Abstract
Splenic iron decreased whereas liver iron was stable during luspatercept therapy in some individuals with thalassemia. This suggests a reduction of ineffective erythropoiesis changes the organ distribution of iron and demonstrates that liver iron concentration alone may not accurately reflect total body iron content. This article describes data from subjects enrolled in BELIEVE (NCT02604433) and BEYOND (NCT03342404).
TO THE EDITOR:
β-thalassemia is an inherited hemoglobin disorder with defective production of β-globin chains, with phenotypes ranging from mild to severe anemia with or without transfusion dependence.1 Luspatercept is a recombinant fusion protein that enhances late-stage erythropoiesis and reduces transfusion burden compared with placebo in patients with transfusion-dependent β-thalassemia.2 With such a powerful reduction of the iron loading rate, one would anticipate a corresponding reduction in liver iron concentration (LIC) by magnetic resonance imaging (MRI)3; however, this has not been observed.2,4,5
We postulated that luspatercept therapy alters the relationship between total body iron concentration and LIC through systematic changes in the liver and spleen volume or in the spleen iron concentration, thus masking the expected reduction in LIC with a lower iron loading rate in patients treated with luspatercept. To explore this possibility, we examined the liver and spleen iron concentration and volume in 11 subjects enrolled in the BELIEVE trial, which evaluated the efficacy and safety of luspatercept vs placebo in adults with transfusion-dependent β-thalassemia.2 To better characterize possible iron distribution changes in the absence of transfusion therapy, we also measured the same parameters in 4 subjects enrolled in the BEYOND trial, which evaluated luspatercept vs placebo in adults with nontransfusion-dependent β-thalassemia.5
Both clinical trials were approved by the Institutional Review Board of Children’s Hospital Los Angeles. Experimental procedures were performed under informed consent. All subjects in this substudy were in the luspatercept arm of either study and were considered responders with respect to the primary outcome.2,5 MRI examinations conducted immediately before active drug initiation and most recently while taking study drug were included in the analysis. Liver and spleen R2∗ images were collected using single breath-hold, multiecho, gradient echo imaging using standard clinical imaging parameters. LIC was derived by previously validated R2∗ calibration.6 Calibration of spleen iron concentration to R2∗ has not been biopsy validated but has been extrapolated from liver iron calibrations based on physical principles.7 Liver and spleen volumes were calculated using planimetry by an experienced MRI radiologist. Organ iron content was calculated as the product of organ volume multiplied by the organ iron concentration.8 One BEYOND subject had prior splenectomy, but liver parameters were retained in the analyses. Changes in plasma iron indices were abstracted from the medical record.
Subjects were adults with a median age of 27 years at luspatercept initiation, treated for a median of 40 months with MRIs, and examined over a median of 30.9 months. No significant change was observed in ferritin, total iron binding capacity (TIBC), or transferrin saturation. Table 1 summarizes initial liver and spleen iron concentration, organ volume, organ iron content, and organ iron content ratios for subjects in both studies as well as the interval changes. No change in liver parameters was noted in both groups. Spleen iron content was 21% of the liver iron content in BELIEVE participants, and 28% in BEYOND participants. Initial spleen iron content was correlated to liver iron content, with an r2 = 0.78 (P < .0001; β = 0.42 [95% confidence interval, 0.28-0.56]). Spleen iron concentration and spleen iron content decreased ∼50% in BELIEVE participants and ∼20% in BEYOND study participants, with Cohen D values of 1.45 for BELIEVE and 1 for BEYOND. Although these changes were not statistically significant for this small sample, the Cohen D values suggest large effect sizes. Spleen volume was stable. At the end of treatment, spleen iron content remained correlated to liver iron content, but the r2 had dropped to 0.32 (P = .05), and the slope was significantly decreased from the baseline relationship (β = 0.11 [95% confidence interval, 0.01-0.21]).
Change in the liver and spleen iron concentration, volume, and content
| . | BELIEVE study + extension . | BEYOND study + extension . | ||||
|---|---|---|---|---|---|---|
| Starting (± StD) . | Δ (± StE) . | P . | Starting (± StD) . | Δ (± StE) . | P . | |
| Spleen iron conc (mg/g) | 10.8 ± 15.6 | −4.9 ± 3.4 | .18 | 2.0 ± 0.9 | −0.4 ± 0.2 | .18 |
| Spleen volume (mL) | 672 ± 242 | −41 ± 67 | .42 | 678 ± 266 | 7 ± 76 | .93 |
| Spleen iron content (g) | 1.97 ± 3.11 | −1.09 ± 0.75 | .18 | 0.29 ± 0.14 | −0.07 ± 0.07 | .40 |
| LIC (mg/g) | 12.7 ± 14.0 | 0.1 ± 4.9 | .99 | 6.2 ± 6.7 | 0.3 ± 0.5 | .63 |
| Liver volume (mL) | 1822 ± 400 | 13 ± 97 | .90 | 1677 ± 497 | 10 ± 32 | .78 |
| Liver iron content (g) | 6.05 ± 7.53 | −0.02 ± 2.75 | .99 | 3.00 ± 3.92 | −0.01 ± 0.29 | .97 |
| Spleen/liver content (%) | 21 ± 20 | −4 ± 5 | .43 | 28 ± 10 | −10 ± 7 | .28 |
| Ferritin (ng/mL) | 2176 ± 2060 | −394 ± 615 | .54 | 659 ± 289 | 154 ± 184 | .56 |
| TIBC (μg/dL) | 396 ± 125 | 90 ± 63 | .19 | 245 ± 15 | 3 ± 11 | .83 |
| Transferrin sat (%) | 61 ± 18 | 1.6 ± 9.1 | .87 | 48 ± 28 | −7.5 ± 7.5 | .50 |
| . | BELIEVE study + extension . | BEYOND study + extension . | ||||
|---|---|---|---|---|---|---|
| Starting (± StD) . | Δ (± StE) . | P . | Starting (± StD) . | Δ (± StE) . | P . | |
| Spleen iron conc (mg/g) | 10.8 ± 15.6 | −4.9 ± 3.4 | .18 | 2.0 ± 0.9 | −0.4 ± 0.2 | .18 |
| Spleen volume (mL) | 672 ± 242 | −41 ± 67 | .42 | 678 ± 266 | 7 ± 76 | .93 |
| Spleen iron content (g) | 1.97 ± 3.11 | −1.09 ± 0.75 | .18 | 0.29 ± 0.14 | −0.07 ± 0.07 | .40 |
| LIC (mg/g) | 12.7 ± 14.0 | 0.1 ± 4.9 | .99 | 6.2 ± 6.7 | 0.3 ± 0.5 | .63 |
| Liver volume (mL) | 1822 ± 400 | 13 ± 97 | .90 | 1677 ± 497 | 10 ± 32 | .78 |
| Liver iron content (g) | 6.05 ± 7.53 | −0.02 ± 2.75 | .99 | 3.00 ± 3.92 | −0.01 ± 0.29 | .97 |
| Spleen/liver content (%) | 21 ± 20 | −4 ± 5 | .43 | 28 ± 10 | −10 ± 7 | .28 |
| Ferritin (ng/mL) | 2176 ± 2060 | −394 ± 615 | .54 | 659 ± 289 | 154 ± 184 | .56 |
| TIBC (μg/dL) | 396 ± 125 | 90 ± 63 | .19 | 245 ± 15 | 3 ± 11 | .83 |
| Transferrin sat (%) | 61 ± 18 | 1.6 ± 9.1 | .87 | 48 ± 28 | −7.5 ± 7.5 | .50 |
Conc, concentration; sat, saturation; StD, standard deviation; StE, standard error.
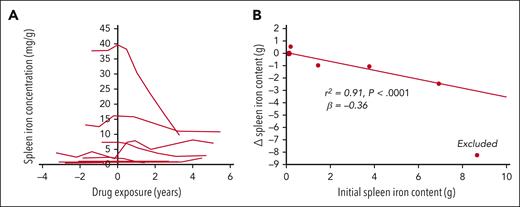
Figure 1A illustrates individual changes in the spleen iron concentration from the initial to final MRI examination for the 11 BELIEVE subjects. Four subjects exhibit steep drops in spleen iron concentration, whereas 1 subject’s spleen iron concentration increased. Figure 1B demonstrates that the change in spleen iron content was highly correlated with initial spleen iron content (r2 = 0.91; P < .0001); change in the spleen iron content was otherwise independent of change in liver iron content. One subject markedly had improved chelation adherence during the study and was excluded from analysis, whereas chelation was stable for the remaining subjects. The ratio of spleen iron content to liver iron content decreased with treatment in both the BEYOND and BELIEVE study subjects (Table 1), but this did not reach statistical significance.
Splenic iron concentration decreased in subjects with high levels at baseline, and the change in the splenic iron content is highly correlated with initial splenic iron content. (A) Splenic iron concentration as a function of drug exposure. Time zero represents the time of drug initiation. Patients with clinically relevant spleen iron concentration exhibited marked improvements. Only 1 patient had significantly increased spleen iron content. (B) Reductions in spleen iron content as a function of the initial spleen iron content. The patient with the highest initial spleen iron content was excluded because their spleen iron change could be attributed to a marked change in chelator compliance (LIC dropped by ∼40 mg/g over the study interval). After doing so, Δ spleen iron content was independent of Δliver iron content (r2 = 0.007; P = .78).
Splenic iron concentration decreased in subjects with high levels at baseline, and the change in the splenic iron content is highly correlated with initial splenic iron content. (A) Splenic iron concentration as a function of drug exposure. Time zero represents the time of drug initiation. Patients with clinically relevant spleen iron concentration exhibited marked improvements. Only 1 patient had significantly increased spleen iron content. (B) Reductions in spleen iron content as a function of the initial spleen iron content. The patient with the highest initial spleen iron content was excluded because their spleen iron change could be attributed to a marked change in chelator compliance (LIC dropped by ∼40 mg/g over the study interval). After doing so, Δ spleen iron content was independent of Δliver iron content (r2 = 0.007; P = .78).
MRI LIC values typically vary proportionally to the total body iron concentration.3 However, any therapy that changes organ size or iron distribution in the body could disrupt this proportionality. We found no liver or spleen volume changes that would explain the lack of improvement in LIC over the BELIEVE study period and its extension. We also did not observe any change in LIC, similar to previous reports of BELIEVE(2) and BEYOND.5 In contrast, spleen iron content declined dramatically in subjects who had increased values at baseline (Figure 1A), with reductions of 1 to 8 g of elemental iron. The decline in spleen iron content could not be explained by changes in the LIC, liver size, or spleen size. Thus, luspatercept appears to alter iron distribution in the body and the relationship between LIC and total body iron content. Because our observations are based on a small portion of the BELIEVE and BEYOND subjects, a larger study sample is needed to confirm these observations.
The mechanism of this change is unknown. Although luspatercept has been shown to reduce transfusion burden in subjects with or without splenectomy,4 the effect of luspatercept on spleen iron or volume, to our knowledge, has not previously been described. Iron loading in the spleen is primarily due to erythrophagocytosis; thus, reduced transfusion intensity will decrease splenic iron influx.9 Iron egress from the spleen is negatively regulated through hepcidin.10 In the BELIEVE study, hepcidin levels declined 53% with therapy and erythroferrone levels rose 51%,11 suggesting that improved red-cell maturation was driving increased iron mobilization from the reticuloendothelial system.12 We saw greater reduction in the spleen iron content in the BELIEVE subjects than in the BEYOND subjects in this substudy, so both transfusion intensity and hepcidin regulation are likely involved. Iron is also removed from the spleen by iron chelation.13 However, the splenic iron changes in this study were out of proportion to liver iron changes, suggesting that iron chelation could not explain the observations.
Iron loading in the liver and spleen occurs through receptor-mediated and nonreceptor-mediated processes that reflect, in part, the degree of ineffective erythropoiesis.14 Without speculating the precise mechanism, it is clear that luspatercept, an agent thought to decrease ineffective erythropoiesis,11,15,16 changes the relative iron distribution between the 2 dominant iron storage organs. Importantly, the current data show that ∼21% to 28% of the total iron content is in the spleen in thalassemia. Although precise estimates of this ratio would require independent-validation of the spleen R2∗-iron calibration, the profound change in spleen or liver iron loading over time suggests that LIC measurement alone does not properly reflect total body iron3 in patients treated with agents, such as luspatercept.
Practical measures of ineffective erythropoiesis are sorely lacking.16 Plasma biomarkers of erythropoiesis fluctuate rapidly, are difficult to standardize, and are not universally available. Hepatic and splenic iron represent the time average of iron balance over weeks to months. Thus, it is worth exploring whether the ratio of splenic iron to liver iron obtained during routine MRI surveillance might serve as a surrogate for the change in ineffective erythropoiesis.
Acknowledgment
This work was supported by Bristol Myers Squibb.
Authorship
Contribution: J.C.W. designed the research study; C.C.D., S. Veluswamy, T.C.H., and T.D.C. performed the research; J.C.W. analyzed the data; C.C.D., S. Vodala, T.D.C., and J.C.W. wrote the paper; and all authors contributed to the revision and approved the submission of the paper.
Conflict-of-interest disclosure: J.C.W. consults for Celgene and Bristol Myers Squibb. T.D.C. consults for Bristol Myers Squibb, Agios Pharma, and Chiesi Farmaceutici. S. Vodala is employed by Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: John C Wood, Department of Pediatrics, Division of Cardiology, Children’s Hospital Los Angeles, 4650 Sunset Blvd, CHLA Mailstop 34, Los Angeles, CA 90027; e-mail: jwood@chla.usc.edu.
References
Author notes
∗C.C.D. and S. Vodala contributed equally to this work.
†T.D.C. and J.C.W. are joint senior authors.
Qualified researchers may request access to individual patient level data through the Bristol Myers Squibb data request platform at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html
There is a Blood Commentary on this article in this issue.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal