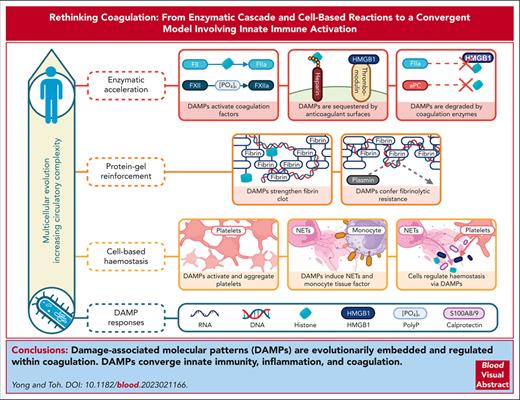
Visual Abstract
Advancements in the conceptual thinking of hemostasis and thrombosis have been catalyzed by major developments within health research over several decades. The cascade model of coagulation was first described in the 1960s, when biochemistry gained prominence through innovative experimentation and technical developments. This was followed by the cell-based model, which integrated cellular coordination to the enzymology of clot formation and was conceptualized during the growth period in cell biology at the turn of the millennium. Each step forward has heralded a revolution in clinical therapeutics, both in procoagulant and anticoagulant treatments to improve patient care. In current times, the COVID-19 pandemic may also prove to be a catalyst: thrombotic challenges including the mixed responses to anticoagulant treatment and the vaccine-induced immune thrombotic thrombocytopenia have exposed limitations in our preexisting concepts while simultaneously demanding novel therapeutic approaches. It is increasingly clear that innate immune activation as part of the host response to injury is not separate but integrated into adaptive clot formation. Our review summarizes current understanding of the major molecules facilitating such a cross talk between immunity, inflammation and coagulation. We demonstrate how such effects can be layered upon the cascade and cell-based models to evolve conceptual understanding of the physiology of immunohemostasis and the pathology of immunothrombosis.
Introduction
The cascade1 and cell-based models2 of coagulation are 2 key milestones in the history of hematology and medicine. Both have been pivotal in framing global understanding of hemostasis and thrombosis. Each, in turn, has laid the foundation upon which clinical quandaries are rationalized. Additionally, these conceptual advancements catalyzed therapeutic development in both bleeding and thrombotic diseases, ranging from initial clotting factor replacements to manipulation of key coagulation reactions, with the impact extending from rare diseases into population health.3-5
Each, in turn, has also revealed areas that did not fit neatly with the model and gaps in our knowledge. Catalyzed by our shared experiences during the COVID-19 pandemic, it is increasingly apparent that there are clinically important linkages or cross talk involving coagulation that now need to be better understood to improve patient care. The rapid gain in information presents a timely challenge to join fragmented evidence and a timely opportunity to reconcile old with new knowledge in a revised concept of coagulation. As with the impact of the cascade and the cell-based models, a new model must form the foundation on which innovations arise to help patients with hemostatic and thrombotic challenges. We present a look back at coagulation history to discern the patterns, lessons, and pitfalls to inform how meaningful progress can evolve our thinking and clinical approach.
Historical perspectives
Lessons from advances in biochemistry
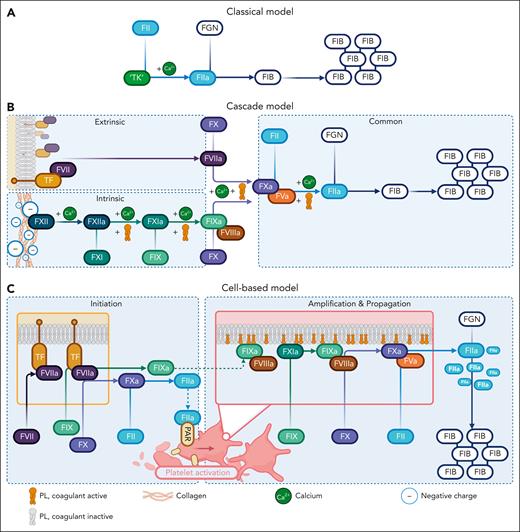
The cascade or waterfall model defined coagulation understanding in the 1960s during a period of significant advancements in biochemistry.6 It supplanted Morawitz’s classical theory of coagulation as a two-step process.7 This involved thrombokinase, which is a tissue extract containing tissue factor (TF) and coagulant-active phospholipids (PLs), reacting with prothrombin in the presence of calcium to generate thrombin. Thrombin then converts fibrinogen into fibrin (Figure 1A).7 However, there were inconsistencies, including bleeding that was not corrected by prothrombin extracts but by normal plasma in patients with hemophilia.6 Availability of coagulation times, that is, prothrombin time and activated partial thromboplastin time (aPTT), and new protein purification techniques enabled identification of new coagulation factors.7,8 The enzymological nature of coagulation was uncovered with the preceding coagulation enzyme sequentially activating the next by limited proteolysis of its zymogen (Figure 1B).6 Two important corrections came later: linking of intrinsic and extrinsic pathways at the level of factor X (FX),9 and the recognition of FV and FVIII as cofactors rather than enzymes.10
Historical models of coagulation. (A) Morawitz classical theory demonstrating prothrombin (FII) activation to thrombin (FIIa) by thrombokinase (TK) in the presence of calcium. Thrombin converts fibrinogen (FGN) into a fibrin (FIB) clot. (B) The cascade or waterfall model, illustrating sequential enzyme activation initiated by extrinsic and intrinsic triggers, which merge into the common pathway at FX to generate thrombin. (C) The cell-based model. The TFase and phosphatidylserine (PS) surfaces coordinate enzymology in 3 overlapping stages to generate a thrombin surge required for clot formation. The figure highlights how each concept builds on preexisting models to advance coagulation understanding.
Historical models of coagulation. (A) Morawitz classical theory demonstrating prothrombin (FII) activation to thrombin (FIIa) by thrombokinase (TK) in the presence of calcium. Thrombin converts fibrinogen (FGN) into a fibrin (FIB) clot. (B) The cascade or waterfall model, illustrating sequential enzyme activation initiated by extrinsic and intrinsic triggers, which merge into the common pathway at FX to generate thrombin. (C) The cell-based model. The TFase and phosphatidylserine (PS) surfaces coordinate enzymology in 3 overlapping stages to generate a thrombin surge required for clot formation. The figure highlights how each concept builds on preexisting models to advance coagulation understanding.
Although the science was driven by a procoagulant focus, the need for an anticoagulant “circuit breaker” was hypothesized by MacFarlane at inception of the cascade model.1 A decade later, key procoagulant factors and cofactors were found to be inactivated by proteolytic cleavage, for example, thrombin and FXa by antithrombin (AT) and FVa and FVIIIa by activated protein C (APC). Lessons from coagulation enzymology also led to identification of anticoagulant cofactors (eg, protein S and heparin).
In tandem with these significant biochemical advancements, therapeutic developments arose, especially with replacement therapeutics in congenital hemophilia, first with cryoprecipitates, followed by plasma-purified factor concentrates from the mid-1960s.11 In the then nascent field of anticoagulation, warfarin and heparin had already entered the clinic by 1960 although their mechanistic actions remained elusive for another 3 decades.12,13 It is now common knowledge that warfarin interferes with the vitamin K–dependent biosynthesis of γ-carboxyglutamic acid (Gla) residues on FX, FIX, FVII and prothrombin.8 Clustered within the N-terminus of these factors, Gla chelation of calcium facilitates charge-dependent binding to the anionic phosphate headgroups on PLs.14 Additionally, Gla-calcium binding changes the N-terminus conformation to expose a hydrophobic segment, which its crucial for PL binding.14
The cascade model provided a dependable platform to rationalize abnormalities in coagulation times. Inconsistencies soon arose, including why deficiencies of proximal factors of the intrinsic pathway resulted in highly variable hemorrhage risks, unrepresented by the degree of aPTT prolongation,2 and why an intact extrinsic pathway could not offset the intrinsic pathway defects of hemophilia, given the important role of TF-FVIIa in clot formation.
Lessons from advances in cellular biology
Inception of the cell-based model of coagulation at the turn of the millennium followed a period of rapid advancements in cellular biology. Through 3 overlapping stages of initiation, amplification, and propagation, the cell-based model highlighted how different cell surfaces coordinate coagulation enzymology toward clot formation (Figure 1C).15 Some of the groundwork had already been laid, particularly on the role of Gla domains in membrane-dependent complex assembly. The importance of coagulant-active PL was highlighted by Giles and Nesheim,16 who uncovered the prothrombotic factor responsible for the bypassing activity of prothrombin complex concentrates in hemophilia patients with inhibitors as PL contaminants, which accelerated the prothrombinase potency of FXa. In further progress, Hoffman et al17 resolved the role of TFase-generated FIXa. Upon injury, TFase-FXa generates a thrombin spark, which activates platelets via protease activated receptors (PARs) but is by itself incapable of forming fibrin.15,17 FXa is restricted to TFase surfaces because it is rapidly digested by AT and tissue factor pathway inhibitor (TFPI) in the fluid phase.17 By contrast, TFase-generated FIXa is stable in solution, allowing the thrombin spark to transmit onto activated platelet surfaces. This causes a thrombin surge via the extrinsic pathway, which is sufficient to tip toward clot formation.17 Later, Hoffman2 demonstrated how platelet surfaces underpinned the hemostatic efficacy of purified FVIIa as a bypassing agent. Despite not expressing TF, activated platelets at the site of injury provides a surface for FVIIa to localize and generate sufficient FXa to fuel prothrombinase.2
The charge dependence of coagulation complex assembly is also highlighted in the role of cofactors. Calcium bridges light and heavy chains with the heavy chain affecting cofactor activity18 and the light chain interacts with PL membranes.14 This fundamental interaction between proteins and lipids is critical in defining binding affinities and enzyme kinetics.19 With cell-derived microparticles extending the availability of membrane surfaces, PL composition also affects the response, with enrichment of phosphatidylserine and phosphatidylinositol over phosphatidylcholine favoring thrombogenesis.20
Advancements in cell biology also led to increased understanding of cellular receptors in both localizing reactions and signaling functions. The procoagulant receptors include TF and glycoprotein 1b/9/5, whereas anticoagulant counterparts include heparin (a glycosaminoglycan), thrombomodulin (TM), and endothelial protein C receptor. Knowledge of their signaling properties helped unravel links to other processes, for example, inflammation.
Therapeutic advancements in this period fused both enzymological and cellular knowledge. This included development of direct anticoagulants targeting the active sites of key coagulation enzymes in Xase and prothrombinase complexes.3,5 Advancements in molecular genetics ushered in the era of recombinant therapeutics for factor replacement therapy, reducing immunogenicity and avoiding horizontal transmission of blood-borne diseases while opening the door toward prolonged half-life products, designer noncoagulation factor therapeutics, for example, emicizumab, and cure-oriented gene therapy.4
Lessons from the COVID-19 pandemic
The coexistence of thrombogenicity and hyperinflammation was evident early on in the COVID-19 pandemic. The thrombotic profile was unusual, with evidence of pulmonary thromboses rather than emboli21 at unusual sites and with unexpected demographics, for example, large-vessel strokes in the young.22 The laboratory findings were also atypical, with elevated fibrinogen, von Willebrand factor (VWF), and D-dimer levels when coagulation times, platelet levels, and anticoagulant concentrations remained relatively unchanged. The importance of D-dimer elevation was highlighted by its association with an increased need for intensive care unit admission, mechanical ventilation, and mortality, which led to its use to stratify patients with high-risk COVID-19.23
Initially, enthusiasm for heparinization at higher doses was driven by reported high-breakthrough venous thromboembolism rates among patients with COVID-19 receiving thromboprophylaxis requiring intensive care unit admission although similar rates had been found during the H1N1 influenza epidemic.24,25 Early observational trials showed that prophylactic heparinization reduced 28-day mortality in those with either COVID-19 coagulopathy or greater than sixfold D-dimer elevation.26 However, large-scale, randomized, controlled trials showed escalated-dose prophylaxis to be ineffective in critically ill cohorts.23 Conversely, benefit was seen for those with less severe disease.27 Antiplatelet drugs were also tested and ultimately disproved.28
These findings point to inconsistencies or gaps in our conventional thinking. However, it is well established that the coexistence of coagulopathy worsens outcome of the primary condition even in the absence of overt disseminated intravascular coagulation (DIC).29 COVID-19 is no different and highlights how our current concepts of innate immunity and coagulation as largely separate processes needs revising. The pathological consequences of this cross talk are encapsulated by the term immunothrombosis, coined by Engelmann and Massberg a decade ago and well reviewed elsewhere.30-32 Immunothrombotic involvement has been increasingly evidenced in COVID-19, vaccine-induced immune thrombotic thrombocytopenia and other critical illness.31,32 Key mediators are host-derived, damage-associated molecular patterns (DAMPs), for example, cell-free histones (CFHs), cell-free DNA (cfDNA), and cell-free RNA (cfRNA) released after cell injury (Figure 2) and in response to pathogen-associated molecular patterns (PAMPs), for example, lipopolysaccharides (LPSs) and β-D-glucan.33 Like DAMPs, PAMPs possess evolutionary conserved characteristics that are immediately recognized by pattern recognition receptors, for example, toll-like receptors (TLRs), which trigger common procoagulant, proinflammatory, and immunological responses toward injury.34
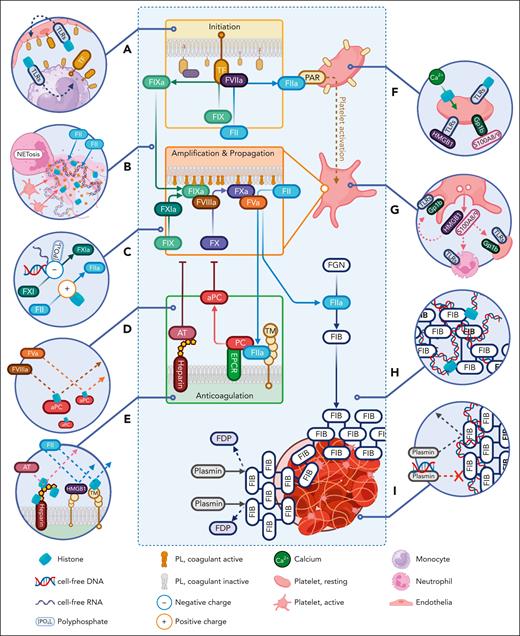
DAMPs and immunothrombosis. (A) In an overlay of the cell-based model, DAMPs (eg, CFHs) trigger clot formation by inducing TF expression. (B) DAMPs induce NETs, which release procoagulant material and structurally facilitates clot assembly. (C) DAMP-mediated charged interactions directly activate coagulation enzymes, for example, prothrombin activation by positively charged CFHs and contact activation of FXI and FXII by the negative charge of polyphosphates, cfDNA, and cfRNA. (D) DAMPs antagonize the innate anticoagulant system; CFH neutralizes APC. (E) CFH and HMGB1 electrostatically bind to TM, inhibiting protein C (PC) activation. CFH also binds heparin-glycosaminoglycans to inhibit AT potentiation. (F) Via DAMP receptors, cfDNA, CFHs, HMGB1, and calprotectin (S100A8/9) activate and aggregate platelets. CFHs also activate platelets by directly forming calcium ionophores on platelet membranes. (G) After activation, platelets express and secrete HMGB1 and calprotectin, which further augment the procoagulant signal in an automated/paracrine manner. (H) Integration of CFH and cfDNA into a FIB (composite) clot enhances its biomechanical properties. (I) This composite clot itself is resistant to lysis by plasmin. cfDNA also directly inhibits plasmin. EPCR, endothelial protein C receptor; FDP, fibrin degradation product; S100A8/9, calprotectin.
DAMPs and immunothrombosis. (A) In an overlay of the cell-based model, DAMPs (eg, CFHs) trigger clot formation by inducing TF expression. (B) DAMPs induce NETs, which release procoagulant material and structurally facilitates clot assembly. (C) DAMP-mediated charged interactions directly activate coagulation enzymes, for example, prothrombin activation by positively charged CFHs and contact activation of FXI and FXII by the negative charge of polyphosphates, cfDNA, and cfRNA. (D) DAMPs antagonize the innate anticoagulant system; CFH neutralizes APC. (E) CFH and HMGB1 electrostatically bind to TM, inhibiting protein C (PC) activation. CFH also binds heparin-glycosaminoglycans to inhibit AT potentiation. (F) Via DAMP receptors, cfDNA, CFHs, HMGB1, and calprotectin (S100A8/9) activate and aggregate platelets. CFHs also activate platelets by directly forming calcium ionophores on platelet membranes. (G) After activation, platelets express and secrete HMGB1 and calprotectin, which further augment the procoagulant signal in an automated/paracrine manner. (H) Integration of CFH and cfDNA into a FIB (composite) clot enhances its biomechanical properties. (I) This composite clot itself is resistant to lysis by plasmin. cfDNA also directly inhibits plasmin. EPCR, endothelial protein C receptor; FDP, fibrin degradation product; S100A8/9, calprotectin.
Current perspectives in immunothrombosis
NETs and innate cellular activation
The discovery of neutrophil extracellular traps (NETs) highlighted the additional importance of innate immune activation to the bidirectional relationship between coagulation and inflammation.35 The process of NET formation (NETosis) involves extrusion of nuclear and cytoplasmic content in a web-like amalgam to immobilize and kill invading pathogens. However, NETs have been implicated in venous and arterial thrombogenesis.36,37 The prothrombotic effects are through both its components and structural surfaces.38 The juxtapositioning of blood cells and procoagulant factors accelerates thrombin generation and fibrin formation, which entwines into the NETs scaffold to reinforce occlusive effects. The bidirectional relationship between NETs and platelets further underpins the integrative properties of NETs. Activated platelets adhere to neutrophils through P-selectin39 as platelet-derived high mobility group box-1 (HMGB1) and calprotectin stimulate NETosis via paracrine effects.40 Reciprocally, platelets are activated by NET-tethered CFHs or C3b.35 NET components that are not DAMPs also participate, for example, digestion of TFPI and AT by neutrophil proteases.41
In addition to neutrophils, the monocyte-macrophage axis is another innate cellular mediator of immunothrombosis, by providing both TFase and phosphatidylserine surfaces. Emerging evidence suggests type 1 interferon (IFN) as a key inducer of TF activity in macrophages.42 Type 1 IFN signaling is procoagulant via hepatocyte release of a HMGB1 intermediary, which, in the presence of LPS cosignaling, induces noncanonical nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain–containing protein 3 (NLRP3) inflammasome–dependent macrophage pyroptosis to expose phosphatidylserine and upregulate TF activity.43,44 IFN-NLRP3 inflammasome activity is further augmented by the transcriptional upregulation of its components by damage signals, both DAMPs and PAMPs, via the nuclear factor κB pathway.45
Nuclear DAMPs
During injury, NETosis and cell death are major contributors of DAMPs, of which CFH is an abundant component.46,47 The strong cationic charge of histones confers avid affinity toward anionic phosphate groups. Although this is vital for intranuclear DNA condensation, CFH binding to phosphate-rich lipid membranes causes pore formation and damaging calcium fluxes. These effects are enhanced by parallel signaling through TLRs. CFH has a well characterized procoagulant profile, which has been comprehensively reviewed.48
CFH release is inextricably paired with cfDNA as chromatin. cfDNA is itself procoagulant, which is thought to require intracirculatory dissociation from CFH.38 Alongside cfRNA, cfDNA presents a polyanionic scaffold that promotes contact activation of FXI and FXII.49 However, this procoagulant mechanism is possibly overstated, being artefactually enhanced during in vitro evaluation by highly procoagulant silica contaminants in aPTT assays.50 Silica-free preparations of cfDNA possess weak contact activating capability, whereas those of cfRNA are highly dependent on its base composition.50,51 Although incompletely elucidated, cfDNA and cfRNA have other procoagulant effects, for example, cfDNA enhancement of TFPI degradation by neutrophil elastase41 and the inhibition of fibrinolysis.52,53 Additionally, cfDNA binds and induces thrombin autoproteolysis, generating thrombin-derived C-terminal fragments. These fragments bind and protect cfDNA (including those in NETs) from nuclease degradation to promote thrombogenicity.54
Silica-based methods also copurify cytoplasmic polyphosphates alongside nucleotides, which like DNA and RNA are polyanionic polymers released as DAMPs or PAMPs.50,55 When directly compared in vitro, polyphosphates are significantly more potent contact activators of coagulation than nucleotides.50 Polyphosphates also accelerate procoagulant reactions by facilitating downstream coagulation complex assembly,55,56 enhance HMGB1-mediated endothelial VWF release,57 and augment VWF-platelet glycoprotein 1b interactions.57 Polyphosphates also possess proinflammatory and complement regulatory effects, as comprehensively reviewed by Conway58 and Baker et al.59
HMGB1 are nonhistone nuclear proteins whose functions include DNA chaperoning and regulation.60 Its cationic C-terminus decondenses chromosomes through charge-dependent interactions with linker DNA segments. Extracellularly, HMGB1 are DAMPs with redox-dependent effects signaled through the receptor for advanced glycation end products and TLRs 2 and 4.60 Its primary procoagulant mechanism is platelet dependent.40,61,62 Activated platelets also secrete and express surface HMGB1 despite being anuclear, suggesting that HMGB1 is integral to platelet function through an automated/paracrine manner to confer early injury enhancement of platelet function.61,62 In a mouse model engineered to lack HMGB1 in platelets, bleeding time is prolonged, whereas thrombus formation, inflammation, and organ damage are reduced during experimental shock.61 In a thrombin-induced DIC rat model, HMGB1 coinfusion accelerates development of CD41+ small vessel thrombosis.62
Through platelet-independent mechanisms, HMGB1 stimulates NETosis40 and monocyte TF expression.40,62 HMGB1 also inhibits protein C activation by binding TM to prevent thrombin-TM interactions.62 Thrombin-HMGB1 procoagulant synergism has also been suggested,62 although the exact mechanism of this remains unclear.
Cytosolic DAMPs
These include members of the S100 family of cytosolic calcium modulators, S100A8 and S100A9, which heterodimerize to form calprotectin.63 Calprotectin is an important regulator of myeloid function. Intracellularly, it modulates calcium signaling and cytoskeletal reorganization, whereas extracellularly, it is both a chemoattractant and a proinflammatory activator of leucocytes.64 Activated neutrophils transfer calprotectin to platelets after necrosis or NETosis, with platelet calprotectin content being proportionate to neutrophil counts.65 Calprotectin also induces platelet thrombogenicity. Platelets adhere to immobilized recombinant calprotectin under venous shear to generate fibrin, and calprotectin knockout reduces platelet responsiveness to collagen.63,64 Calprotectin also dose-dependently induces platelet PS exposure and PS-positive microparticle release.63 Glycoprotein 1b is the primary platelet receptor for calprotectin, and the binding potentiates VWF-mediated platelet aggregation ex vivo.63
Evidence suggests that the diverse effects of extracellular calprotectin bridge the links between cellular effectors of inflammation, innate immunity, and coagulation.66 In vivo, calprotectin knockout impairs leucocyte recruitment and transendothelial migration67 to reduce infiltration and cellular proliferation in response to injury.66 Calprotectin also enhances endotoxin-induced phagocytosis via TLR4.68 Similar effects have been described for other cytosolic molecules extracellularly, for example, adenosine triphosphate, cathepsin G, elastase, and myeloperoxidase, and these have been fully reviewed elsewhere.69
Future outlook and the convergent model of coagulation
Patterns in evolutionary hemostasis
Molecules classified as DAMPs extracellularly have vital intracellular roles. In encoding life itself, DNA is phylogenetically the oldest (Figure 3). DNA chaperones, for example, histones and HMGB1, facilitate intranuclear genomic organization required for evolution toward multicellularity. To meet the metabolic demands of increasing complexity, the need for an injury response to preserve circulatory integrity has led to the same vital molecules responsible for cell health also becoming endogenous injury signals. Additional layering and refinements along the evolutionary timeline have deeply embedded DAMPs within the physiology of injury responses.
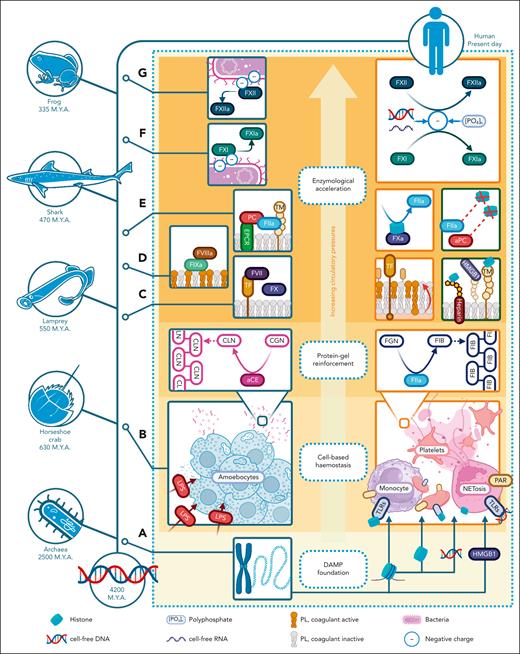
Evolutionary hemostasis in the response to injury. This figure shows how DAMPs, being the foundation upon which other hemostatic components are layered, have been evolutionarily embedded and regulated as a component of coagulation. The injury response coevolved in tandem with the complexity of the multicellular organism and its circulatory tree. Evolutionary emergence of key hemostatic components, based on comparative studies in extant animals performed by Doolittle and others (referenced in main text), is charted on a timeline (left) in parallel with DAMP interactions with each emerging hemostatic component (right). (A) DAMPs have vital intracellular function. Some, for example, RNA, DNA, and histones, are the very foundation of multicellular life. (B) Exemplified by the horseshoe crab, the most primitive injury response is facilitated by cells, undifferentiated by hemostatic or immune purpose. Injury-derived LPS activates and aggregates circulating amoebocytes. LPS also triggers formation of a coagulin (CLN) protein gel from amoebocyte-released coagulation proteins. (C) TF, FVII, FX, prothrombin (FII), and FGN evolved before the appearance of jawed animals, followed by FV, FIX (D), and the TM-PC pathway (E). (F-G) The emergence of FXII and FXI is more recent and links coagulation to the contact pathway, activatable by negatively charged DAMPs, including polyphosphates, cfRNA, and cfDNA. aCE, activated coagulogen enzyme; CGN, coagulogen; M.Y.A., million years ago.
Evolutionary hemostasis in the response to injury. This figure shows how DAMPs, being the foundation upon which other hemostatic components are layered, have been evolutionarily embedded and regulated as a component of coagulation. The injury response coevolved in tandem with the complexity of the multicellular organism and its circulatory tree. Evolutionary emergence of key hemostatic components, based on comparative studies in extant animals performed by Doolittle and others (referenced in main text), is charted on a timeline (left) in parallel with DAMP interactions with each emerging hemostatic component (right). (A) DAMPs have vital intracellular function. Some, for example, RNA, DNA, and histones, are the very foundation of multicellular life. (B) Exemplified by the horseshoe crab, the most primitive injury response is facilitated by cells, undifferentiated by hemostatic or immune purpose. Injury-derived LPS activates and aggregates circulating amoebocytes. LPS also triggers formation of a coagulin (CLN) protein gel from amoebocyte-released coagulation proteins. (C) TF, FVII, FX, prothrombin (FII), and FGN evolved before the appearance of jawed animals, followed by FV, FIX (D), and the TM-PC pathway (E). (F-G) The emergence of FXII and FXI is more recent and links coagulation to the contact pathway, activatable by negatively charged DAMPs, including polyphosphates, cfRNA, and cfDNA. aCE, activated coagulogen enzyme; CGN, coagulogen; M.Y.A., million years ago.
An example of the earliest cell-mediated response to injury is in the invertebrate Limulus polyphemus (the Atlantic horseshoe crab), which has remained relatively unchanged since the time of trilobites. Amoebocytes are its sole circulating cells, which aggregate, degranulate, and phagocytose microbes in response to exogenous injury signals. Amoebocyte granules contain coagulation factors, which autoactivate upon contact with LPS and β-D-glucan to proteolyze soluble coagulogen into insoluble coagulin gel.70 Factor C, which recognizes LPS, contains short consensus repeat domains found in mammalian complement proteins.70 The dual role of amoebocytes also hints at a common ancestry of cell-mediated responses, which later diverged in vertebrates into more elaborate hemostatic and immunological roles.71
The enzymology of vertebrate coagulation is phylogenetically less old. Core components are highly conserved, and TF, FVII, FX, prothrombin, and fibrinogen are detected in the most primitive extant vertebrate, that is, jawless fish (eg, lampreys).72 The sequence of emergence is unclear, although it is likely that thrombin was the first, by being an agglutinator of platelet ancestors through proteolysis of PARs.72 The later appearance of fibrinogen as an alternative thrombin substrate provided fibrin gel reinforcement.72 Later evolutionary additions to vertebrate coagulation also demonstrate relevant links with DAMPs. Key injury signals, for example, cfDNA and LPS, are highly charged and can autoactivate contact pathway enzymes. FXI, as the “youngest” coagulation enzyme phylogenetically, links the contact pathway to the extrinsic cascade whils being capable of contact activation itself.
Connected pathways
Crossregulation between seemingly distinct injury responses also points toward common evolutionary roots. Although the connection between coagulation and complement is well described, there is crossregulation between DAMPs and complement (Figure 4; supplemental Table 1, available on the Blood website). CFH electrostatically binds C4 to inhibit progression along the classical and mannose-binding lectin cascades. This binding results in both a reduction in membrane attack complex formation73 and in CFH-mediated cytotoxicity. C1q also modulates HMGB1 signaling by forming a quaternary complex alongside their receptors (leucocyte-associated immunoglobulin-like receptor-1 and receptor for advanced glycation end products) on monocytes surfaces to shift differentiation toward an anti-inflammatory M2 phenotype.74 These checks and balances across pathways indicate the permeating role of DAMPs across all injury responses.
Crossregulation of DAMPs by the complement and coagulation pathways. Circulating protein sensors (eg, C3, C1q, mannose-binding lectin [MBL], or FVII) bind injury-associated ligands (eg, microbial surfaces, microbial proteins, or TF) to trigger a biochemical response, amplified through a protease cascade and reinforced by cross talk between pathways. The final protein product mounts the requisite injury responses of cytotoxicity or coagulation. This is subjected to homeostatic regulation by noninjured surfaces, which suppresses these responses. As part of the injury signal, DAMPs modulate both complement and coagulation. (A) DAMPs can enhance complement activation. HMGB-1 binds to C1q and activates C2 and C4. (B) Conversely, DAMPs also neutralize complement-associated inflammatory signaling to prevent excessive collateral damage. HMGB1–receptor for advanced glycation end product and C1q–LAIR-1 complexing on monocyte surfaces promotes anti-inflammatory differentiation. (C) Binding of CFH at the level of C4 inhibits downstream complement activation. CFH-C4 binding reciprocally neutralizes CFH toxicity. (D) FIIa and APC can also neutralize DAMPs via proteolysis. (E) Anticoagulant surfaces can bind and sequester DAMPs, although higher DAMP doses competitively inhibit these anticoagulant properties. LAIR-1, leucocyte-associated immunoglobulin-like receptor-1MAC, membrane attack complex.
Crossregulation of DAMPs by the complement and coagulation pathways. Circulating protein sensors (eg, C3, C1q, mannose-binding lectin [MBL], or FVII) bind injury-associated ligands (eg, microbial surfaces, microbial proteins, or TF) to trigger a biochemical response, amplified through a protease cascade and reinforced by cross talk between pathways. The final protein product mounts the requisite injury responses of cytotoxicity or coagulation. This is subjected to homeostatic regulation by noninjured surfaces, which suppresses these responses. As part of the injury signal, DAMPs modulate both complement and coagulation. (A) DAMPs can enhance complement activation. HMGB-1 binds to C1q and activates C2 and C4. (B) Conversely, DAMPs also neutralize complement-associated inflammatory signaling to prevent excessive collateral damage. HMGB1–receptor for advanced glycation end product and C1q–LAIR-1 complexing on monocyte surfaces promotes anti-inflammatory differentiation. (C) Binding of CFH at the level of C4 inhibits downstream complement activation. CFH-C4 binding reciprocally neutralizes CFH toxicity. (D) FIIa and APC can also neutralize DAMPs via proteolysis. (E) Anticoagulant surfaces can bind and sequester DAMPs, although higher DAMP doses competitively inhibit these anticoagulant properties. LAIR-1, leucocyte-associated immunoglobulin-like receptor-1MAC, membrane attack complex.
As for the bidirectional connection between inflammation and coagulation, these are well described75 and summarized in supplemental Table 1. Increasingly, DAMPs are recognized as mediators of the cross talk. Its procoagulant effects can be both direct and indirect via its proinflammatory actions, for example, via tumor necrosis factor α and interleukin-1 (IL-1). Its physiological role, rather than its role only in disease pathogenesis, is indicated by involvement in the homeostatic mechanisms that regulate the intensity and duration of the coagulant-inflammatory healing response to injury. The thrombin-APC axis is of major importance, and the balance between procoagulant/anticoagulant effects and pro/anti-inflammatory signals via PAR1 can pivot accordingly, for example, presence of CFH and degradation by thrombin or APC, and histone 2B-PAR1 interactions in facilitating plasmin-induced cell migration during wound healing.76,77
Many of the homeostatic mechanisms use the charged nature of DAMPs. An example is CFH binding to anionic monocyte membranes to cause release of IL-6, which stimulates the hepatic acute phase response to increase circulating C-reactive protein to electrostatically neutralize CFHs.78 The highly oxidative milieu generated by neutrophil-derived reactive oxidative species also affects charge in redox-sensitive DAMPs. In vivo, oxidation of S100A8 nullifies its chemotactic capabilities,79 whereas oxidation of HMGB1 neutralizes its immunostimulatory function.80 Conversely, oxidation of cfDNA confers resistance to DNase degradation.81 These will also modulate their procoagulant effects, as detailed earlier. In ischemic stroke models, clearance of HMGB1 and S100A9 through electrostatic neutralization by cystine-rich domains in macrophage-derived apoptosis inhibitor promotes tissue healing.82
Evolving models of coagulation and the convergence of pathways
DAMPs participate at every stage of hemostatic clot formation (Table 1).48 Although our knowledge stemmed from observations in pathology, that is, at high circulating concentrations, the local physiological relevance of DAMPs is becoming clearer in light of its dose-dependent effects. In vitro, subcytotoxic doses of CFH (<30 μg/mL) increases TF expression and decryption in both endothelia and monocytes while suppressing TM expression.83,84 CFH doses as low as 5 to 10 μg/mL induce thrombin generation and platelet aggregation and inhibit APC generation ex vivo.56,93 Plasma from patients with sepsis with low cfDNA concentrations (<5 μg/mL) have significantly enhanced thrombin-generating capability compared with plasma from healthy patients.46 With circulating HMGB1 levels in patients with sepsis or DIC ranging between 0 and 10 nM, 1 nM HMGB1 inhibits APC activation by 10%.62
Procoagulant effects of key DAMPs
| DAMP . | Procoagulant mechanisms . | Reference . |
|---|---|---|
| CFHs | Upregulates TF expression and decryption on endothelial and monocytic surfaces | 83,84 |
| Autoactivates FVII-activating protease, which activates FVII and degrades TFPI | 85 | |
| Autoactivates prothrombin | 86 | |
| Binds to prothrombin and facilitates thrombin generation through the alternative prothrombinase complex | 87 | |
| Activates platelets, resulting in platelet degranulation and aggregation | 56 | |
| Induces endothelial Weibel-Palade body degranulation and ultralarge VWF release | 88 | |
| Induces coagulant-active phosphatidylserine expression on platelet, endothelial, and erythrocyte surfaces | 89,90 | |
| Increases clot robustness and fibrinolytic resistance by covalent and noncovalent incorporation | 52,91 | |
| Competitive inhibition of plasmin | 91 | |
| Induces NET formation | 92 | |
| Downregulates TM expression on endothelial surfaces | 83 | |
| Inhibits TM function by binding to extracellular domain, inhibiting PC activation | 93 | |
| Competitive inhibition of APC as an APC substrate | 47 | |
| Competitive inhibition of AT potentiation by binding to glycosaminoglycans including heparan sulfate | 94 | |
| NETs | A source of procoagulant DAMPs including CFHs, HMGB1, calprotectin, and cfDNA | 95 |
| Allows for the accumulation and juxtaposing of procoagulant cells and material | 35,41 | |
| Confers fibrinolytic resistance through clot incorporation | 35 | |
| Extruded neutrophil proteases degrade innate anticoagulants, including TFPI and AT | 41 | |
| cfDNA | Contact activation of FXI and FXII | 50 |
| Enhances TFPI degradation by neutrophil elastase | 41 | |
| Confers fibrinolytic resistance when incorporated into clots | 52 | |
| Inhibition of plasmin by competitive binding or by the formation of a plasmin-fibrin-cfDNA ternary complex | 53,96 | |
| Induces thrombin autoproteolysis, generating thrombin C-terminal fragments which protect cfDNA against degradation | 54 | |
| cfRNA | Contact activation of FXI and FXII | 50 |
| HMGB1 | Stimulates monocyte TF expression | 40,62 |
| Activates platelets, resulting in platelet degranulation and aggregation | 40,61,62 | |
| Induces NET formation | 40 | |
| Inhibits TM function by binding to extracellular domain, inhibiting PC activation | 62 | |
| Calprotectin | Augments platelet activation and aggregation | 63,64 |
| Polyphosphates | Contact activation of FXI and FXII | 50,55,56 |
| Inhibits TFPI activity and enhances its inactivation by FXIa | 50 | |
| Enhances HMGB1-mediated endothelial VWF release | 57 | |
| Augments VWF-GP1b interactions | 57 |
| DAMP . | Procoagulant mechanisms . | Reference . |
|---|---|---|
| CFHs | Upregulates TF expression and decryption on endothelial and monocytic surfaces | 83,84 |
| Autoactivates FVII-activating protease, which activates FVII and degrades TFPI | 85 | |
| Autoactivates prothrombin | 86 | |
| Binds to prothrombin and facilitates thrombin generation through the alternative prothrombinase complex | 87 | |
| Activates platelets, resulting in platelet degranulation and aggregation | 56 | |
| Induces endothelial Weibel-Palade body degranulation and ultralarge VWF release | 88 | |
| Induces coagulant-active phosphatidylserine expression on platelet, endothelial, and erythrocyte surfaces | 89,90 | |
| Increases clot robustness and fibrinolytic resistance by covalent and noncovalent incorporation | 52,91 | |
| Competitive inhibition of plasmin | 91 | |
| Induces NET formation | 92 | |
| Downregulates TM expression on endothelial surfaces | 83 | |
| Inhibits TM function by binding to extracellular domain, inhibiting PC activation | 93 | |
| Competitive inhibition of APC as an APC substrate | 47 | |
| Competitive inhibition of AT potentiation by binding to glycosaminoglycans including heparan sulfate | 94 | |
| NETs | A source of procoagulant DAMPs including CFHs, HMGB1, calprotectin, and cfDNA | 95 |
| Allows for the accumulation and juxtaposing of procoagulant cells and material | 35,41 | |
| Confers fibrinolytic resistance through clot incorporation | 35 | |
| Extruded neutrophil proteases degrade innate anticoagulants, including TFPI and AT | 41 | |
| cfDNA | Contact activation of FXI and FXII | 50 |
| Enhances TFPI degradation by neutrophil elastase | 41 | |
| Confers fibrinolytic resistance when incorporated into clots | 52 | |
| Inhibition of plasmin by competitive binding or by the formation of a plasmin-fibrin-cfDNA ternary complex | 53,96 | |
| Induces thrombin autoproteolysis, generating thrombin C-terminal fragments which protect cfDNA against degradation | 54 | |
| cfRNA | Contact activation of FXI and FXII | 50 |
| HMGB1 | Stimulates monocyte TF expression | 40,62 |
| Activates platelets, resulting in platelet degranulation and aggregation | 40,61,62 | |
| Induces NET formation | 40 | |
| Inhibits TM function by binding to extracellular domain, inhibiting PC activation | 62 | |
| Calprotectin | Augments platelet activation and aggregation | 63,64 |
| Polyphosphates | Contact activation of FXI and FXII | 50,55,56 |
| Inhibits TFPI activity and enhances its inactivation by FXIa | 50 | |
| Enhances HMGB1-mediated endothelial VWF release | 57 | |
| Augments VWF-GP1b interactions | 57 |
GP1b, glycoprotein 1b; PC, protein C.
In this immunohemostatic response, DAMPs invoke both enzyme- and cell-based coagulation responses. Collectively, DAMPs augment the sequential enzymatic reactions and recruit surfaces for clot assembly (Figure 5). However, DAMPs can initiate thrombin generation independently of TF availability. cfDNA can trigger the intrinsic pathway and CFH can bind prothrombin and FXa in the absence of PL surfaces to generate sufficient thrombin to convert FV to FVa. This activates the intrinsic pathway and the amplification phase of coagulation. The physiological relevance of this CFH-mediated alternative prothrombinase has been demonstrated by the correction of hemostasis in a hemophilia mouse model.87
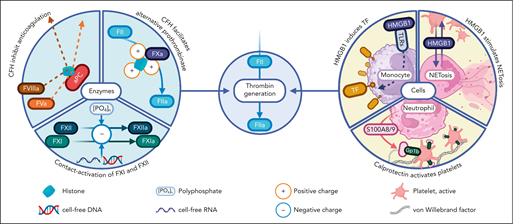
Thrombin generation by DAMPs. DAMPs promote thrombin generation through contact activation of FXI and FXII by negatively charged polyanions (eg, polyphosphates, cfRNA, and DNA), the alternative prothrombinase pathway mediated by histones, and inhibiting anticoagulant pathways. DAMP signals also trigger the cell-mediated injury response, promoting procoagulant factor release and procoagulant surfaces. DAMPs are expressed and/or released as part of the cell-mediated response to amplify the injury signal. GP1b, glycoprotein 1b.
Thrombin generation by DAMPs. DAMPs promote thrombin generation through contact activation of FXI and FXII by negatively charged polyanions (eg, polyphosphates, cfRNA, and DNA), the alternative prothrombinase pathway mediated by histones, and inhibiting anticoagulant pathways. DAMP signals also trigger the cell-mediated injury response, promoting procoagulant factor release and procoagulant surfaces. DAMPs are expressed and/or released as part of the cell-mediated response to amplify the injury signal. GP1b, glycoprotein 1b.
In parallel with how the ubiquity of thrombin modulates and integrates the dynamics of procoagulant/anticoagulant and fibrinolytic forces, DAMPs also converge such effects to ensure that the overall response is localzsed and proportionate to the hemostatic, antimicrobial, and healing requirements of injury. Incorporation of CFH and cfDNA into clots confers added strength and fibrinolytic resistance.48 High doses of cfDNA also inhibit plasmin activity, through either competitive inhibition96 or formation of an inactive fibrin-cfDNA-plasmin ternary complex.53
Current evidence, therefore, points to the omnipresence of DAMPs throughout the coagulation process, as an instigator, amplifier, propagator, and composite of the end product. The convergent model of coagulation proposes that innate immunity, inflammation, and coagulation are intertwined processes, which can inform development of new approaches to contemporary clinical challenges. Proposed new therapies have included DAMP inhibition (eg, noncoagulant heparin,97 polyanions,98 or thrombomodulin99) and the systemic use of extracorporeal molecular filters.100 Equally, this concept converges the cascade and cell-based models to highlight the potential relevance of targeting coagulation in multiple medical conditions.
Discussion
In piecing together the history of coagulation through its conceptual advancements and evolutionary stages, 3 themes have emerged. First is the integrated and pattern recognition nature of the survival response to injury. Second is the integral role of charge, localizing structures, and surfaces. Third is the impact of flourishing scientific fields and contemporaneous technological developments.
The danger signal and injury response is fundamental to survival and has existed from time immemorial. From rudimentary and shared beginnings, the response that respective disciplines have delineated as innate immunity, inflammation, and coagulation pathways have coevolved with circulatory development to meet the needs of multicellular organisms. This immunohemostatic response to pathogen invasion and fluid loss is deeply entwined and hard to unpick because of the crossregulatory effects. The heterogenous histopathology of thrombotic lesions, which can be platelet, fibrin, or NET-rich, likely reflects the underlying hemostatic response of the clinical condition or its anatomical location.
The fundamental importance of charge in key interactions extends beyond that of its earliest description in the modeling of coagulation. The recurring themes in all the (anti)coagulation enzymatic reactions of a vitamin K–dependent protein, a cofactor, calcium, and PL suggest evolutionary selection. The cell-based model further emphasizes its importance through increased availability of anionic PL surfaces for localizing enzymatic components. The proposed convergence model takes this further by highlighting how charge in DAMPs is innate to its multiple functions, including homing onto bacteria. This new model does not debunk its precedents but adds and evolves how we understand hemostatic plug formation and its resolution or pathological transition.
Development of the cascade model of coagulation arose during the heyday of biochemistry in the 1960s. Similarly, the cell-based model arose during major advances in cell and molecular biology around the 1990s. Both turning points in the history of coagulation have heralded therapeutic successes. Thirty years on, there is burgeoning crossdisciplinary science and technological advances in stereochemistry, artificial intelligence-assisted modeling, and whole genome sequencing to shift the understanding beyond single molecule structure and function to deciphering multiple interacting molecular and functional effects. This environment together with the multiplicity of new data makes it timely to rethink coagulation. In developing our perspective and proposal of a new model, we acknowledge the limitations of appraising literature that can be disjointed or contradictory and the challenges of assessing robustness in claims of relevant connections to coagulation. Nonetheless, there is a coherent concept built upon solid foundations that we believe can trigger the necessary debate, discussion, and delivery of new information that can fill the gaps in our knowledge.
With the present memory of the pandemic and the need for resilience building into the future, the lessons of antibody-mediated thrombotic syndromes, and ongoing concerns of vaccine-induced immune thrombotic thrombocytopenia–like presentations underscore the importance of improving knowledge around how, why, and when the injury response becomes dysfunctional. This would likely have implications beyond infection and autoimmune conditions and impact more broadly whenever there is associated endotheliopathy. Such conditions in which there are surges in DAMPs include new interventional modalities, for example, chimeric antigen receptor T-cell therapy. New diagnostics and therapeutics are needed and will require refreshed efforts that cross the conventional boundaries between coagulation, innate immune activation, and inflammation.
Acknowledgments
The authors are grateful to Colin Downey, Simon Abrams, and Guozheng Wang for many helpful discussions. The illustrations were created with BioRender.com.
This work was supported by Liverpool University Hospitals NHS Foundation Trust, the British Heart Foundation (PG/14/19/30751 and PG/16/65/32313), the Department of Health and Social Care. This study was supported by the National Institute for Health Research (NIHR135073).
The views expressed are those of the authors and not necessarily those of Liverpool University Hospitals NHS Foundation Trust, the British Heart Foundation, Department of Health and Social Care, or the National Institute for Health Research.
Authorship
Contribution: J.Y. and C.-H.T. contributed equally to the conceptualization of this review and in revising and finalizing the manuscript and figures for submission; and J.Y. reviewed the literature base and drafted the manuscript and figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cheng-Hock Toh, Department of Clinical Infection, Microbiology and Immunology, University of Liverpool, Ronald Ross Building, 8 West Derby St, Liverpool L69 7BE, United Kingdom; email: c.h.toh@liverpool.ac.uk.
References
Author notes
All data are available on request from corresponding author, Cheng-Hock Toh (c.h.toh@liverpool.ac.uk).
The online version of this article contains a data supplement.

![Crossregulation of DAMPs by the complement and coagulation pathways. Circulating protein sensors (eg, C3, C1q, mannose-binding lectin [MBL], or FVII) bind injury-associated ligands (eg, microbial surfaces, microbial proteins, or TF) to trigger a biochemical response, amplified through a protease cascade and reinforced by cross talk between pathways. The final protein product mounts the requisite injury responses of cytotoxicity or coagulation. This is subjected to homeostatic regulation by noninjured surfaces, which suppresses these responses. As part of the injury signal, DAMPs modulate both complement and coagulation. (A) DAMPs can enhance complement activation. HMGB-1 binds to C1q and activates C2 and C4. (B) Conversely, DAMPs also neutralize complement-associated inflammatory signaling to prevent excessive collateral damage. HMGB1–receptor for advanced glycation end product and C1q–LAIR-1 complexing on monocyte surfaces promotes anti-inflammatory differentiation. (C) Binding of CFH at the level of C4 inhibits downstream complement activation. CFH-C4 binding reciprocally neutralizes CFH toxicity. (D) FIIa and APC can also neutralize DAMPs via proteolysis. (E) Anticoagulant surfaces can bind and sequester DAMPs, although higher DAMP doses competitively inhibit these anticoagulant properties. LAIR-1, leucocyte-associated immunoglobulin-like receptor-1MAC, membrane attack complex.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/25/10.1182_blood.2023021166/2/m_blood_bld-2023-021166-c-gr4.jpeg?Expires=1768821877&Signature=t1iO-smY8RSa-Z3YpC4vLzTod9zaeSOS~1TFrKI12QzJedbhux9r7I-E5zvXepgIZvFkBHVQkryofZW77h9E1SHDumOCFibQJsoqp-C7zCQp4Y~WK9x1Eta9s9QsJLCIn6TsVJOQwsfd7i0qfiZDsXayE9rW6X33BW8EekkzUafYQbK41ZZcRoKxFrX34LxgbaBjSQ6DGLRhlrqenDZzW~K~dLR4UkCHwnUrEZsJj9tWvp9hPDqyWI7A4v17spX74HBjW7OZk819AohFyeZV57XylMIGKMXmq8nGYgwS0yacSB227lOrehbB3SS9krmlsKdrSmCruTKGlxQNDBp3Pw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal