efforts are ongoing to determine the utility of the measures and subscales for both clinical trials and clinical decision-making.59 Attempts to address prognosis in this way, apart from the aforementioned systems that focus on disease characteristics, have taken into account patient characteristics other than age that are integral to decision-making in patients with MDS, most of whom are older adults. This patient-centric approach, combined with other prognostic efforts focused on disease biology, is gaining traction in this field.
Tools incorporating molecular information
Established prognostic models before the mid-2010s relied primarily on clinical variables derived from peripheral blood counts, cytogenetics, and marrow pathology to categorize patients into risk categories. More recently, somatic mutations have been found to be valuable prognostic biomarkers for assessing MDS clinical outcomes60-65 and have been included in several initial prognostic systems.64,65 Multiple groups have similarly pursued the addition of genetics to their scoring systems (Table 1).
Nazha et al65 applied a machine learning algorithm for personalized prognosis. This approach involved more than 1200 patients with clinical data to calculate the IPSS and IPSS-R, and targeted deep sequencing was performed on 38 genes commonly reported in commercial laboratories’ genomic panels and shown to have clinical impact in MDS and other myeloid malignancies. The algorithm identified chromosomal karyotype, platelet, hemoglobin levels, BM blast percentage, age, 7 discrete gene mutations, and the number of mutations as having a prognostic impact on the OS and leukemia-free survival. Ultimately, the new model, which was validated in an external cohort of patients from an interventional clinical trial, accounted for all noted variables as well as their interactions with each other to provide a personalized prognostic model for each patient. This group went on to use a similar approach to model the prediction of patients treated with hypomethylating agents and BM transplantation.66 The model is also shown to be dynamically useful.
The EuroMDS consortium studied a population of more than 2000 patients with MDS and developed a prognostic tool that incorporated a large number (63) of clinical and genomic variables.64 Their computationally-complex approach defined genotype-phenotype correlations in MDS and leveraged them to measure the combined prognostic information of gene mutations with clinical variables. Eight subgroups were identified: patients with SF3B1 mutations, TP53 mutations and/or a complex karyotype, SRSF2 mutations, U2AF1 mutations associated with the deletion of chromosome 20q and/or abnormalities of chromosome 7, and AML-like mutation patterns and those without specific genomic profiles. A feature evident from this study was the heterogenous distribution of the WHO subtypes across their new genomic categories, emphasizing the increasing need to capture all relevant and distinct MDS biological features. The GenoMed4All consortium67 published 2 stratification tools that incorporated sex, 1 of which incorporated molecular features. This group used 4 different MDS cohorts, with a total of more than 13 000 patients, to evaluate the prognostic effect of sex on outcomes in MDS. In all cohorts studied, men had a worse median OS than women. However, this has not been formally assessed in other cohorts.
From the IPSS-R to IPSS-M
The IPSS-R has been a global standard for patient risk stratification since 2012 for clinical trial design, correlative analyses, and treatment recommendations, relying on hematologic and cytogenetic features; however, it does not consider the potential role of gene mutations. Multiple publications from the latter half of the 2010s contributed to the knowledge about the prognostic impact of molecular mutations and spurred a large international effort to develop a clinical molecular prognostic model. This effort required nearly a decade to come to fruition, because of the detailed approach applied. Marrow samples from 2701 patients with MDS were profiled for mutations in 156 genes.68 Clinical and molecular variables were evaluated for their associations with leukemia-free survival, leukemic transformation, and OS. Feature selection was applied to determine the set of independent molecular IPSS (IPSS-M) prognostic variables. These features included the depth of cytopenia, marrow blast percentage, IPSS-R version of cytogenetic risk groups, and relatively new genomic variables. Using hematologic parameters, cytogenetic abnormalities, and somatic mutations of 31 genes, the IPSS-M resulted in a unique risk score for individual patients (Figure 2). The relative weights of the selected variables were estimated using a robust multivariable model adjusted for confounders and generated the IPSS-M (Table 1).68
IPSS-M prognostic risk schema. The main effect genes are shown on the left in a statistically weighted order (top having the most adverse risk). Residual genes are included as additives (maximum of 2 genes). These mutations are incorporated into the algorithm, which also includes cytopenias, marrow blasts, and cytogenetic abnormalities, to calculate the IPSS-M score and risk category. SF3B15q = SF3B1 mutation with isolated del(5q) or with 1 additional aberration excluding –7/del(7q). SF3B1α = SF3B1 mutation without comutations in BCOR, BCORL1, RUNX1, NRAS, STAG2, SRSF2, or del(5q). H, high; L, low; MH, moderate high; ML, moderate low; VH, very high; VL, very low risk. Adapted with permission from Bernard et al.68 These results may be accessed via a web-based URL: https://mds-risk-model.com.
IPSS-M prognostic risk schema. The main effect genes are shown on the left in a statistically weighted order (top having the most adverse risk). Residual genes are included as additives (maximum of 2 genes). These mutations are incorporated into the algorithm, which also includes cytopenias, marrow blasts, and cytogenetic abnormalities, to calculate the IPSS-M score and risk category. SF3B15q = SF3B1 mutation with isolated del(5q) or with 1 additional aberration excluding –7/del(7q). SF3B1α = SF3B1 mutation without comutations in BCOR, BCORL1, RUNX1, NRAS, STAG2, SRSF2, or del(5q). H, high; L, low; MH, moderate high; ML, moderate low; VH, very high; VL, very low risk. Adapted with permission from Bernard et al.68 These results may be accessed via a web-based URL: https://mds-risk-model.com.
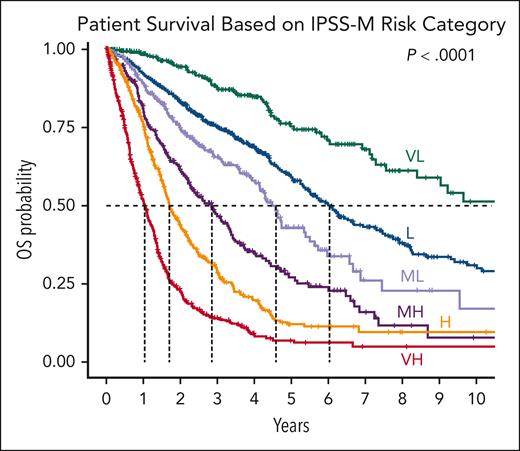
The IPSS-M demonstrated at least 1 oncogenic genomic alteration in 94% of patients with MDS. Multivariable analysis identified TP53multihit, FLT3 mutations, and MLLPTD (also termed KMT2A) as the highest genetic predictors of adverse outcomes. Conversely, SF3B1 mutations were associated with favorable outcomes, but this was modulated by patterns of comutation. Furthermore, 6 IPSS-M risk categories with prognostic differences regarding OS, leukemia-free survival, and risk of AML transformation were derived (Table 1; Figure 3). Compared with the IPSS-R, the IPSS-M improved prognostic discrimination across all clinical end points and restratified 46% of patients, 74% upstaged and 26% downstaged.68 The IPSS-M was found to be applicable for patients with primary and secondary/therapy-related MDS. By combining genomic profiling with hematologic and cytogenetic parameters, the IPSS-M demonstrated improved risk stratification of patients with MDS and, now, represents a useful tool for clinical decision-making in 2023 and beyond. Additionally, the IPSS-M was shown to be useful prognostically for both patients who were untreated and those who were HMA-treated and underwent stem cell transplantion.68 Recent studies have independently verified the utility of this schema,69,70 with additional ongoing extensions, which importantly require the addition of methods for using IPSS-M as a dynamic tool.
IPSS-M and risk categories. Kaplan-Meier probability estimates of OS of the 2701 patients in the International Working Group for Prognosis in MDS cohort are presented across IPSS-M risk categories. Dashed lines highlight the median values. Adapted with permission from Bernard et al.68
IPSS-M and risk categories. Kaplan-Meier probability estimates of OS of the 2701 patients in the International Working Group for Prognosis in MDS cohort are presented across IPSS-M risk categories. Dashed lines highlight the median values. Adapted with permission from Bernard et al.68
A review of the specific somatic mutations associated with the current prognostic systems demonstrated consistency regarding the specific genes and their comutations possessing risk relationships for patients’ leukemia-free survival and OS when evaluated in combination with clinical and cytogenetic features (Table 2).64,65,68Table 2 demonstrates the relative hazard of specific gene mutations for determining adverse clinical outcomes via the IPSS-M and provides websites for accessing patient’s personalized risk data. Additionally, the low incidence (≤5%) of some of these genes within the MDS population indicates the need for evaluating data from relatively large patient groups in order to gauge the clinical impact of certain low incidence genes. Of global relevance, it is necessary to recognize that extensive molecular genetic testing may not be attainable in resource-poor settings, although IPSS-M can calculate score ranges even if some mutation data are missing.
Somatic mutations associated with clinical outcomes in the current prognostic systems for MDS
| Mutation . | Bernard et al, IPSS-M68 Leukemia-free survival N = 2428 . | Bersanelli et al64 survival N = 2043 . | Nazha et al65 survival N = 479 . | |
|---|---|---|---|---|
| Incidence, % . | Hazard ratio∗ . | |||
| TP53multi-hit | 11 | 3.27 | +† | +‡ |
| MLLPTD | 2 | 2.22 | ||
| FLTITD+TKD | 1 | 2.22 | +§ | |
| SF3B15q | 1 | 1.66 | +‖ | |
| NPM1 | 1 | 1.54 | +§ | |
| RUNX1 | 16 | 1.53 | + | + |
| NRAS | 5 | 1.52 | + | + |
| ETV6 | 2 | 1.48 | ||
| IDH2 | 6 | 1.46 | + | |
| CBL | 6 | 1.34 | + | + |
| EZH2 | 7 | 1.31 | + | + |
| U2AF1 | 11 | 1.28 | +¶ | |
| SRSF2 | 18 | 1.27 | +# | + |
| DNMT3A | 19 | 1.25 | + | |
| ASXL1 | 30 | 1.24 | + | + |
| KRAS | 4 | 1.22 | + | |
| SF3B1α | 28 | 0.92 | + | + |
| STAG2 | +∗∗ | |||
| RAD21 | + | |||
| Mutation . | Bernard et al, IPSS-M68 Leukemia-free survival N = 2428 . | Bersanelli et al64 survival N = 2043 . | Nazha et al65 survival N = 479 . | |
|---|---|---|---|---|
| Incidence, % . | Hazard ratio∗ . | |||
| TP53multi-hit | 11 | 3.27 | +† | +‡ |
| MLLPTD | 2 | 2.22 | ||
| FLTITD+TKD | 1 | 2.22 | +§ | |
| SF3B15q | 1 | 1.66 | +‖ | |
| NPM1 | 1 | 1.54 | +§ | |
| RUNX1 | 16 | 1.53 | + | + |
| NRAS | 5 | 1.52 | + | + |
| ETV6 | 2 | 1.48 | ||
| IDH2 | 6 | 1.46 | + | |
| CBL | 6 | 1.34 | + | + |
| EZH2 | 7 | 1.31 | + | + |
| U2AF1 | 11 | 1.28 | +¶ | |
| SRSF2 | 18 | 1.27 | +# | + |
| DNMT3A | 19 | 1.25 | + | |
| ASXL1 | 30 | 1.24 | + | + |
| KRAS | 4 | 1.22 | + | |
| SF3B1α | 28 | 0.92 | + | + |
| STAG2 | +∗∗ | |||
| RAD21 | + | |||
Adjusted hazard ratio via Cox regression for the risk of leukemic transformation or death, adjusted for age, sex, and secondary/therapy-related vs primary MDSs. Mutations were also used for OS and generated 6 risk categories defining the IPSS-M: https://mds-riskmodel.com/. SF3B15q = SF3B1 mutation with isolated del(5q) or with 1 additional aberration excluding del(7q). SF3B1α= SF3B1 mutation without comutations in BCOR, BCORL1, RUNX1, NRAS, STAG2, SRSF2, or del(5q). Residual genes (Nres), the number of mutated genes in the following list: STAG2, BCOR, BCORL1, CEBPA, ETNK1, GATA2, GNB1, IDH1, NF1, PHF6, PPM1D, PRPF8, PTPN11, SETBP1, and WT1. This variable (≤2 genes) has a hazard ratio of 1.26.
Includes all TP53 mutations ± complex karyotype.
Includes all TP53 mutations.
With AML-like mutations.
SF3B1 with del5q and/or ASXL1 and/or RUNX1 mutations.
Associated with del(20q) and/or chromosome 7 abnormalities.
Associated with TET2 or separately with ASXL1, RUNX1, IDH2, and EZH2 mutations; https://mds.itb.cnr.it/#/mds.
Also present in the report by Bernard et al68 as a residual gene (Nres).
Relationship between prognostic classifications and recent morphologic classifications
The WHO 2016 provided a useful morphologic classification of MDS subtypes. Because virtually 90% of patients with MDS exhibit genetic and/or cytogenetic abnormalities, the reclassification of MDS by the WHO 2022 update and the International Consensus Classification valuably incorporates both genetically defined and morphologically defined patient subgroups,71,72 which were published contemporaneously (Figure 1). Generally, patients with SF3B1 mutations or del(5q) abnormalities (as sole lesions) have better prognostic risk, and those with TP53multihit have poorer prognoses. However, importantly, although when using morphologic subgroups, generally, patients with lower or higher marrow blast counts have better or poorer prognostic risks, respectively, when applying the IPSS-M molecular features, a clear heterogeneity of prognostic risk within the morphologic subgroups has been demonstrated.68,73 Further molecular analysis of these morphologic subgroups is warranted to aid clinical evaluation and understanding of their biologic classification and prognostic risk.
Future of prognostication in MDS
The trajectory of prognostic classification for MDS has followed similar modes of development for most classifications and is dependent on progress in methods of evaluation of the specific process being analyzed.74 In the case of MDS, this refinement has been provided by statistical analyses of improved morphologic and clinical assessments (FAB cooperative group, WHO, and clinical trials) and technologic advances in biology (cytogenetics and mutational analyses; Figure 1). Further evaluation of molecular heterogeneity is anticipated, with additional mutational subclassification of patients with MDS.75 In addition, prior investigations of transcriptomic abnormalities within MDS CD34+ cells have provided data indicating prognostic differences based on differing gene expression profiles that were additive to the patients’ mutational features.76-78 The extension of such studies should determine the potential future contribution of integrating exomic and transcriptomic data for assessing prognostic information. These approaches may include further integration of whole-genome analysis and implementation of artificial intelligence. However, there is an ongoing need for less costly and more efficient molecular investigation as the field of MDS evolves. To address this concern, using targeted prognostic panels with a limited number of relevant genes (∼30-40) would be beneficial.
The field is also moving forward by going back to prognosticating the potential evolution to MDS from early myeloid clonal hematopoiesis (CH.) This approach can provide information that allows for proactive identification of predisposition states for MDS. As such, following several earlier studies,79-81 the ability to risk-stratify and prognosticate these patients is now being assessed in the CH arena. A recent study82 investigated the risk factors for CH progression to MDS, establishing a molecularly based CH prognostic model. The resultant CH risk score represents an additional step toward improving somatic myeloid disease prognostication. Analogous efforts are warranted for assessing the role of germ line mutations in myeloid disease prognostication.
Importantly, additional progress in the future of our field requires extensive collaboration from all stakeholders, including patients and advocacy organizations, clinicians in the community and in academics, and pharmaceutical companies engaged in myeloid drug development. The goals for our patients include QoL and quantity of life, both of which can be enhanced through simpler and more cost-effective diagnostic testing and prognostic algorithms that can be implemented worldwide. Such approaches should aid in the more effective development of drugs and their usage capable of altering the natural history of MDS.
Conclusions
The ability to risk-stratify MDS patients with the potential to evolve to frank AML or lose meaningful hematopoietic capacity is essential to manage the expectations of patients and providers alike. Over the years, progressive iterations of prognostic scoring systems have been developed for clinical care of these patients. The recently expanding compendium of sequencing analysis offers insights into the pathobiology of MDS. In 2023, the IPSS-M is a timely and valuable tool to tailor clinical decision-making based on each patient’s hematologic, morphologic, and molecular profiles. Such improvements in risk stratification/prognostication for MDS applied along with further molecular characterization of this heterogeneous spectrum of disease should prove useful for providing additional therapeutic interventions to alter the natural history of MDS.
Authorship
Contribution: A.E.D. and P.L.G. reviewed the literature and wrote the manuscript.
Conflict-of-interest disclosure: A.E.D. received consulting fees from Geron, Bristol Myers Squibb, Novartis, Regeneron, Sobi, Caribou, and Gilead. P.L.G. received consulting fees from Bristol Myers Squibb, Novartis, and Gilead and research study funding from Gilead and Bristol Myers Squibb.
Correspondence: Amy E. DeZern, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans St, CRBI Room 3M87, Baltimore, MD 21287; email: adezern1@jhmi.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal