Key Points
Children with T-ALL treated in the COG AALL0434 had excellent EFS and OS independent of the ETP status.
Persistent leukemia after induction was a strong predictor of inferior outcome in patients with non-ETP T-ALL.
Abstract
The early thymic precursor (ETP) immunophenotype was previously reported to confer poor outcome in T-cell acute lymphoblastic leukemia (T-ALL). Between 2009 and 2014, 1256 newly diagnosed children and young adults enrolled in Children’s Oncology Group (COG) AALL0434 were assessed for ETP status and minimal residual disease (MRD) using flow cytometry at a central reference laboratory. The subject phenotypes were categorized as ETP (n = 145; 11.5%), near-ETP (n = 209; 16.7%), or non-ETP (n = 902; 71.8%). Despite higher rates of induction failure for ETP (6.2%) and near-ETP (6.2%) than non-ETP (1.2%; P < .0001), all 3 groups showed excellent 5-year event-free survival (EFS) and overall survival (OS): ETP (80.4% ± 3.9% and 86.8 ± 3.4%, respectively), near-ETP (81.1% ± 3.3% and 89.6% ± 2.6%, respectively), and non-ETP (85.3% ± 1.4% and 90.0% ± 1.2%, respectively; P = .1679 and P = .3297, respectively). There was no difference in EFS or OS for subjects with a day-29 MRD <0.01% vs 0.01% to 0.1%. However, day-29 MRD ≥0.1% was associated with inferior EFS and OS for patients with near-ETP and non-ETP, but not for those with ETP. For subjects with day-29 MRD ≥1%, end-consolidation MRD ≥0.01% was a striking predictor of inferior EFS (80.9% ± 4.1% vs 52.4% ± 8.1%, respectively; P = .0001). When considered as a single variable, subjects with all 3 T-ALL phenotypes had similar outcomes and subjects with persistent postinduction disease had inferior outcomes, regardless of their ETP phenotype. This clinical trial was registered at AALL0434 as #NCT00408005.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) accounts for ∼15% of childhood ALL cases.1 Historically, event-free survival (EFS) and overall survival (OS) rates in T-ALL have been inferior to those in B-ALL, despite intensified therapy.2-6 Age, presenting leukocyte count, and recurrent genomic alterations are minimally prognostic in T-ALL. Minimal residual disease (MRD), however, is strongly prognostic and used for risk stratification.7-10
In 2009, a novel T-ALL subtype with features similar to normal early T-cell precursors (ETPs) and with a distinctive gene expression profile and immunophenotype was described in 17 patients; it was termed ETP ALL.11 Normal ETPs are primitive thymocytes arising from hematopoietic stem cells, retaining multilineage differentiation potential, and maturing stepwise to become lineage-committed T progenitors. ETP lymphoblasts are immunophenotypically defined by absent CD8 and CD1a expression, weak CD5 expression, and robust expression of 1 or more myeloid and/or stem cell markers.11 Several studies have demonstrated an incomplete overlap between the immunophenotype and ETP gene signature, suggesting that strict application of immunophenotypic criteria may under-represent the ETP spectrum.11-13 The term “near ETP ALL” (near-ETP) has been used to describe cases that otherwise meet the immunophenotypic criteria for ETP ALL but express higher levels of CD5.14,15 In normal thymocyte development, CD5 is expressed at a low level in immature double-negative thymocytes, with its expression increasing at the more mature double-positive and single-positive stages.16,17 As such, near-ETP likely represents a maturational state between ETP and the more mature stages of thymocyte transformation, here termed non-ETP (supplemental Figure 1, available on the Blood website).18
Small retrospective analyses found that patients with ETP ALL had markedly inferior outcomes compared with patients without ETP; thus, experimental therapies or first-remission hematopoietic stem cell transplantation (HSCT) were recommended.11,12,14,19 Although patients with ETP often have high MRD levels,20,21 more recent small studies (COALL-97 [10 patients],13 DFCI05-001/11-001 [21 patients],22 UKALL2003 [35 patients],23 and AIEOP-BFM 2000 and 2009 [47 patients])24 suggested that ETP and non-ETP ALL have similar outcomes. However, the prognostic importance of the ETP status in a large, uniformly treated, and centrally classified contemporary T-ALL cohort remains unknown.
The Children’s Oncology Group (COG) AALL0434 is a large multicenter randomized phase 3 trial for patients newly diagnosed with T-ALL.7,25 This study used a single centralized reference laboratory to prospectively test the ETP status and postinduction MRD. Importantly, MRD status, but not ETP status, was used for risk stratification in this trial. A preliminary analysis of outcomes by ETP status showed significantly higher rates of induction failure (IF) for those with ETP and near-ETP compared with non-ETP, but the data were not mature enough to evaluate EFS or OS.26 We now report mature outcome data from all subjects in AALL0434 for whom ETP status was established.
Methods
Subject eligibility and protocol ethics approvals
AALL0434 (NCT00408005) enrolled 1562 patients with T-ALL from January 2007 to July 2014 and used a prednisone-based chemotherapy backbone.7,25 During the 7-year accrual period, the study was amended 11 times to accommodate administrative changes or clarify study-related procedures, but no amendments were made to change the protocol-specified therapies. The 306 subjects enrolled from 2007 to 2009, before the description of the ETP phenotype by Coustan-Smith et al11 did not have an ETP assessment. The 1256 newly diagnosed subjects had complete ETP data evaluated at a single COG Reference Laboratory (CONSORT diagram; Figure 1). The eligibility criteria included all subjects with newly diagnosed, untreated disease between ages 1 and 31 years. The characteristics of the ETP, near-ETP, and non-ETP groups are provided in supplemental Table 1. AALL0434 was approved by the NCI Cancer Evaluation and Therapeutic Program, the Food and Drug Administration, the Pediatric Central Institutional Review Board (PedCIRB), and by local institutional review boards at participating centers. Written informed consent/assent was obtained from the study participants and, when appropriate, their legal guardians, in accordance with the Declaration of Helsinki.
CONSORT diagram of participant allocation. alloHSCT, allogeneic hematopoietic stem-cell transplantation; MRD, minimal residual disease; T-ALL, T-cell acute lymphoblastic leukemia; T-NHL, T-cell non-Hodgkin lymphoma.
CONSORT diagram of participant allocation. alloHSCT, allogeneic hematopoietic stem-cell transplantation; MRD, minimal residual disease; T-ALL, T-cell acute lymphoblastic leukemia; T-NHL, T-cell non-Hodgkin lymphoma.
Evaluation of the ETP phenotype
ETP, near-ETP, and non-ETP statuses were assessed in diagnostic bone marrow (BM) or peripheral blood (PB) samples using 8- to 9-color multiparameter flow cytometry, as previously described.27 Those classified as having ETP had T-lymphoblasts that were CD8– and CD1a– (<5% positive), weakly expressed CD5 (either <75% positive or median intensity >1 log less than that in mature T cells), and expressed 1 or more myeloid or stem cell markers (>25% positive), including CD13, CD33, CD34, CD117, and HLA-DR.11 A significant fraction of cases showed stronger CD5 expression but otherwise met the ETP immunophenotypic criteria; these were classified as having near-ETP. The remaining cases were defined as having non-ETP phenotype (supplemental Figure 1).
Assessment of minimal residual disease
Using established methods,27-30 MRD testing was performed predominantly at the COG flow cytometry reference laboratory at the University of Washington (B.L.W.), with a small number of initial specimens assayed at Johns Hopkins University (M.J.B.). MRD was assessed using 8- and 9-color flow cytometry in the PB on day 8, in the BM on day 15, and in the BM on day-29 end of induction (EOI) and, again, at day-57 end of consolidation (EOC) in all subjects with high-risk disease (EOI M2 marrow or MRD ≥1.0%) or IF (EOI M3 marrow).
Statistical analyses
Survival rates were estimated using the Kaplan-Meier method and the standard errors reported by Peto et al.31,32 Survival rates are presented as rates ± standard error. EFS was defined as the time from study enrollment to the first event (IF, induction death, relapse, second malignant neoplasm, or remission death) or date of last contact. OS was defined as the time from study enrollment to death or date of last contact. Disease-free survival was defined as the time from postinduction study entry at randomization to the first event (relapse, second malignant neoplasm, or remission death) or date of last contact. Proportions were compared between groups using the χ2 test or Fisher exact test. Cumulative incidence rates were computed using the cumulative incidence function for competing risks of IF, induction death, second malignant neoplasm, and remission death, with comparisons between groups performed using the K-sample test.33 A P < .05 was considered significant for all comparisons. All analyses were performed using SAS software version 9.4, and graphics were generated with R version 2.13.1 (http://www.r-project.org). Data as of 30 June 2018 are included in this report, consistent with our previous reports of study-related outcomes.
Results
ETP status and end-induction MRD
ETP status was determined via multiparameter flow cytometry in 1256 of 1526 (82.3%) enrolled patients, with the ETP status unknown for the 306 patients enrolled before 2009. One hundred forty-five patients (11.5%) had ETP, 209 (16.7%) had near-ETP, and 902 (71.8%) had non-ETP (Figure 1). After 4 weeks of induction, MRD levels were assessed before randomization. Three hundred seventy-three subjects did not participate in the second-stage consent; the treatment received by these patients after removal from the protocol therapy was at the discretion of the treating physician (supplemental Table 2). Subjects with intermediate- and high-risk disease participated in a randomized 2 × 2 study design, whereas those with low-risk disease did not. All subjects with IF were assigned to receive high-dose methotrexate (HDMTX) with nelarabine. Per the protocol, no participants were assigned to receive an allogenic hematopoietic stem cell transplant (allo-HSCT); those who did were taken off the study and managed at the discretion of the treating investigator. Subjects with ETP were significantly more likely to be stratified as having high risk; subjects with ETP and near-ETP (6.2%) were 5 times more likely to be in the IF group (P < .0001) than those with non-ETP (1.2%; Table 1). The ETP and near-ETP groups were more likely to have day-29 MRD ≥0.1% (75.5% and 57.1%, respectively) than the non-ETP group (23.5%; P < .0001). Nearly 60% of patients with ETP ALL had a day-29 MRD ≥1%, compared with 37.8% of those with near-ETP and 13.2% of those with non-ETP (P < .0001) (supplemental Table 1). These data emphasize the significantly inferior early response of ETP and near-ETP ALL compared with non-ETP ALL. Only 9.0% of those with ETP had an initial white blood cell (WBC) count ≥200 000/μL, whereas those with near-ETP and non-ETP had a similar frequency of WBC ≥200 000/μL (29.7% vs 29.9%, respectively).
ETP status based on the risk group
| Risk group . | ETP, n = 145 (%) . | Near-ETP, n = 209 (%) . | Non-ETP, n = 902 (%) . | Statistical comparisons . |
|---|---|---|---|---|
| Induction∗ | 38 (26.2%) | 65 (31.1%) | 206 (22.8%) | P < .0001 |
| LR | 5 (3.5%) | 10 (4.8%) | 75 (8.3%) | |
| IR | 38 (26.2%) | 78 (37.3%) | 520 (57.7%) | |
| HR | 55 (37.9%) | 43 (20.6%) | 90 (10.0%) | |
| IF | 9 (6.2%) | 13 (6.2%) | 11 (1.2%) |
| Risk group . | ETP, n = 145 (%) . | Near-ETP, n = 209 (%) . | Non-ETP, n = 902 (%) . | Statistical comparisons . |
|---|---|---|---|---|
| Induction∗ | 38 (26.2%) | 65 (31.1%) | 206 (22.8%) | P < .0001 |
| LR | 5 (3.5%) | 10 (4.8%) | 75 (8.3%) | |
| IR | 38 (26.2%) | 78 (37.3%) | 520 (57.7%) | |
| HR | 55 (37.9%) | 43 (20.6%) | 90 (10.0%) | |
| IF | 9 (6.2%) | 13 (6.2%) | 11 (1.2%) |
HR, high risk; IR, intermediate risk; LR, low risk.
Patients who were enrolled in the study and received induction therapy but did not participate in the second-stage consent were not risk-stratified and were removed from protocol therapy at the EOI. The treatment received by patients taken off the protocol therapy at the EOI was at the discretion of the treating physician and, therefore, varied. For all COG studies, patients were followed up for disease and vital status after they were removed from the protocol therapy. Off-study patients were no longer followed up if they met any of the following criteria: lost to follow-up, withdrawal of consent for further follow-up, enrollment in another COG therapeutic study, death, or the reaching the maximum follow-up 10-year period.
ETP status does not predict the outcome
All 3 immunophenotypic T-ALL subtypes showed not statistically significant and excellent 5-year EFS and OS rates: ETP (80.4% ± 3.9% and 86.8 ± 3.4%, respectively), near-ETP (81.1% ± 3.3% and 89.6 ± 2.6%, respectively), and non-ETP (85.3% ± 1.4% and 90.0% ± 1.2%, respectively; Figure 2A-B). However, the timing of events differed according to the ETP status. Among patients with near-ETP and non-ETP, most events occurred within 18 months of diagnosis and plateaued after 2 years. In contrast, patients with ETP continued to experience events throughout the follow-up period, with no clear plateaus (Figure 2A). Further, 5-year cumulative incidence of relapse rates were not statistically different: 6.7% ± 2.2% for ETP, 10.1% ± 2.1% for near-ETP, and 8.8% ± 1.0% for non-ETP (P = .5411; Figure 2C). The 5-year cumulative incidence of remission death rates did not differ based on ETP status: 5.3% ± 2.0% for ETP, 1.0% ± 0.7% for near-ETP, and 3.2% ± 0.6% for non-ETP (P = .0959; Figure 2D). We also used multivariable Cox regression analysis to determine whether the ETP status was an independent predictor of outcome (Table 2). In this analysis, only initial WBC ≥200 000/μL (hazard ratio, 1.61; 95% confidence interval [CI], 1.19-2.18) and day-29 MRD ≥0.1% (hazard ratio, 3.06; 95% CI, 2.18-4.28) retained prognostic significance. However, neither ETP (hazard ratio, 0.80; 95% CI, 0.51-1.28) nor near-ETP (hazard ratio, 0.79; 95% CI, 0.53-1.17) statuses were prognostic.
EFS, OS, and cumulative incidence of relapse (CIR) as per ETP status. (A) Five-year EFS rates were 80.4% ± 3.9% for ETP, 81.1% ± 3.3% for near-ETP, and 85.3% ± 1.4% for non-ETP (P = .1679). (B) Five-year OS rates were 86.8% ± 3.4% for ETP, 89.6% ± 2.6% for near-ETP, and 90.0% ± 1.2% for non-ETP (P = .3297). (C) Five-year CIR rates were 6.7% ± 2.2% for ETP, 10.1% ± 2.1% for near-ETP, and 8.8% ± 1.0% for non-ETP (P = .5411). (D) Five-year cumulative incidence of remission death rates were 5.3% ± 2.0% for ETP, 1.0% ± 0.7% for near-ETP, and 3.2% ± 0.6% for non-ETP (P = .0959).
EFS, OS, and cumulative incidence of relapse (CIR) as per ETP status. (A) Five-year EFS rates were 80.4% ± 3.9% for ETP, 81.1% ± 3.3% for near-ETP, and 85.3% ± 1.4% for non-ETP (P = .1679). (B) Five-year OS rates were 86.8% ± 3.4% for ETP, 89.6% ± 2.6% for near-ETP, and 90.0% ± 1.2% for non-ETP (P = .3297). (C) Five-year CIR rates were 6.7% ± 2.2% for ETP, 10.1% ± 2.1% for near-ETP, and 8.8% ± 1.0% for non-ETP (P = .5411). (D) Five-year cumulative incidence of remission death rates were 5.3% ± 2.0% for ETP, 1.0% ± 0.7% for near-ETP, and 3.2% ± 0.6% for non-ETP (P = .0959).
Multivariable Cox regression analysis of EFS
| Covariate . | Hazard ratio (95% CI) . | P value . |
|---|---|---|
| Age (ref ≤10 y) | 0.92 (0.69-1.23) | .573 |
| Sex (ref = male) | 1.21 (0.89-1.65) | .224 |
| Race (ref = White) | ||
| Black | 1.10 (0.73-1.65) | .647 |
| Other | 1.18 (0.70-1.99) | .533 |
| WBC (ref ≤200 × 103) | 1.61 (1.19-2.18) | .002 |
| Day-29 MRD (ref ≤0.1%) | 3.06 (2.18-4.28) | <.0001 |
| ETP status (ref = non-ETP) | ||
| ETP | 0.80 (0.51-1.28) | .358 |
| Near-ETP | 0.79 (0.53-1.17) | .238 |
| Day-8 PB MRD (ref ≤0.01%) | 1.18 (0.63-2.22) | .597 |
| Day-15 BM MRD (ref ≤0.001%) | 1.08 (0.67-1.74) | .750 |
| Covariate . | Hazard ratio (95% CI) . | P value . |
|---|---|---|
| Age (ref ≤10 y) | 0.92 (0.69-1.23) | .573 |
| Sex (ref = male) | 1.21 (0.89-1.65) | .224 |
| Race (ref = White) | ||
| Black | 1.10 (0.73-1.65) | .647 |
| Other | 1.18 (0.70-1.99) | .533 |
| WBC (ref ≤200 × 103) | 1.61 (1.19-2.18) | .002 |
| Day-29 MRD (ref ≤0.1%) | 3.06 (2.18-4.28) | <.0001 |
| ETP status (ref = non-ETP) | ||
| ETP | 0.80 (0.51-1.28) | .358 |
| Near-ETP | 0.79 (0.53-1.17) | .238 |
| Day-8 PB MRD (ref ≤0.01%) | 1.18 (0.63-2.22) | .597 |
| Day-15 BM MRD (ref ≤0.001%) | 1.08 (0.67-1.74) | .750 |
ref, reference.
MRD identifies inferior outcomes based on the ETP status
Although day-29 MRD ≥0.1% was associated with inferior EFS and OS rates for the whole cohort (Figure 3), no significant difference in the EFS (P = .313) or OS (P = .946) was observed when 0.01% was used as a cutoff compared with 0.1%; therefore, all day-29 MRD comparisons we made using this cutoff. When analyzed based on ETP status (supplemental Figure 2), day-29 MRD ≥0.1% was associated with inferior EFS (70.0% ± 3.8% vs 90.6% ± 1.4%; P < .0001) and OS (81.7% ± 3.3% vs 93.2% ± 1.2%; P < .0001) in non-ETP and inferior EFS (73.8% ± 4.8% vs 93.8% ± 3.2%; P = .0003) and OS (87.6% ± 3.7% vs 96.3% ± 2.5%; P = .046) in near-ETP, but MRD showed no significant difference in the EFS or OS in ETP. Day-29 MRD was predictive of relapse in non-ETP, but not in patients with ETP or near-ETP (Figure 4), with a cumulative incidence of relapse rate for patients with non-ETP 4 times higher for the day-29 MRD ≥0.1%.
EFS and OS based on the MRD status at the EOI. (A) Five-year EFS was 73.1% ± 2.3% for patients with EOI MRD ≥0.1% and 90.6% ± 1.1% for patients with an EOI MRD <0.1% (P < .0001). (B) Five-year OS was 84.3% ± 1.9% for patients with an EOI MRD ≥0.1% and 93.4% ± 1.0% for patients with EOI MRD <0.1% (P < .0001).
EFS and OS based on the MRD status at the EOI. (A) Five-year EFS was 73.1% ± 2.3% for patients with EOI MRD ≥0.1% and 90.6% ± 1.1% for patients with an EOI MRD <0.1% (P < .0001). (B) Five-year OS was 84.3% ± 1.9% for patients with an EOI MRD ≥0.1% and 93.4% ± 1.0% for patients with EOI MRD <0.1% (P < .0001).
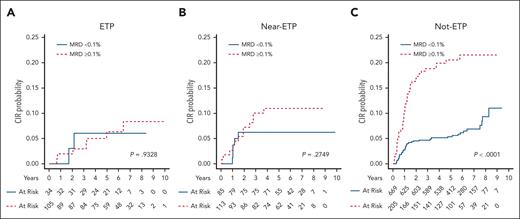
CIR based on MRD status at EOI in patients with ETP, near-ETP, and non-ETP. (A) Five-year CIR rates for patients with ETP were 6.3% ± 2.7% for MRD <0.1% and 11.0% ± 3.0% for MRD ≥0.1%. (P = .9328). (B) Five-year CIR rates for patients with near-ETP were 6.3% ± 2.7% for MRD <0.1% and 11.0% ± 3.0% for MRD ≥0.1%. (P = .2749). (C) Five-year CIR rates for patients with non-ETP were 5.4% ± 0.9% for MRD <0.1% and 20.5% ± 2.9% for MRD ≥0.1%. (P < .0001).
CIR based on MRD status at EOI in patients with ETP, near-ETP, and non-ETP. (A) Five-year CIR rates for patients with ETP were 6.3% ± 2.7% for MRD <0.1% and 11.0% ± 3.0% for MRD ≥0.1%. (P = .9328). (B) Five-year CIR rates for patients with near-ETP were 6.3% ± 2.7% for MRD <0.1% and 11.0% ± 3.0% for MRD ≥0.1%. (P = .2749). (C) Five-year CIR rates for patients with non-ETP were 5.4% ± 0.9% for MRD <0.1% and 20.5% ± 2.9% for MRD ≥0.1%. (P < .0001).
In patients with ETP, day-29 MRD ≥10% was the only MRD cutoff predictive of an inferior outcome (supplemental Figure 2A-B). In all subgroups, patients with MRD >10% had an EFS of 40% to 50%, similar to that seen in patients with IF treated with HDMTX and nelarabine on arm D of AALL0434.25 For subjects randomly assigned to receive nelarabine, there were improved outcomes for the non-ETP group, a trend to benefit in the near-ETP group, and no benefit for the ETP group (supplemental Figure 3A-C). There were no differences in disease-free survival for subjects with ETP, near-ETP, or non-ETP based on methotrexate randomization (capizzi methotrexate vs HDMTX; supplemental Figure 3D-F).
Only subjects with high risk or IF underwent end-consolidation MRD testing in AALL0434. We found that an EOC MRD ≥0.01% was highly predictive of treatment failure for the entire group (5-year EFS, 52.4% ± 8.1% compared with 80.9% ± 4.1% for EOC MRD <0.01%; P = .0001) and also within the non-ETP group but not within the ETP and near-ETP groups (Figure 5). For patients with an EOI MRD ≥10%, an EOC MRD <0.01% or ≥0.01% did not impact EFS or OS (P = not significant; data not shown).
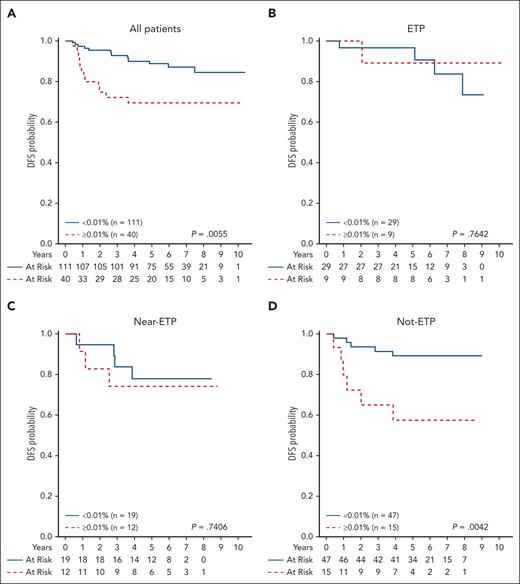
Disease-free survival (DFS) rates based on EOC MRD in patients with ETP, near-ETP, and non-ETP with a day-29 MRD ≥1%. Five-year DFS for (A) total cohort: 69.6% ± 8.6% for patients with EOC MRD ≥0.01% and 88.7% ± 3.4% for patients with EOC MRD <0.01% (P = .0055); (B) ETP: 88.9% ± 10.5% for EOC MRD ≥0.01% and 90.4% ± 7.2% for EOC MRD <0.01% (P = .7642); (C) near-ETP: 75.0% ± 15.3% for EOC MRD ≥0.01% and 78.6% ± 10.5% for EOC MRD <0.01% (P = .7406); and (D) non-ETP: 58.2% ± 18.8% for EOC MRD ≥0.01% and 89.3% ± 5.0% for EOC MRD <0.01% (P = .0042).
Disease-free survival (DFS) rates based on EOC MRD in patients with ETP, near-ETP, and non-ETP with a day-29 MRD ≥1%. Five-year DFS for (A) total cohort: 69.6% ± 8.6% for patients with EOC MRD ≥0.01% and 88.7% ± 3.4% for patients with EOC MRD <0.01% (P = .0055); (B) ETP: 88.9% ± 10.5% for EOC MRD ≥0.01% and 90.4% ± 7.2% for EOC MRD <0.01% (P = .7642); (C) near-ETP: 75.0% ± 15.3% for EOC MRD ≥0.01% and 78.6% ± 10.5% for EOC MRD <0.01% (P = .7406); and (D) non-ETP: 58.2% ± 18.8% for EOC MRD ≥0.01% and 89.3% ± 5.0% for EOC MRD <0.01% (P = .0042).
Association between clinical and early response features and outcomes
We assessed whether additional risk factors could be used to identify the ETP phenotype-driven outcomes. In addition to presenting WBC in the risk-group assignment, all subjects had MRD levels assessed on induction days 8 and 15. We assessed whether an initial WBC count ≥200 000/μL was associated with an inferior outcome. Diagnostic WBC ≥200 000/μL predicted inferior EFS and OS rates for those with near-ETP (P = .002 and P = .0085, respectively) and non-ETP (P = .0045 and P = .006, respectively) but not for those with ETP (supplemental Figure 4). This was maintained using a diagnostic WBC cutoff count of 100 000/μL; however, using a cutoff of 50 000/μL and 10 000/μL, significance was lost in the near-ETP group but preserved in the non-ETP group (supplemental Table 3). Day 8 PB and day 15 BM MRD ≥0.01% were both associated with inferior EFS (supplemental Figure 5) but not OS. We further found that central nervous system involvement status at diagnosis did not portend a risk of relapse in the ETP and near-ETP groups, whereas CNS3 status was associated with an increased risk of relapse in the non-ETP group (supplemental Figure 6).
Discussion
In AALL0434, we used a prospective, intent-to-treat analytic design with centrally determined ETP status and MRD measurement to investigate treatment-related outcomes for 1256 T-ALL subjects, 354 of whom had the ETP or near-ETP phenotype. The use of a central laboratory is important in a rare disease, such as T-ALL, to provide consistent classification and MRD assessment, particularly given the posttherapy immunophenotypic changes known to occur.27 We have established that subjects with ETP and near-ETP ALL are more likely to have elevated end-induction MRD levels, with the rate of IF being 5 times higher in those with ETP and near-ETP than in those with non-ETP ALL. Nevertheless, the ETP status was not predictive of EFS and OS, with all groups achieving excellent outcomes. However, because patients with ETP were more frequently removed from protocol therapy, their EFS/OS may be more reflective of their off-protocol treatment. Our analysis of MRD categorical data and ETP status demonstrated that an end-induction MRD ≥0.1% is likely to capture most patients at risk for relapse, regardless of ETP status. The high rate of day-29 MRD ≥0.1% in T-ALL, overall, is driven by its very high rate in patients with ETP/near-ETP. The rate in the non-ETP group was comparable with that reported for high-risk B-ALL in COG protocols.34,35 Unexpectedly, an EOC MRD ≥0.01% was not predictive of outcomes in patients with ETP or near-ETP. In those with near-ETP, events occurred relatively early in both patients with EOC MRD–positive and those with EOC MRD–negative status. In contrast, late events in the ETP EOC MRD–negative group contributed to inferior outcomes. Two subjects developed secondary malignant neoplasms, and 1 relapsed during follow-up. We, previously, reported that patients with ETP treated in AALL0434 were more likely to have received off-protocol allo-HSCT;25 therefore, we hypothesized that late events in patients with EOC MRD–negative status may be related to off-protocol or posttransplant mortality. Another possibility is that EOC may be too early a time point to discriminate outcomes based on MRD in patients with ETP, given the slower kinetics of disease response. Other than WBC count ≥200 000/μL or elevated levels of postinduction MRD, we could not identify any biologic or demographic features to explain the relapses in AALL0434. Overall, we found that the diagnosis of ETP or near-ETP alone was not sufficiently strong to merit use as an independent predictor of inferior EFS or OS in the context of contemporary T-ALL therapy.
ETP and near-ETP represent a continuum of maturational states in stem-like leukemias. The flow cytometric diagnosis of ETP is subjective because it is based on an arbitrary cutoff for CD5 expression. Other groups have proposed nonimmunophenotypic definitions for immature T-ALL subtypes that overlap with the ETP immunophenotype. Expression of LYL1 has been associated with immature immunophenotypic features,36 such as the expression of MEF2C.37 Another approach has emphasized the absence of a biallelic T-cell receptor–γ deletion (ABD) phenotype.12 Zuurbier et al investigated the overlap between immunophenotypic ETP, the ABD phenotype, and immature-cluster T-ALL, as defined by MEF2C expression, and found that the use of immunophenotype alone underestimated the number of cases with a corresponding ETP gene signature. Furthermore, they found that ABD phenotype overlapped substantially with ETP phenotype cases.13 Given the potential of underestimating ETP phenotype cases through the strict application of immunophenotypic criteria, diagnostic gene expression profiling may play a complementary role in the determination of ETP status in future studies.
An end-induction MRD ≥10% was a striking predictor of inferior outcomes in all patients. This finding, coupled with the high prevalence of IF, and the limited predictive value of EOC MRD ≥0.01% in patients with ETP, provides a rationale for intensification of therapy during induction or consolidation for all patients but may be targeted to disease resistance pathways that are unique to patients with ETP. We speculate that along the ETP maturational continuum, various gene programs may mediate responsiveness to antiproliferative therapy, leading to our finding that some subjects with ETP had MRD-negative status by the EOI or cleared their persistent MRD by the EOC.
We were not able to systematically correlate genetic features with ETP or near-ETP in this study. However, other investigators have described the heterogeneous genetic landscape of ETP ALL in a subset of 264 AALL0434 study participants for whom molecular testing could be performed.38,39 Liu et al39 found that when compared with those with near-ETP and non-ETP, 19 patients with ETP predominantly had translocations involving LMO2/LYL1, with alterations in JAK/STAT signaling, Ras signaling, and epigenetic dysregulation, consistent with prior reports.38,40 None of the recurrent alterations found in the ETP group in this study was associated with relapse in the univariate analysis.39 More recent reports have noted that a subset of ETP ALL shows activation of BCL11B either through chromosomal rearrangements or focal amplifications, leading to a distal neoenhancer.41-44 A study of another cohort of patients from AALL0434, enriched for IF cases, found an association between KMT2A-rearrangements (KMT2A-r) and ETP status.45 Additionally, KMT2A-r status was associated with IF and risk of relapse/refractory disease, but in particular ETP phenotype cases, KMT2A was associated with MRD positivity.45 Chonghaile et al also demonstrated that samples of patient with ETP are preferentially dependent on BCL-2 for survival compared with samples of those with non-ETP and are sensitive to treatment with the BCL-2 inhibitor venetoclax,46 whereas Maude et al found that the preferential activation of JAK/STAT signaling in ETP phenotype samples was abrogated by treatment with the JAK1/2 inhibitor ruxolitinib.40 As a potential avenue for future exploration, venetoclax and ruxolitinib sensitize intrinsically glucocorticoid-resistant ETP ALL to treatment with dexamethasone.47,48 Additional genomic characterization of the AALL0434 cohort is ongoing; preliminary results demonstrate that cases of ETP ALL are frequently associated with deregulation of HOXA13 and HOXA9 and that the HOXA9 subgroup is associated with inferior EFS.49,50
We, previously, reported that subjects with ETP were more frequently taken off the study during induction and referred for allo-HSCT than those without ETP.25 This likely reflects the disease kinetics of having ETP, because clinicians might have been more likely to alter therapy during induction in patients with persistently high levels of disease on day-8 or day-15 disease assessments. Although not powered for efficacy, our analyses of 73 randomized subjects did not show any benefit of nelarabine within the ETP phenotype. Similarly, nelarabine improved EFS only in adults with non-ETP when treated with the hyper-CVAD (cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride [adriamycin], dexamethasone) regimen.15
Given their preclinical efficacy in ETP ALL,40,46-48 the addition of targeted agents such as ruxolitinib or venetoclax directed at the JAK/STAT or BCL-2 pathways may offer a unique opportunity to intensify induction therapy while limiting excess toxicity.51,52 Our novel finding that near-ETP has equivalent rates of IF to ETP demonstrates that defining ETP ALL immunophenotypically may underestimate the genetically heterogeneous group of patients with T-ALL at risk of IF. Therefore, diagnostic genomic profiling should be considered in future trials to further refine risk stratification in pediatric T-ALL.
Acknowledgments
The authors acknowledge the support and assistance of the COG ALL committee, the Cancer Evaluation and Therapeutic Program section of the National Institutes of Health, and the Pediatric Central Central Institutional Review Board. The authors thank the patients and families who supported this research through their consent to participate in the COG ALL clinical trials AALL03B1, AALL008B1, and AALL0434.
This work was supported by grants from the National Institutes of Health (NIH), National Cancer Institute (NCI), National Clinical Trials Network (NCTN) Operations Center (U10CA180886 and U10 CA98543); NIH, NCI, NCTN Statistics and Data Center (U10CA180899 and U10 CA98413); NIH, NCI, NCTN Banking (U24 CA114766); and St Baldrick’s Foundation. M.L.L. is an Endowed Professor of Pediatric Cancer Research, The Aldarra Foundation Endowed Chair, and Bill and June Boeing, Founders. E.A.R. is a KiDS of the NYU Foundation Professor at NYU Langone Health. S.P.H. has the Jeffrey E. Perelman Distinguished Chair in Pediatrics at the Children’s Hospital of Philadelphia.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: B.L.W., M.D., R.J.S., B.A., K.R.R., P.A.Z.-M., N.J.W., M.J.B., W.L.C., E.A.R., M.L.L., S.P.H., K.P.D., D.T.T., and S.S.W. designed the research; B.L.W., R.J.S., and S.S.W. analyzed and interpreted the data; M.D. and Z.C. performed statistical analysis; R.J.S. and Z.C. generated figures; B.L.W., R.J.S., and S.S.W. wrote the manuscript; and all authors revised the manuscript.
Conflict-of-interest disclosure: S.P.H. has received honoraria from Amgen, Jazz, and Servier; received consulting fees from Novartis; and owns common stock in Amgen. The remaining authors declare no competing financial interests.
Correspondence: Brent L. Wood, Laboratory Medicine, Children's Hospital Los Angeles, 4620 Sunset Blvd MS32, Los Angeles, CA 90027; e-mail: bwood@chla.usc.edu.
References
Author notes
Presented in a plenary session at the 2014 American Society of Hematology Annual Meeting, San Francisco, CA, 6-9 December 2014.
Clinical trial access and individual participant data are available through the Children’s Oncology Group (datarequest@childrensoncologygroup.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal