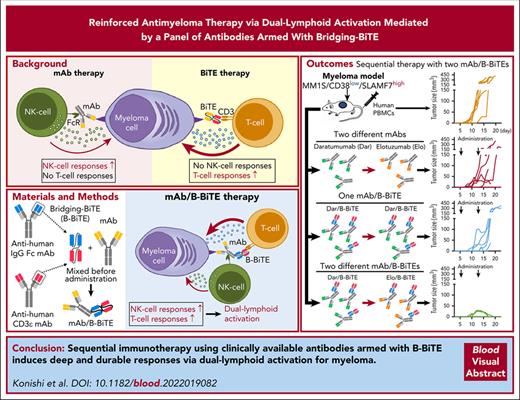
Key Points
Clinically available antibodies armed with B-BiTE successfully activated both human T cells and NK cells for myeloma cells.
Immunotherapy using 2 new different B-BiTE–based bispecifics induced deep and durable responses via dual-lymphoid activation for myeloma.
Abstract
Immunotherapy using bispecific antibodies including bispecific T-cell engager (BiTE) has the potential to enhance the efficacy of treatment for relapsed/refractory multiple myeloma. However, myeloma may still recur after treatment because of downregulation of a target antigen and/or myeloma cell heterogeneity. To strengthen immunotherapy for myeloma while overcoming its characteristics, we have newly developed a BiTE-based modality, referred to as bridging-BiTE (B-BiTE). B-BiTE was able to bind to both a human immunoglobulin G–Fc domain and the CD3 molecule. Clinically available monoclonal antibodies (mAbs) were bound with B-BiTE before administration, and the mAb/B-BiTE complex induced antitumor T-cell responses successfully while preserving and supporting natural killer cell reactivity, resulting in enhanced antimyeloma effects via dual-lymphoid activation. In contrast, any unwanted off-target immune-cell reactivity mediated by mAb/B-BiTE complexes or B-BiTE itself appeared not to be observed in vitro and in vivo. Importantly, sequential immunotherapy using 2 different mAb/B-BiTE complexes appeared to circumvent myeloma cell antigen escape, and further augmented immune responses to myeloma relative to those induced by mAb/B-BiTE monotherapy or sequential therapy with 2 mAbs in the absence of B-BiTE. Therefore, this modality facilitates easy and prompt generation of a broad panel of bispecific antibodies that can induce deep and durable antitumor responses in the presence of clinically available mAbs, supporting further advancement of reinforced immunotherapy for multiple myeloma and other refractory hematologic malignancies.
Introduction
The last few decades have seen progress in new therapeutic strategies for multiple myeloma (MM), such as the use of monoclonal antibodies (mAbs) specific for antigens expressed by myeloma cells in combination with immunomodulatory drugs and proteasome inhibitors.1-3 However, despite promising results of clinical trials, MM still appears to be an incurable hematologic malignancy.4
Myeloma cells show both interindividual and intraindividual heterogeneity,5-7 which is likely one of the reasons for their ability to gain resistance to antimyeloma therapy. Even though myeloma cells are considered to originate from individual clones, MM finally develops through different plasma cell clones with a variety of abnormalities and characteristics.5-7 As a consequence, it has been observed that myeloma cells showing reduced expression of a target antigen can appear and/or that preexisting resistant clones can be selected and are able to survive and grow after treatment with mAbs, immunomodulatory drugs, and/or proteasome inhibitors.8-11 To overcome these obstacles, switching of a series of therapeutic regimens is usually adopted for later-line antimyeloma therapy, based on the results of clinical trials. Another approach has been the development of next-generation immunotherapy that can activate human T cells against myeloma cells with drug resistance to strengthen antimyeloma effectiveness and achieve deep responses.1
Chimeric antigen receptor (CAR)–redirected T cells and bispecific antibodies targeting G-protein coupled receptor family C group 5 member D (GPRC5D) as well as B-cell maturation antigen (BCMA) have induced clinical responses in patients with drug-resistant and refractory MM.12-18 In comparison with CAR T-cell therapy, bispecifics including BiTE would be more applicable to many patients with MM because it is a recombinant protein with off-the-shelf availability.19 However, for further improvement of treatment efficacy, a number of points pertaining to bispecifics need to be addressed. First, to our knowledge, they appear to stimulate mostly human T cells against myeloma cells by using a silenced Fc domain to decrease the possibility of nonspecific stimulation between T cells and Fc receptor–expressing cells in the absence of tumor cells.20,21 If both T cells and natural killer (NK) cells could be safely and specifically activated against myeloma cells, this would likely boost the efficacy of antimyeloma therapy. Second, as they basically bind to a single antigen expressed by myeloma cells, targeting of the whole myeloma cell population might be difficult in view of its heterogeneity.16 This would seem to be one of the main concerns pertaining to both CAR T cells, bispecifics, and other forms of targeted immunotherapy.
Against this background, we have newly designed a BiTE-based modality, referred to as bridging-BiTE (B-BiTE). B-BiTE is able to bind to both a human immunoglobulin G (IgG)–Fc domain and the CD3 molecule. Once clinically available mAbs have become bound to B-BiTE before administration, the resulting mAb/B-BiTE complex would successfully induce antitumor T-cell responses while preserving and supporting NK cell reactivity, resulting in concurrent dual-lymphoid activation against myeloma cells. By rapidly preparing a panel of mAb/B-BiTE complexes, we have investigated the efficacy and safety of this treatment modality, which makes it possible to target MM over a broad range of cell heterogeneity.
Materials and methods
Human samples and cell lines
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy volunteers and patients using Ficoll-Paque (GE Healthcare) and stored until use. Primary lymphoma cells and myeloma cells were obtained from patients at the time of diagnosis. All experiments using human samples were approved by the certified review board of Ehime University. All experiments were performed in accordance with the relevant guidelines and regulations, and all of the donors provided written informed consent. Details of the cell lines used in this study are mentioned in supplemental Methods, available on the Blood website.
Generation of mAb/B-BiTE complexes
Each mAb/B-BiTE complex was generated as follows. Briefly, antihuman CD19 mAb (clone FMC63) and isotype mAb (clone MOPC21), which possess the Fc fragment derived from human IgG1, were purchased (Absolute Antibody). Clinically available antihuman CD20 mAb (rituximab), antihuman CD38 mAb (daratumumab), and antihuman signaling lymphocytic activation molecule family member 7 (SLAMF7) mAb (elotuzumab) were also purchased from pharmaceutical companies. Purified B-BiTE protein was mixed with a mAb in phosphate-buffered saline without serum at a molar ratio of 1:1. After incubation for 1 hour at room temperature, the mixtures were used as mAb/B-BiTE complexes for further experiments.
Flow cytometry
Target-specific binding of each mAb/B-BiTE complex was confirmed as follows. Briefly, target cells were incubated with the mAb/B-BiTE complex, then washed and stained with phycoerythrin-conjugated anti-His mAb (clone GG11-8F3.5.1) or phycoerythrin-conjugated F(ab’)2 goat antihuman Fcγ polyclonal antibody (Jackson ImmunoResearch) for detection. Expression of cell surface proteins was assessed by fluorochrome-conjugated antibodies indicated in supplemental Methods. For staining of human T cells and NK cells, a panel of fluorochrome-conjugated antibodies (described in “T-cell functional assays” and “NK cell functional assays” mentioned in supplemental Methods) was used. A panel of multiple human cytokines in murine sera was measured by LEGENDplex multianalyte flow assay kit (BioLegend). All samples were analyzed using a Gallios flow cytometer (Beckman Coulter) and FlowJo Version 10.8.1 software (Becton Dickinson).
Cytotoxicity assays
Standard cytotoxicity assays were performed as described previously.22,23 Briefly, 5.0 × 103 target cells were labeled with chromium-51 (51Cr) for 1.5 hours at 37°C. CD19+ B-cell–depleted or nondepleted PBMCs were prepared as responder cells and incubated with target cells in the presence of a mAb or a mAb/B-BiTE complex at various effector-to-target ratios for 18 hours. The supernatants were collected and their counts per minute (cpm) rates were measured. The percentage of specific lysis was calculated as: (experimental release cpm – spontaneous release cpm)/(maximal release cpm – spontaneous release cpm) × 100 (%).
In vivo murine experiments
All of the murine experiments in this study were approved by the Ehime University Animal Care Committee. All experiments were performed in accordance with the relevant guidelines and regulations. NOD/Shi-scid interleukin 2r gamma (IL-2rγ) (null) female mice aged 5 weeks were purchased from In-Vivo Science Inc. Then, in vivo potential off-target reactivity and antimyeloma effects induced by a mAb/B-BiTE complex were assessed as described in supplemental Methods. All the experiments were performed at least twice, and representative data are shown in the figures.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 9.1.2. The paired t test (2-sided) or the Welch t test (2-sided) was used to examine whether 2 groups subjected to parametric variation were significantly different for a given variable. Murine survival was expressed in the form of Kaplan-Meier curves, and the log-rank (Mantel-Cox) test was performed. For each statistical analysis, differences at P <.05 were considered significant.
Results
Efficacy and safety of the mAb/B-BiTE complex as a new bispecific antibody
Two single-chain variable fragments (scFvs), 1 encoding an scFv specific for the Fc domain of human IgG and the other an scFv bound to human CD3ε, were prepared (supplemental Figure 1A). Based on the framework of BiTE, we newly designed B-BiTE (supplemental Figure 1B) and then hypothesized that a panel of antitumor antibodies armed with B-BiTE would induce human T-cell reactivity while preserving human NK cell reactivity.
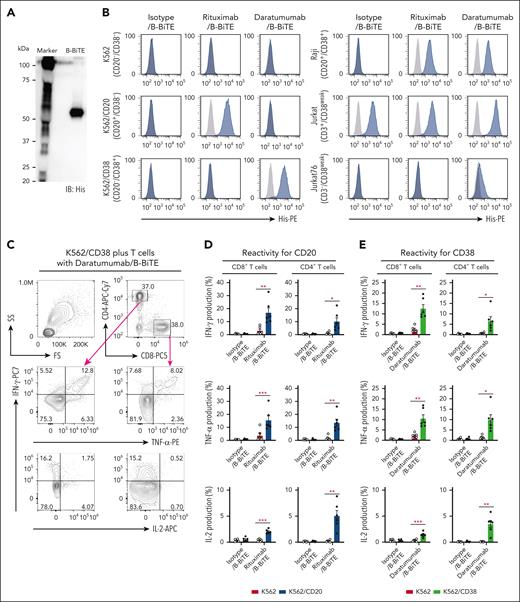
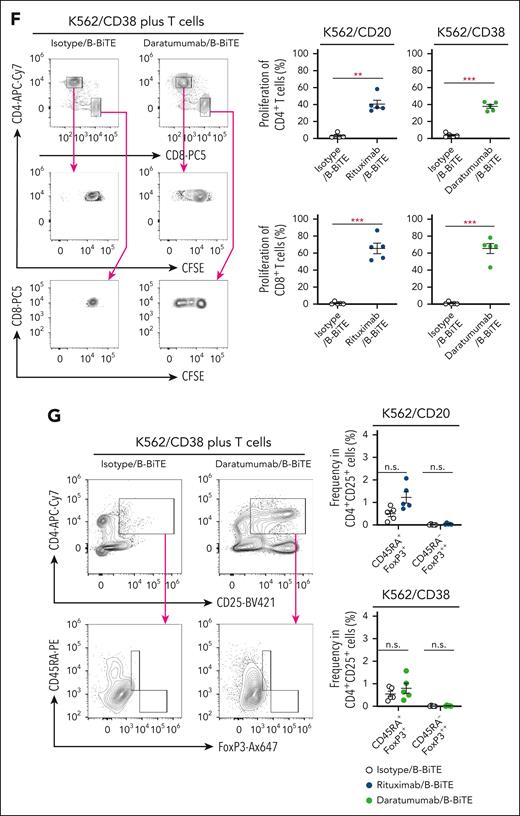
After purification of B-BiTE (Figure 1A), it was mixed with a human mAb to generate a mAb/B-BiTE complex before performing the following series of experiments. It was found that the CD19 mAb/B-BiTE complex bound successfully to CD19+ target cells and CD3-expressing cells and isolated human peripheral blood T cells produced a panel of cytokines against the CD19+ target cells in the presence of the CD19 mAb/B-BiTE complex but not an isotype/B-BiTE complex (supplemental Figures 2 and 3A). Clinically available mAbs armed with B-BiTE, such as rituximab/B-BiTE and daratumumab/B-BiTE, also bound to CD20+, CD38+, and CD3+ target cells (Figure 1B). Indeed, purified human T cells responded to target cells in the presence of rituximab/B-BiTE and daratumumab/B-BiTE (Figure 1C-E; supplemental Figure 3A). The proliferation capacity of human T cells activated by a mAb/B-BiTE complex was also investigated. As expected, human CD8+ T cells as well as CD4+ T cells proliferated well upon recognition of CD20+ and CD38+ target cells in the presence of rituximab/B-BiTE and daratumumab/B-BiTE, respectively (Figure 1F). We also confirmed that the frequency of regulatory T cells in the activated CD4+CD25+ T-cell population, which was determined by CD45RA+FoxP3+ or CD45RA–FoxP3++ cells (supplemental Figure 3B), did not increase (Figure 1G). These results suggest that B-BiTE binds to a human IgG-based mAb, resulting in successful activation of human T cells as a newly generated bispecific antibody.
B-BiTE and specific activation of human T cells by a mAb/B-BiTE complex. (A) B-BiTE was purified and then detected using anti-His mAb. (B) K562, K562/CD20, K562/CD38, Raji, Jurkat, and Jurkat 76 cells were stained with each mAb/B-BiTE complex (blue), containing 10 μg/mL isotype, rituximab, or daratumumab bound to 3.3 μg/mL B-BiTE. K562/CD20 and K562/CD38 cells were established by transduction with the CD20 or CD38 gene, respectively. The gray histogram shows the staining with PE-anti-His mAb alone. (C-E) Freshly isolated human peripheral blood CD3+ T cells derived from 6 different donors were cocultured with K562 (red), K562/CD20 (blue), or K562/CD38 (green) cells in the presence of isotype/B-BiTE, rituximab/B-BiTE, or daratumumab/B-BiTE (mAb, 7.5 μg/mL; B-BiTE, 2.5 μg/mL). Multiple cytokine production by CD8+ T cells and CD4+ T cells was evaluated by intracellular staining assay. The gating strategy and representative cytokine plots of CD8+ T cells and CD4+ T cells against K562/CD38 cells are shown (C), and the results for K562/CD20 cells (D) and K562/CD38 cells (E) are summarized. Each dot represents a donor, and an isotype/B-BiTE complex was prepared as a negative control. (F) Freshly isolated human peripheral blood CD3+ T cells derived from 5 different donors were labeled with CFSE, and stimulated with irradiated K562/CD20 and K562/CD38 cells at an E:T ratio of 10:1 in the presence of rituximab/B-BiTE, daratumumab/B-BiTE, or isotype/B-BiTE complex (mAb, 7.5 μg/mL; B-BiTE, 2.5 μg/mL). After 5 days of incubation, the mean fluorescence intensity (MFI) of CFSE–labeled CD4+ T cells and CD8+ T cells was examined. Representative stainings of CD4+ T cells and CD8+ T cells derived from the same donor are depicted (left), and their proliferation (%) against indicated target cells is summarized (right). The percentage was calculated as: (control MFI – experimental MFI)/(control MFI) × 100 (%). (G) CD45RA+FoxP3+ and CD45RA–FoxP3++ populations among CD4+CD25+ T cells after stimulation, similar to panel F, were measured. Representative stainings are shown (left), and the frequency of the indicated population from each donor is depicted (right). All paired data were evaluated statistically by a paired t test (2-sided). Error bars show the SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. CFSE, carboxyfluorescein diacetate succinimidyl ester; E:T, effector to target; MFI, mean fluorescence intensity; n.s., not significant; PE, phycoerythrin; SD, standard deviation.
B-BiTE and specific activation of human T cells by a mAb/B-BiTE complex. (A) B-BiTE was purified and then detected using anti-His mAb. (B) K562, K562/CD20, K562/CD38, Raji, Jurkat, and Jurkat 76 cells were stained with each mAb/B-BiTE complex (blue), containing 10 μg/mL isotype, rituximab, or daratumumab bound to 3.3 μg/mL B-BiTE. K562/CD20 and K562/CD38 cells were established by transduction with the CD20 or CD38 gene, respectively. The gray histogram shows the staining with PE-anti-His mAb alone. (C-E) Freshly isolated human peripheral blood CD3+ T cells derived from 6 different donors were cocultured with K562 (red), K562/CD20 (blue), or K562/CD38 (green) cells in the presence of isotype/B-BiTE, rituximab/B-BiTE, or daratumumab/B-BiTE (mAb, 7.5 μg/mL; B-BiTE, 2.5 μg/mL). Multiple cytokine production by CD8+ T cells and CD4+ T cells was evaluated by intracellular staining assay. The gating strategy and representative cytokine plots of CD8+ T cells and CD4+ T cells against K562/CD38 cells are shown (C), and the results for K562/CD20 cells (D) and K562/CD38 cells (E) are summarized. Each dot represents a donor, and an isotype/B-BiTE complex was prepared as a negative control. (F) Freshly isolated human peripheral blood CD3+ T cells derived from 5 different donors were labeled with CFSE, and stimulated with irradiated K562/CD20 and K562/CD38 cells at an E:T ratio of 10:1 in the presence of rituximab/B-BiTE, daratumumab/B-BiTE, or isotype/B-BiTE complex (mAb, 7.5 μg/mL; B-BiTE, 2.5 μg/mL). After 5 days of incubation, the mean fluorescence intensity (MFI) of CFSE–labeled CD4+ T cells and CD8+ T cells was examined. Representative stainings of CD4+ T cells and CD8+ T cells derived from the same donor are depicted (left), and their proliferation (%) against indicated target cells is summarized (right). The percentage was calculated as: (control MFI – experimental MFI)/(control MFI) × 100 (%). (G) CD45RA+FoxP3+ and CD45RA–FoxP3++ populations among CD4+CD25+ T cells after stimulation, similar to panel F, were measured. Representative stainings are shown (left), and the frequency of the indicated population from each donor is depicted (right). All paired data were evaluated statistically by a paired t test (2-sided). Error bars show the SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. CFSE, carboxyfluorescein diacetate succinimidyl ester; E:T, effector to target; MFI, mean fluorescence intensity; n.s., not significant; PE, phycoerythrin; SD, standard deviation.
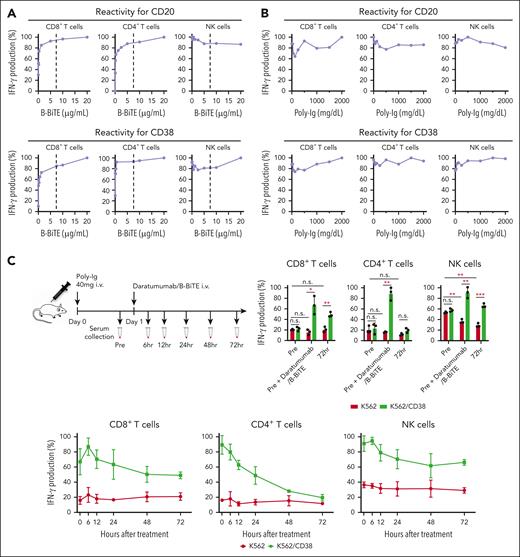
Next, we assessed whether isolated human NK cells are also activated by mAb/B-BiTE. Purified human peripheral blood NK cells similarly recognized target cells in the presence of an antitumor mAb/B-BiTE as well as the mAb alone (supplemental Figure 4A-B). The concentration of B-BiTE lower than that of rituximab and daratumumab appeared to be sufficient and saturated to induce human T-cell responses. Importantly, even in the presence of much higher concentrations of B-BiTE than that of rituximab or daratumumab, NK cell reactivity did not decrease and was sufficiently preserved relative to that induced by an antitumor mAb itself (Figure 2A). These results suggest that B-BiTE appears not to interfere with recognition of the human IgG-Fc domain by NK cells.
Preserved NK cell reactivity and stability of a mAb/B-BiTE complex. (A) IFN-γ production by isolated human CD3+ T cells including CD8+ T cells (left) and CD4+ T cells (middle), and human NK cells (right) against K562/CD20 cells (top) or K562/CD38 cells (bottom), was measured in the presence of 7.5 μg/mL rituximab or daratumumab together with graded concentrations of B-BiTE. The concentration of mAb is indicated as a dotted line in each graph. The maximal IFN-γ production by responder cells against target cells was considered as 100%, and each reactivity was calculated relative to this value. (B) Human PBMCs were incubated with K562/CD20 cells (top) or K562/CD38 cells (bottom) in the presence of rituximab/B-BiTE or daratumumab/B-BiTE complex (mAb, 7.5 μg/mL; B-BiTE, 2.5 μg/mL) with different concentrations of human Poly-Ig. Competitive assays were performed, and the IFN-γ production by human CD8+ T cells (left), CD4+ T cells (middle), and NK cells (right) was measured by intracellular cytokine assay. IFN-γ production (%) in the presence of each concentration of Poly-Ig was calculated relative to that induced by rituximab/B-BiTE or daratumumab/B-BiTE for K562/CD20 cells or K562/CD38 cells in the absence of Poly-Ig and is plotted. (C) Poly-Ig (20 mg) was IV injected into irradiated NOD/Shi-scid IL-2rγ(null) mice (n = 3) twice with a 12-hour interval, on day 0 (Poly-Ig, 40 mg/mouse). Daratumumab/B-BiTE complex (daratumumab, 30 μg; B-BiTE, 10 μg) was similarly injected into the mice on day 1. Murine serum was collected at each time point (before treatment and 6, 12, 24, 48, and 72 hours after treatment) (top left). Then, human PBMCs were cocultured with K562 cells or K562/CD38 cells in wells containing each collected serum. A sample just after administration of daratumumab/B-BiTE (0 hour) was prepared by adding it to pretreated serum in vitro (40 μg/mL). IFN-γ production by CD8+ T cells (bottom left), CD4+ T cells (bottom middle), and NK cells (bottom right) against target cells was measured. The maximal IFN-γ production by responder cells was considered as 100%, and each reactivity was calculated relative to this value. The values obtained before treatment, at 0 hour, and at 72 hours after treatment were also compared directly (top right). Error bars show the SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; n.s., not significant; Poly-Ig, polyclonal immunoglobulin.
Preserved NK cell reactivity and stability of a mAb/B-BiTE complex. (A) IFN-γ production by isolated human CD3+ T cells including CD8+ T cells (left) and CD4+ T cells (middle), and human NK cells (right) against K562/CD20 cells (top) or K562/CD38 cells (bottom), was measured in the presence of 7.5 μg/mL rituximab or daratumumab together with graded concentrations of B-BiTE. The concentration of mAb is indicated as a dotted line in each graph. The maximal IFN-γ production by responder cells against target cells was considered as 100%, and each reactivity was calculated relative to this value. (B) Human PBMCs were incubated with K562/CD20 cells (top) or K562/CD38 cells (bottom) in the presence of rituximab/B-BiTE or daratumumab/B-BiTE complex (mAb, 7.5 μg/mL; B-BiTE, 2.5 μg/mL) with different concentrations of human Poly-Ig. Competitive assays were performed, and the IFN-γ production by human CD8+ T cells (left), CD4+ T cells (middle), and NK cells (right) was measured by intracellular cytokine assay. IFN-γ production (%) in the presence of each concentration of Poly-Ig was calculated relative to that induced by rituximab/B-BiTE or daratumumab/B-BiTE for K562/CD20 cells or K562/CD38 cells in the absence of Poly-Ig and is plotted. (C) Poly-Ig (20 mg) was IV injected into irradiated NOD/Shi-scid IL-2rγ(null) mice (n = 3) twice with a 12-hour interval, on day 0 (Poly-Ig, 40 mg/mouse). Daratumumab/B-BiTE complex (daratumumab, 30 μg; B-BiTE, 10 μg) was similarly injected into the mice on day 1. Murine serum was collected at each time point (before treatment and 6, 12, 24, 48, and 72 hours after treatment) (top left). Then, human PBMCs were cocultured with K562 cells or K562/CD38 cells in wells containing each collected serum. A sample just after administration of daratumumab/B-BiTE (0 hour) was prepared by adding it to pretreated serum in vitro (40 μg/mL). IFN-γ production by CD8+ T cells (bottom left), CD4+ T cells (bottom middle), and NK cells (bottom right) against target cells was measured. The maximal IFN-γ production by responder cells was considered as 100%, and each reactivity was calculated relative to this value. The values obtained before treatment, at 0 hour, and at 72 hours after treatment were also compared directly (top right). Error bars show the SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; n.s., not significant; Poly-Ig, polyclonal immunoglobulin.
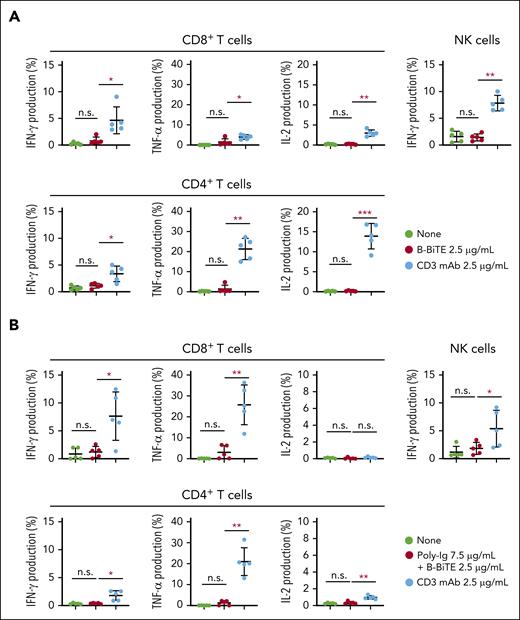
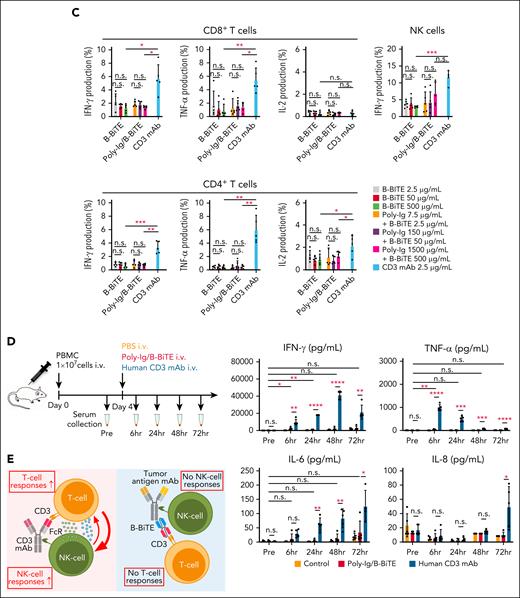
We then investigated further characteristics of the mAb/B-BiTE complex. Both rituximab/B-BiTE and daratumumab/B-BiTE were still able to activate T cells against target cells in the presence of human serum polyclonal Ig (Figure 2B). To assess the in vivo stability of the mAb/B-BiTE complexes, NOD/Shi-scid IL-2rγ(null) mice were injected with human polyclonal Ig before the administration of daratumumab/B-BiTE. Because 3 log high amounts of human Ig hampered its specific detection, in vitro human T-cell reactivity induced by each murine serum was used as a surrogate marker. Daratumumab/B-BiTE decreased after 72 hours but a certain amount still appeared to remain in murine serum (Figure 2C). Importantly, the murine serum collected after the injection of daratumumab/B-BiTE did not activate human T cells against irrelevant target cells (Figure 2C). Furthermore, human polyclonal Ig armed with B-BiTE, or B-BiTE itself, did not induce any T-cell/NK cell reactivity among human PBMCs. In contrast, antihuman CD3 mAb with the human Fc domain was able to activate human immune cells including T cells and NK cells (Figure 3A-C). Based on these results, we also investigated off-target cytokine production in vivo. After engraftment of freshly isolated human PBMCs in NOD/Shi-scid IL-2rγ(null) mice, human polyclonal Ig/B-BiTE or antihuman CD3 mAb was injected IV into each mouse. In this model, dissociation and clearance of the mAb/B-BiTE complex from murine sera became evident 24 to 72 hours after injection, and its half-life measured by direct binding of the mAb/B-BiTE was 11.13 hours, even though human Ig as well as antihuman CD3 mAb was preserved at 72 hours after injection (supplemental Figure 4C-E). The levels of most of the human cytokines in mice treated with Ig/B-BiTE were not significantly elevated, but those with antihuman CD3 mAb were observed, suggesting absence of off-target immune-cell reactivity because of B-BiTE (Figure 3D-E; supplemental Figure 4F).
Assessment of potential off-target reactivity induced by a mAb/B-BiTE complex. (A-C) Fresh human PBMCs isolated from 5 different donors were incubated in the presence of 2.5 μg/mL B-BiTE alone (A), 7.5 μg/mL Poly-Ig with 2.5 μg/mL B-BiTE (B). Much higher concentrations of B-BiTE alone or Poly-Ig with B-BiTE were also tested as indicated (C). Cytokine production by CD8+ T cells, CD4+ T cells, and NK cells was assessed by intracellular cytokine staining. Human IgG1-based antihuman CD3 mAb (clone OKT3) was used as a positive control. Each dot represents each donor. A paired t test (2-sided) was performed for comparison, and error bars show the SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; n.s., not significant. (D) Ten million freshly isolated human PBMCs were injected IV into NOD/Shi-scid IL-2rγ(null) mice (n = 5). After 4 days of PBMC injection, these mice were administered indicated antibodies (antibody, 30 μg/mL; B-BiTE, 10 μg/mL). A series of human cytokines that were contained in each murine serum were measured by cytokine beads assays. Each dot represents each mouse. The Welch t test (2-sided) was performed for comparison, and error bars show the SD. Each group as well as each of the time points in the group treated with Poly-Ig/B-BiTE complexes were compared. The decrease in cytokines after treatment was not assessed statistically. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; n.s., not significant. (E) Antihuman CD3 mAb with the human Fc domain is able to activate T cells and NK cells in the absence of target cells, resulting in induction of off-target reactivity (left). In contrast, the mAb/B-BiTE is able to activate neither human T cells nor NK cells without target cells (right). Poly-Ig, polyclonal immunoglobulin; SD, standard deviation.
Assessment of potential off-target reactivity induced by a mAb/B-BiTE complex. (A-C) Fresh human PBMCs isolated from 5 different donors were incubated in the presence of 2.5 μg/mL B-BiTE alone (A), 7.5 μg/mL Poly-Ig with 2.5 μg/mL B-BiTE (B). Much higher concentrations of B-BiTE alone or Poly-Ig with B-BiTE were also tested as indicated (C). Cytokine production by CD8+ T cells, CD4+ T cells, and NK cells was assessed by intracellular cytokine staining. Human IgG1-based antihuman CD3 mAb (clone OKT3) was used as a positive control. Each dot represents each donor. A paired t test (2-sided) was performed for comparison, and error bars show the SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; n.s., not significant. (D) Ten million freshly isolated human PBMCs were injected IV into NOD/Shi-scid IL-2rγ(null) mice (n = 5). After 4 days of PBMC injection, these mice were administered indicated antibodies (antibody, 30 μg/mL; B-BiTE, 10 μg/mL). A series of human cytokines that were contained in each murine serum were measured by cytokine beads assays. Each dot represents each mouse. The Welch t test (2-sided) was performed for comparison, and error bars show the SD. Each group as well as each of the time points in the group treated with Poly-Ig/B-BiTE complexes were compared. The decrease in cytokines after treatment was not assessed statistically. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; n.s., not significant. (E) Antihuman CD3 mAb with the human Fc domain is able to activate T cells and NK cells in the absence of target cells, resulting in induction of off-target reactivity (left). In contrast, the mAb/B-BiTE is able to activate neither human T cells nor NK cells without target cells (right). Poly-Ig, polyclonal immunoglobulin; SD, standard deviation.
Human T-cell and NK cell responses induced by a clinically available mAb armed with B-BiTE
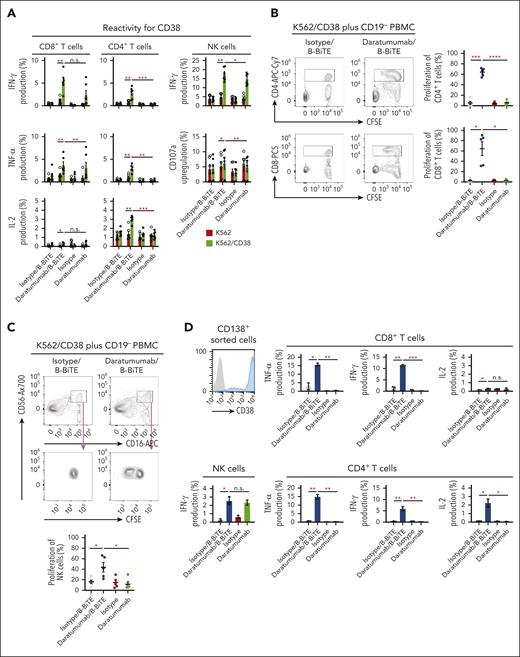
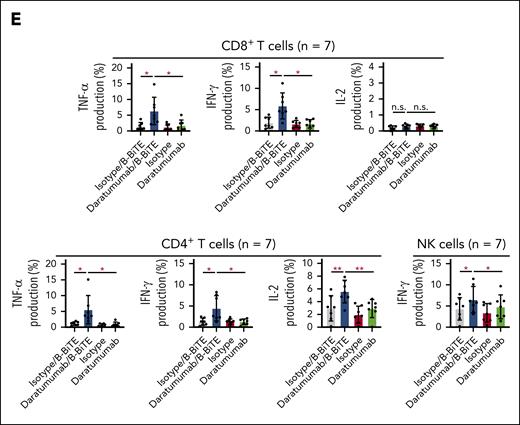
We then examined in detail the immune responses of coexisting human T cells and NK cells against target cells induced by a clinically available antitumor mAb bound to B-BiTE. Using daratumumab as a model, human CD8+ T cells, CD4+ T cells, as well as NK cells among CD19+ B-cell–depleted PBMCs (CD19– PBMCs) successfully recognized CD38+ target cells in the presence of the daratumumab/B-BiTE complex, resulting in the production of cytokines and upregulation of CD107a (Figure 4A). Interestingly, interferon gamma production and degranulation by NK cells induced by daratumumab/B-BiTE tended to be higher than those mediated by the mAb alone, and proliferation of human NK cells in the presence of daratumumab/B-BiTE was supported by T cells simultaneously stimulated with daratumumab/B-BiTE, as evidenced by a higher proliferation rate than that mediated by mAb itself (Figure 4B-C). To investigate B-BiTE for the treatment for MM, clinical samples obtained from patients with MM were also tested. Patients’ CD8+ T cells, CD4+ T cells, and NK cells responded to their own myeloma cells in the presence of daratumumab/B-BiTE (Figure 4D-E). Similar results were obtained using CD19– PBMCs with rituximab/B-BiTE and clinical samples obtained from a patient with lymphoma (supplemental Figure 5), also suggesting the clinical availability of the mAb/B-BiTE complex. Collectively, these results suggest that the mAb/B-BiTE complex is able to successfully activate human T cells as well as NK cells against tumor cells, and simultaneous stimulation of both T cells and NK cells induced by the mAb/B-BiTE is able to enhance the antitumor effects further.
Antitumor reactivity and proliferation capacity of human T cells and NK cells induced by a mAb/B-BiTE complex. (A) CD19+ B-cell–depleted human PBMCs (CD19– PBMCs) derived from 6 different donors were incubated with K562 or K562/CD38 cells in the presence of the indicated antibodies. Multiple cytokine production by peripheral blood CD8+ T cells, CD4+ T cells, and NK cells was assessed using intracellular cytokine assays. The concentration of daratumumab and B-BiTE was 7.5 μg/mL and 2.5 μg/mL, respectively. Each dot represents each donor. (B-C) CD19– PBMCs derived from 5 different donors were labeled with CFSE and stimulated as above. After 5 days of incubation with the indicated antibodies, the MFI of CFSE–labeled human CD8+ T cells, CD4+ T cells (B), and NK cells (C), which were designated as CD16+CD56+ cells among CD4–CD8– population, was examined, and their proliferation (percentage) against K562/CD38 cells is summarized. Each dot represents each donor. (D-E) CD19– PBMCs from the same patient were incubated with isolated myeloma cells in the presence of the indicated antibodies including daratumumab/B-BiTE (daratumumab, 7.5 μg/mL; B-BiTE, 2.5 μg/mL). Cytokine production by CD8+ T cells, CD4+ T cells, and NK cells against target cells was assessed. Representative data (triplicate) and CD38 expression of CD138+ myeloma cells isolated from a patient are shown (D). This analysis was performed using samples obtained from 7 different patients including the patient in panel D, and the results are summarized (E). Each dot represents each donor. All data were evaluated statistically by a paired t test (2-sided) or a Welch t test (2-sided), and error bars show the SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CFSE, carboxyfluorescein diacetate succinimidyl ester; MFI, mean fluorescence intensity; n.s., not significant; SD, standard deviation.
Antitumor reactivity and proliferation capacity of human T cells and NK cells induced by a mAb/B-BiTE complex. (A) CD19+ B-cell–depleted human PBMCs (CD19– PBMCs) derived from 6 different donors were incubated with K562 or K562/CD38 cells in the presence of the indicated antibodies. Multiple cytokine production by peripheral blood CD8+ T cells, CD4+ T cells, and NK cells was assessed using intracellular cytokine assays. The concentration of daratumumab and B-BiTE was 7.5 μg/mL and 2.5 μg/mL, respectively. Each dot represents each donor. (B-C) CD19– PBMCs derived from 5 different donors were labeled with CFSE and stimulated as above. After 5 days of incubation with the indicated antibodies, the MFI of CFSE–labeled human CD8+ T cells, CD4+ T cells (B), and NK cells (C), which were designated as CD16+CD56+ cells among CD4–CD8– population, was examined, and their proliferation (percentage) against K562/CD38 cells is summarized. Each dot represents each donor. (D-E) CD19– PBMCs from the same patient were incubated with isolated myeloma cells in the presence of the indicated antibodies including daratumumab/B-BiTE (daratumumab, 7.5 μg/mL; B-BiTE, 2.5 μg/mL). Cytokine production by CD8+ T cells, CD4+ T cells, and NK cells against target cells was assessed. Representative data (triplicate) and CD38 expression of CD138+ myeloma cells isolated from a patient are shown (D). This analysis was performed using samples obtained from 7 different patients including the patient in panel D, and the results are summarized (E). Each dot represents each donor. All data were evaluated statistically by a paired t test (2-sided) or a Welch t test (2-sided), and error bars show the SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CFSE, carboxyfluorescein diacetate succinimidyl ester; MFI, mean fluorescence intensity; n.s., not significant; SD, standard deviation.
Enhanced antimyeloma effects induced by the mAb/B-BiTE complex
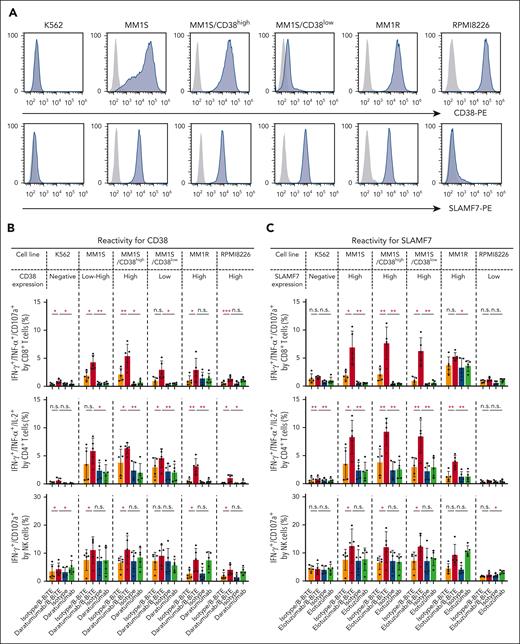
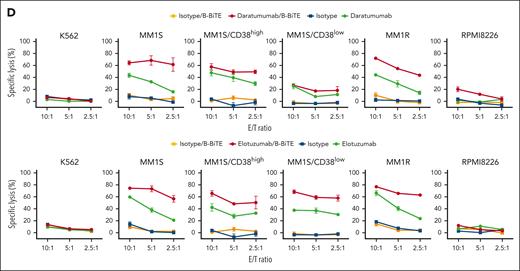
We then applied B-BiTE to generate a panel of new bispecific antibodies for the development of a new MM treatment strategy. First, the expression of CD38 and SLAMF7 on various myeloma cell lines was investigated because these 2 antigens are major targets for immunotherapy against myeloma, and mAbs, such as daratumumab and elotuzumab, are already clinically available. As shown in Figure 5A, CD38lowSLAMF7high cells, CD38highSLAMF7high cells, and CD38highSLAMF7low cells were present. Furthermore, the MM1S cell line contained 2 populations indicated as CD38high and CD38low, suggesting heterogeneity of the myeloma cells. Daratumumab/B-BiTE and elotuzumab/B-BiTE became bound to CD38+ and SLAMF7+ target cells, respectively (supplemental Figure 6A). To clarify the difference between CD38high and CD38low myeloma cells as target cells for the mAb/B-BiTE complex, MM1S/CD38high cells and MM1S/CD38low cells were isolated for further experiments. In the presence of daratumumab/B-BiTE or elotuzumab/B-BiTE, human CD8+ T cells, CD4+ T cells, and NK cells among CD19– PBMCs degranulated or produced cytokines against myeloma cells with low to high expression of CD38 and SLAMF7, respectively (Figure 5B-C). The antigen-specific cytotoxic reactivity of human immune cells induced by the mAb/B-BiTE complex was enhanced in comparison with that mediated by a mAb alone. Note that the killing activity mediated by a mAb/B-BiTE targeting the same antigen tended to correlate with the expression level of the target antigens in each modality, except for RPMI8226 cells targeting CD38 (Figure 5D; supplemental Figure 6B). Examination of specific killing of 1 myeloma cell line expressing different target antigens showed that human T/NK cells effectively killed CD38highSLAMF7low RPMI8226 cells in the presence of daratumumab/B-BiTE, relative to elotuzumab/B-BiTE, and conversely also lysed CD38lowSLAMF7high MM1S/CD38low cells sufficiently (Figure 5D). Although freshly isolated human T cells as well as NK cells expressed CD38 and SLAMF7 to varying degrees, any cytotoxicity of PBMCs against those immune cells in the presence of daratumumab/B-BiTE and elotuzumab/B-BiTE was negligible (supplemental Figure 7). These results suggest that the bispecific antibodies newly generated using B-BiTE are able to induce enhanced antimyeloma responses successfully and effectively.
Antimyeloma effects of mAb/B-BiTE-redirected human T cells and NK cells. (A) Expression patterns of CD38 and SLAMF7 on myeloma cell lines. (B-C) CD19– human PBMCs derived from 5 different donors were incubated with target myeloma cell lines in the presence of the indicated antibodies including daratumumab/B-BiTE (B) or elotuzumab/B-BiTE (C) (mAb, 7.5 μg/mL; B-BiTE, 2.5 μg/mL), and assessed for their multiple cytokine production and CD107a upregulation. CD8+ T cells triple-positive for IFN-γ/TNF-α/CD107a, CD4+ T cells triple-positive for IFN-γ/TNF-α/IL-2, and NK cells double-positive for IFN-γ/CD107a were measured by flow cytometry. Each dot represents each donor. Expression level of CD38 (B) or SLAMF7 (C) in each cell line is labeled according to the result in panel A. The paired t test (2-sided) was performed for comparison, and error bars show the SD. (D) CD19– PBMCs were incubated with target cells in the presence of the indicated antibodies (mAb, 7.5 μg/mL; B-BiTE, 2.5 μg/mL) at different E:T ratios, and their cytotoxicity was examined using chromium-51 (51Cr)–release assays. All the experiments were performed in triplicate, and error bars show the SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; E:T, effector to target; n.s., not significant; SD, standard deviation.
Antimyeloma effects of mAb/B-BiTE-redirected human T cells and NK cells. (A) Expression patterns of CD38 and SLAMF7 on myeloma cell lines. (B-C) CD19– human PBMCs derived from 5 different donors were incubated with target myeloma cell lines in the presence of the indicated antibodies including daratumumab/B-BiTE (B) or elotuzumab/B-BiTE (C) (mAb, 7.5 μg/mL; B-BiTE, 2.5 μg/mL), and assessed for their multiple cytokine production and CD107a upregulation. CD8+ T cells triple-positive for IFN-γ/TNF-α/CD107a, CD4+ T cells triple-positive for IFN-γ/TNF-α/IL-2, and NK cells double-positive for IFN-γ/CD107a were measured by flow cytometry. Each dot represents each donor. Expression level of CD38 (B) or SLAMF7 (C) in each cell line is labeled according to the result in panel A. The paired t test (2-sided) was performed for comparison, and error bars show the SD. (D) CD19– PBMCs were incubated with target cells in the presence of the indicated antibodies (mAb, 7.5 μg/mL; B-BiTE, 2.5 μg/mL) at different E:T ratios, and their cytotoxicity was examined using chromium-51 (51Cr)–release assays. All the experiments were performed in triplicate, and error bars show the SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; E:T, effector to target; n.s., not significant; SD, standard deviation.
Successful treatment of myeloma with low target antigen using a combination of mAb/B-BiTE complexes
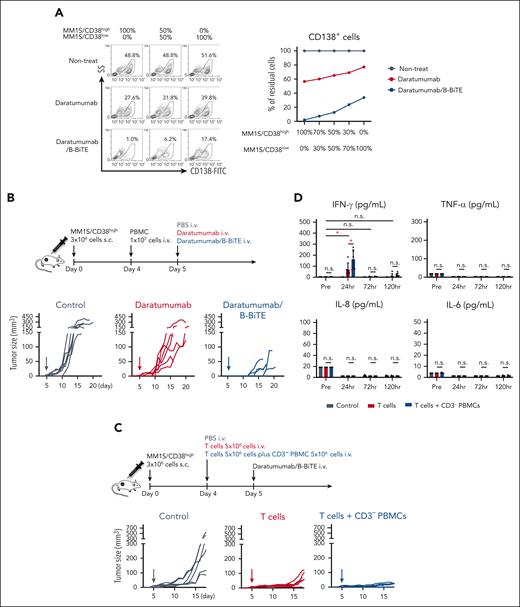
To investigate how heterogeneous myeloma cells with differing expression of a target antigen would affect the cytotoxicity induced by a mAb/B-BiTE or a mAb alone, we first prepared an in vitro model in which target cells contained different ratios of MM1S/CD38high cells and MM1S/CD38low cells. Both myeloma cell lines expressed CD138 at a similar level (supplemental Figure 8A). When the amount of myeloma cells was measured in terms of CD138 expression after incubation with CD19– PBMCs, it was found that MM1S/CD38high cells had almost disappeared, whereas a proportion of MM1S/CD38low cells remained in the presence of daratumumab/B-BiTE. In contrast, a large proportion of MM1S/CD38high cells as well as MM1S/CD38low cells remained viable in the presence of daratumumab alone, indicating enhancement of the antimyeloma T-cell reactivity by B-BiTE (Figure 6A). Thus, the heterogeneity of myeloma cells expressing different levels of a target antigen may account for recurrence after treatment with a single mAb/B-BiTE complex.
In vivo antimyeloma effects and on-target cytokine release mediated by a mAb/B-BiTE complex. (A) Target myeloma cells including MM1S/CD38high and MM1S/CD38low cells at different ratios were prepared. CD19– human PBMCs were cocultured with these target cells at an E:T ratio of 4:1 in the presence of daratumumab, or daratumumab/B-BiTE (daratumumab, 7.5 μg/mL; B-BiTE, 2.5 μg/mL), and the proportion of CD138+ MM1S cells among total cells was measured (left). The percentage of MM1S cells after treatment with antibodies was calculated relative to the frequency of MM1S cells without any antibodies and is plotted as residual cells (right). (B) Three million MM1S/CD38high cells were injected subcutaneously into the right flank of irradiated NOD/Shi-scid IL-2rγ(null) mice. After engraftment of the MM1S/CD38high cells, 1.0 × 107 freshly isolated human PBMCs were injected IV into the mice on day 4. On day 5, daratumumab (30 μg) or daratumumab/B-BiTE complex (daratumumab, 30 μg; B-BiTE, 10 μg) was similarly injected into each mouse, followed by measurement of tumor sizes (n = 6 mice per group). (C) Three million MM1S/CD38high cells were similarly inoculated into NOD/Shi-scid IL-2rγ (null) mice. After engraftment, 5.0 × 106 freshly isolated human T cells or 5.0 × 106 human T cells together with 5.0 × 106 CD3– PBMCs were injected IV into the mice on day 4. On day 5, daratumumab/B-BiTE complex was similarly injected into each mouse, followed by measurement of tumor sizes (n = 6 mice per group). (D) To assess the amounts of cytokines, the experiment in panel C was repeated again, and murine sera were collected at each time point. A series of human cytokines contained in each murine serum were measured using cytokine beads assays. Each dot represents an individual mouse. The Welch t test (2-sided) was performed for comparison, and error bars show the SD. Each of the groups as well as each of the time points in the group treated with both T cells and CD3– PBMCs were compared. Decreases in cytokines after treatment were not assessed statistically. ∗P < .05; E:T, effector to target; n.s., not significant; SD, standard deviation.
In vivo antimyeloma effects and on-target cytokine release mediated by a mAb/B-BiTE complex. (A) Target myeloma cells including MM1S/CD38high and MM1S/CD38low cells at different ratios were prepared. CD19– human PBMCs were cocultured with these target cells at an E:T ratio of 4:1 in the presence of daratumumab, or daratumumab/B-BiTE (daratumumab, 7.5 μg/mL; B-BiTE, 2.5 μg/mL), and the proportion of CD138+ MM1S cells among total cells was measured (left). The percentage of MM1S cells after treatment with antibodies was calculated relative to the frequency of MM1S cells without any antibodies and is plotted as residual cells (right). (B) Three million MM1S/CD38high cells were injected subcutaneously into the right flank of irradiated NOD/Shi-scid IL-2rγ(null) mice. After engraftment of the MM1S/CD38high cells, 1.0 × 107 freshly isolated human PBMCs were injected IV into the mice on day 4. On day 5, daratumumab (30 μg) or daratumumab/B-BiTE complex (daratumumab, 30 μg; B-BiTE, 10 μg) was similarly injected into each mouse, followed by measurement of tumor sizes (n = 6 mice per group). (C) Three million MM1S/CD38high cells were similarly inoculated into NOD/Shi-scid IL-2rγ (null) mice. After engraftment, 5.0 × 106 freshly isolated human T cells or 5.0 × 106 human T cells together with 5.0 × 106 CD3– PBMCs were injected IV into the mice on day 4. On day 5, daratumumab/B-BiTE complex was similarly injected into each mouse, followed by measurement of tumor sizes (n = 6 mice per group). (D) To assess the amounts of cytokines, the experiment in panel C was repeated again, and murine sera were collected at each time point. A series of human cytokines contained in each murine serum were measured using cytokine beads assays. Each dot represents an individual mouse. The Welch t test (2-sided) was performed for comparison, and error bars show the SD. Each of the groups as well as each of the time points in the group treated with both T cells and CD3– PBMCs were compared. Decreases in cytokines after treatment were not assessed statistically. ∗P < .05; E:T, effector to target; n.s., not significant; SD, standard deviation.
To further demonstrate the usefulness of the mAb/B-BiTE as a new bispecific antibody, we performed in vivo side-by-side experiments. NOD/Shi-scid IL-2rγ(null) mice were subcutaneously inoculated into the right flank with MM1S/CD38high myeloma cells, followed by IV injection of human freshly isolated and unstimulated PBMCs on day 4 (supplemental Figure 8B). Daratumumab or daratumumab/B-BiTE complex was then injected once into the tumor-bearing mice on day 5. Treatment of the mice using daratumumab suppressed the tumor growth of CD38high myeloma, and importantly, daratumumab/B-BiTE significantly enhanced the antimyeloma response relative to that mediated by daratumumab alone (Figure 6B). Further examination demonstrated that NK cell reactivity in addition to T-cell reactivity induced by daratumumab/B-BiTE augmented the antimyeloma effects. Interestingly, the extent of cytokine release because of dual-lymphoid activation was similar to that mediated by T cells alone (Figure 6C-D; supplemental figure 8C). When these mice were treated twice with daratumumab/B-BiTE or daratumumab on days 5 and 12, the level of in vivo cytotoxicity against myeloma cells induced by each modality appeared to be the same, suggesting that administration of daratumumab twice would sufficiently enhance the killing activity when targeting CD38high myeloma cells (supplemental Figure 8D).
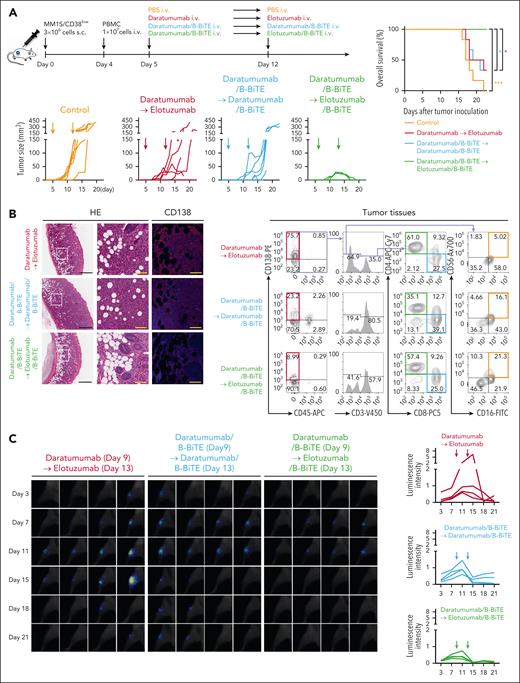
Next, we similarly inoculated MM1S/CD38low myeloma cells into NOD/Shi-scid IL-2rγ(null) mice to establish an in vivo model of the low antigen level for CD38. Based on the results of in vitro and in vivo experiments described above, mice bearing MM1S/CD38low cells were treated with each modality twice on days 5 and 12. Although antitumor effects were induced by daratumumab/B-BiTE, they were not as marked as those achieved by targeting MM1S/CD38high myeloma cells (Figure 6B, 7A; supplemental Figure 8D). There was no significant difference in tumor growth between mice treated twice with daratumumab/B-BiTE and those treated with daratumumab in combination with elotuzumab. Interestingly, after treatment with daratumumab/B-BiTE, elotuzumab/B-BiTE suppressed tumor growth strongly and effectively, extending the survival of tumor-bearing mice (Figure 7A). Histological and cell-based analyses on day 17 revealed that human CD45–CD138+ myeloma cells had largely disappeared, whereas human NK cells along with human T cells had accumulated at tumor sites after treatment with the combination of both daratumumab/B-BiTE and elotuzumab/B-BiTE (Figure 7B; supplemental Figure 9A). Note that human PBMCs were engrafted successfully in each murine group, and human CD45+ cells appeared to increase after administration of the mAb with B-BiTE compared with the mAb alone (supplemental Figure 9B-C). We also confirmed that elotuzumab/B-BiTE exerted direct antimyeloma effects in the absence of daratumumab/B-BiTE in vivo. Two IV injections of elotuzumab/B-BiTE on days 5 and 12 into MM1S/CD38low myeloma-bearing mice delayed tumor growth slightly, whereas elotuzumab alone did not decrease tumor volumes (supplemental Figure 8E).
Enhanced in vivo antimyeloma effects induced by 2 different mAb/B-BiTE complexes. (A) Three million MM1S/CD38lowSLAMF7high cells were injected subcutaneously into NOD/Shi-scid IL-2rγ(null) mice, then 1.0 × 107 freshly isolated PBMCs obtained from the donor were injected IV on day 4. Daratumumab (30 μg) or daratumumab/B-BiTE complex (daratumumab, 30 μg; B-BiTE, 10 μg) was administered IV on day 5. On day 12, 30 μg elotuzumab was administered IV to the mice treated with daratumumab alone. On the other hand, daratumumab/B-BiTE or elotuzumab/B-BiTE (mAb, 30 μg; B-BiTE, 10 μg) was administered to the mice treated with daratumumab/B-BiTE on the same day (top left). Tumor sizes after treatment are depicted (bottom left), and the overall survival of each murine group (n = 6) is summarized in the form of Kaplan-Meier curves (right). Overall survival was compared among the groups using a log-rank (Mantel-Cox) test. ∗P < .05; ∗∗∗P < .001. (B) To perform histological analyses, the experiments in panel A were repeated, and subcutaneous tumors were resected from each murine group on day 17. Representative hematoxylin and eosin staining and fluorescence immunohistochemistry for human CD138 at the tumor sites are shown (left). The images in white rectangles are magnified, and the scale bars in the pictures represent 600 μm (black) and 100 μm (yellow). In addition, tumor tissues were homogenized, and the phenotypes of human cells that accumulated at the tumor sites were also analyzed by flow cytometry. Representative dot plots of human CD45–CD138+ residual MM1S myeloma cells (labeled red), human CD45+CD3+CD8+ T cells (labeled blue), human CD45+CD3+CD4+ T cells (labeled green), as well as human CD45+CD3–CD16+CD56+ NK cells (labeled orange) obtained from each murine group (n = 3) are shown (right). (C) Three hundred thousand MM1S/CD38lowSLAMF7high/SLR+ cells were injected into the right tibia of irradiated NOD/Shi-scid IL-2rγ(null) mice. After confirming tumor engraftment by bioluminescence imaging assays on day 7, a total of 3.0 × 106 human PBMCs were injected IV on day 8. Daratumumab (30 μg) or daratumumab/B-BiTE (daratumumab, 30 μg; B-BiTE, 10 μg) was similarly administered on day 9, followed by administration of elotuzumab (30 μg), daratumumab/B-BiTE, or elotuzumab/B-BiTE (mAb, 30 μg; B-BiTE, 10 μg) on day 13. Total photon counts for each mouse were acquired, and luminescence intensity was calculated as arbitrary units (n = 4 mice per group). Obtained whole images are shown in supplemental Figure 10B. Magnified images of the right tibia from each mouse are displayed on the left, and luminescence data for each murine group are summarized on the right. SLR, stable luciferase red.
Enhanced in vivo antimyeloma effects induced by 2 different mAb/B-BiTE complexes. (A) Three million MM1S/CD38lowSLAMF7high cells were injected subcutaneously into NOD/Shi-scid IL-2rγ(null) mice, then 1.0 × 107 freshly isolated PBMCs obtained from the donor were injected IV on day 4. Daratumumab (30 μg) or daratumumab/B-BiTE complex (daratumumab, 30 μg; B-BiTE, 10 μg) was administered IV on day 5. On day 12, 30 μg elotuzumab was administered IV to the mice treated with daratumumab alone. On the other hand, daratumumab/B-BiTE or elotuzumab/B-BiTE (mAb, 30 μg; B-BiTE, 10 μg) was administered to the mice treated with daratumumab/B-BiTE on the same day (top left). Tumor sizes after treatment are depicted (bottom left), and the overall survival of each murine group (n = 6) is summarized in the form of Kaplan-Meier curves (right). Overall survival was compared among the groups using a log-rank (Mantel-Cox) test. ∗P < .05; ∗∗∗P < .001. (B) To perform histological analyses, the experiments in panel A were repeated, and subcutaneous tumors were resected from each murine group on day 17. Representative hematoxylin and eosin staining and fluorescence immunohistochemistry for human CD138 at the tumor sites are shown (left). The images in white rectangles are magnified, and the scale bars in the pictures represent 600 μm (black) and 100 μm (yellow). In addition, tumor tissues were homogenized, and the phenotypes of human cells that accumulated at the tumor sites were also analyzed by flow cytometry. Representative dot plots of human CD45–CD138+ residual MM1S myeloma cells (labeled red), human CD45+CD3+CD8+ T cells (labeled blue), human CD45+CD3+CD4+ T cells (labeled green), as well as human CD45+CD3–CD16+CD56+ NK cells (labeled orange) obtained from each murine group (n = 3) are shown (right). (C) Three hundred thousand MM1S/CD38lowSLAMF7high/SLR+ cells were injected into the right tibia of irradiated NOD/Shi-scid IL-2rγ(null) mice. After confirming tumor engraftment by bioluminescence imaging assays on day 7, a total of 3.0 × 106 human PBMCs were injected IV on day 8. Daratumumab (30 μg) or daratumumab/B-BiTE (daratumumab, 30 μg; B-BiTE, 10 μg) was similarly administered on day 9, followed by administration of elotuzumab (30 μg), daratumumab/B-BiTE, or elotuzumab/B-BiTE (mAb, 30 μg; B-BiTE, 10 μg) on day 13. Total photon counts for each mouse were acquired, and luminescence intensity was calculated as arbitrary units (n = 4 mice per group). Obtained whole images are shown in supplemental Figure 10B. Magnified images of the right tibia from each mouse are displayed on the left, and luminescence data for each murine group are summarized on the right. SLR, stable luciferase red.
We also assessed antimyeloma effects using an in vivo myeloma model in which NOD/Shi-scid IL-2rγ(null) mice were inoculated intratibially with MM1S/CD38low cells. The luminescence intensity of luciferase-transduced MM1S/CD38low cells revealed that tumor growth was completely suppressed after sequential administration of daratumumab/B-BiTE in combination with elotuzumab/B-BiTE (Figure 7C; supplemental Figure 10). Therefore, these results support the potential effectiveness of sequential treatment using a panel of different bispecific antibodies generated by B-BiTE to enhance in vivo immune responses against myeloma with heterogeneous characteristics.
Discussion
We used clinically available IgG1-based mAbs to confirm the reactivity of the mAb/B-BiTE complex, which possesses 2 antigen binding sites similar to an original mAb. Target-antigen oligomerization owing to binding of mAb/B-BiTE via 2 binding sites might be able to facilitate the accumulation of Fc receptors on NK cells,24 thus inducing successful target-specific NK cell reactivity and proliferation supported by cytokines from simultaneously activated T cells.25 Because the original clone HP6017, used as the scFv of B-BiTE specific for IgG-Fc, appears to bind to the CH3 domain of IgG-Fc,26 B-BiTE itself is unlikely to negatively affect the interaction between the IgG-Fc domain and NK cells.27,28 In contrast to separate administration of a mAb and a bispecific antibody such as BiTE, a mAb/B-BiTE complex might have certain advantages. One is that it would allow simultaneous delivery of both to a tumor site. Another is that it would obviate the need to generate 2 different immunological synapses using a mAb and a BiTE, allowing NK cells and T cells to activate in closer proximity.
When applying a mAb/B-BiTE complex that possesses wild-type Fc region into the clinic, careful investigation of unwanted off-target and on-target cytokine production by immune cells would be necessary. It was reported that bispecific antibodies possessing a wild-type Fc domain might have the potential to engage T cells and NK cells by binding to both CD3 and the Fc receptor without tumor cells. Catumaxomab, the first clinically available bispecific antibody recognizing both epithelial cell adhesion molecule and CD3 with a wild-type Fc domain, can activate both human T cells and NK cells.29,30 In a clinical trial of IV infused Catumaxomab, infiltration of immune cells into the liver occurred, resulting in fulminant acute liver failure. Although the mechanism responsible was not directly demonstrated, pathological analysis suggested that off-target reactivity of Catumaxomab with T cells and Kupffer cells might have been involved, as expression of epithelial cell adhesion molecule in hepatocytes was negative.31 Importantly, in this study, neither human polyclonal Ig with B-BiTE nor B-BiTE itself spontaneously activated human PBMCs, which include human T cells and NK cells, in vitro and in vivo, suggesting the absence of off-target reactivity. Because human IgG-Fc–possessing antihuman CD3 mAb can activate human T cells and NK cells without target cells in vitro and in vivo, the structural difference of mAb/B-BiTE from conventional bispecifics may contribute to activate both T cells and NK cells with target specificity (Figure 3E). Additionally, dissociation and clearance of a mAb/B-BiTE complex might allow to attenuate continuous cytokine release from immune cells (Figures 2C and 3D; supplemental Figure 4C-F). In contrast, because tumor-specific mAb/B-BiTE complex appeared to be delivered at tumor sites to induce antimyeloma effects in vivo (Figures 6 and 7), assessment of on-target adverse events because of dual-lymphoid activation via the mAb/B-BiTE would be also required. However, it would be difficult to confirm the safety profiles of the mAb/B-BiTE complex by direct comparison with other modalities, for example silenced Fc-equipped bispecifics, because these 2 modalities have different affinities for target antigens and different conformations. Our investigation of in vivo on-target cytokine release using mice treated with daratumumab/B-BiTE and human T cells with or without human CD3– PBMCs demonstrated no remarkable cytokine production because of dual-lymphoid activation (Figure 6D; supplemental Figure 8C). However, in the event of significant cytokine release caused by off-target and on-target reactivity because of mAb/B-BiTE complexes in the human body, for example in the form of IL-6 elevation, tocilizumab would be able to suppress the immune reaction. Risks of on-target/off-tumor reactivity targeting CD38 and/or SLAMF7 also need to be considered because a proportion of lymphocytes, including T cells and NK cells, express CD38 and/or SLAMF7.32,33 Thus far, our in vitro analysis has failed to demonstrate any significant cytokine production and cytotoxicity because of fratricidal activity of both T cells and NK cells among PBMCs in the presence of mAb/B-BiTE complexes (Figures 4A and 5B-C; supplemental Figure 7). Although CD38 is also expressed in human hematopoietic progenitor cells and other normal cells,34 clinical trials of CAR T cells targeting CD38 have shown that the treatment was tolerable and manageable in patients.35,36 Apart from CAR T cells with high avidity for CD38/SLAMF7, T cells/NK cells redirected with mAb/B-BiTE complexes might preferentially recognize myeloma cells with high expression of target antigens while sparing normal cells, suggesting tolerability. Importantly, however, application of mAb/B-BiTE complexes for patients with autoreactive IgG-type antibodies and/or autoimmune disorders should be considered with caution, because they might potentially induce unwanted or unknown on-target/off-tumor reactivity upon coincidental binding of B-BiTE to their autoantibodies. Future clinical trials will require very careful monitoring for any unexpected off-target and on-target toxicities in individual patients.
Clinical trials using new modalities, such as CAR T cells and bispecific antibodies, have demonstrated responses to drug-resistant myeloma cells.16,37 As it should be borne in mind that patients recruited for trials have been heavily pretreated, it might be difficult to obtain durable responses for myeloma using a single agent.38-41 Interestingly, ciltacabtagene autoleucel, which expresses 2 heavy chain variable regions specific for 2 different BCMA domains, appears to induce better clinical responses.39,40,42 Furthermore, bispecific CAR T cells targeting BCMA/CD38, BCMA/SLAMF7, or BCMA/GPRC5D have been developed and appear to exert enhanced antimyeloma effects in comparison with single-targeting CAR T cells.35,43-45 The reasons for this may be downregulation of a target antigen/domain on myeloma cells, trogocytosis, and possible lineage switching whereby myeloma clones initially expressing a low level of the target antigen outgrow after treatment with immunological agents.37,46 Our results suggest that a mAb/B-BiTE complex would induce strengthened and deep antimyeloma responses in comparison with a mAb alone. However, mAb/B-BiTE monotherapy for MM did not always induce sufficient in vivo antitumor responses against myeloma cells. In this situation, sequential therapy involving the switching of different mAb/B-BiTE complexes rapidly prepared using the original mAbs was able to overcome the problem without generating patient–derived CAR T-cell products (Figure 7).
In comparison with elotuzumab/B-BiTE monotherapy, daratumumab/B-BiTE monotherapy produced stronger antimyeloma effects in vivo (Figure 6; supplemental Figure 8D-E). Similar trends appear to be observed clinically for daratumumab monotherapy compared with elotuzumab monotherapy.47 This would likely be because of the presence and release of the soluble form of SLAMF7 target protein from myeloma cells,48 which might hamper the antimyeloma reactivity induced by elotuzumab/B-BiTE. Indeed, it is likely that elotuzumab/B-BiTE would mediate sufficient antimyeloma reactivity after reduction of the myeloma cell population in vivo by daratumumab/B-BiTE (Figure 7). These results suggest that the immune responses mediated by a mAb/B-BiTE complex might depend on the selected target antigen, and that elotuzumab/B-BiTE might be appropriate as a sequential therapy to induce sufficient, deep, and durable molecular remission, especially in high-risk patients with MM without relapse. All of the above findings suggest that the development of a new treatment strategy using mAb/B-BiTE complexes in combination with other treatment modalities targeting ≥2 myeloma antigens would yield much better clinical responses for myeloma16,49-54 (supplemental Figure 11).
It is also noteworthy that HP6017 used for B-BiTE binds to human IgG regardless of IgG subclass,55,56 suggesting that other IgG subclasses, such as IgG2, IgG3, and IgG4 as well as IgG1, would also be available for generating a broad range of bispecific antibodies using B-BiTE. Therefore, with the clinical availability of existing mAbs, B-BiTE would be applicable for treatment of various types of refractory tumors. For example, rituximab/B-BiTE (Figure 1; supplemental Figure 5) and other mAb/B-BiTE complexes could be used as a booster therapy for relapsed/refractory B-cell malignancies before and/or after CD19 CAR T-cell therapy. Furthermore, the scFv specific for the IgG-Fc domain could be tethered with scFvs specific for other domains, such as immunomodulatory molecules capable of mediating innate or adoptive immune responses,57 to generate new modalities for strengthening the efficacy of immune therapy for refractory tumors. These possibilities require further investigation.
In conclusion, we have demonstrated that B-BiTE in combination with clinically available mAbs has the potential to become a new modality for strengthening antimyeloma responses via dual-lymphoid activation. This approach would facilitate the rapid preparation of new bispecific antibodies capable of inducing deep and durable antitumor responses, thus further advancing immunotherapy for MM and other refractory hematologic malignancies.
Acknowledgments
The authors thank Kenji Kameda, Division of Analytical Bio-Medicine, Advanced Research Support Center, Ehime University, for his technical assistance.
This work was supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science, grant 18K08331 (T.O.), Takeda Science Foundation (T.O.), SENSHIN Medical Research Foundation (T.O.), and the Center for Clinical and Translational Research of Kyushu University Hospital (T.O.).
Authorship
Contribution: T.K. performed the experimental work, analyzed and interpreted the results, and wrote the manuscript; T.O. conceptualized the study, performed the experimental work, analyzed and interpreted the results, and wrote and edited the manuscript; M.M., K. Tanimoto, Y.M., and C.I. provided the materials and validated the results; T.S. and T.I. helped to perform some of the in vivo murine experiments; M.Y. validated the results, provided financial support, and edited the manuscript; and K. Takenaka supervised the study, provided financial support, and edited the manuscript.
Conflict-of-interest disclosure: Ehime University has filed a patent application related to this study in which T.O., M.Y., and K. Takenaka are named as inventors. The remaining authors declare no competing financial interests.
Correspondence: Toshiki Ochi, Department of Hematology, Clinical Immunology and Infectious Diseases, Ehime University Graduate School of Medicine, Shitsukawa, Toon, Ehime 791-0295, Japan; e-mail: ochi.toshiki.eg@ehime-u.ac.jp; and Katsuto Takenaka, Department of Hematology, Clinical Immunology and Infectious Diseases, Ehime University Graduate School of Medicine, Shitsukawa, Toon, Ehime 791-0295, Japan; e-mail: takenaka.katsuto.hy@ehime-u.ac.jp.
References
Author notes
All data are available in the main text or the supplementary materials or are available from the corresponding authors on reasonable request. Original data are available upon reasonable request from the corresponding author, Toshiki Ochi (ochi.toshiki.eg@ehime-u.ac.jp).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal