In this issue of Blood, Chung et al compare and contrast the effects of plasma low-density lipoprotein (LDL) and high-density lipoprotein (HDL) on von Willebrand factor (VWF) self-association and demonstrate increased hypercoagulable forms of VWF in the setting of elevated LDL-to-HDL ratios.1
VWF is a multimeric plasma glycoprotein that plays a central role in primary hemostasis. Endothelial cells release basal amounts of VWF but also use a specialized secretion system to release massive amounts of procoagulant ultra–high-molecular-weight multimers into the blood when stimulated. In its active form, which is promoted by circulatory shear forces, multimer length, and interaction with other plasma proteins, VWF recruits platelets to sites of vascular injury and forms the initial platelet plug. As platelets and VWF accumulate and activate, the stage is set for thrombin generation and fibrin clot formation. However, the focus of this article is on the influence of plasma factors that promote a specific form of self-associated VWF. This form of VWF may provide clues to VWF’s role in cardiovascular disease and thrombotic microangiopathies.
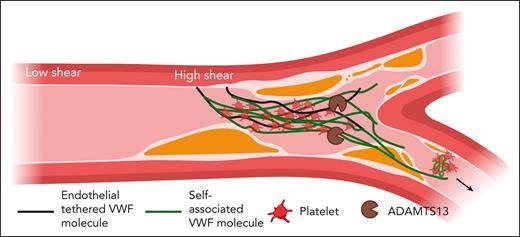
In 2002, Savage et al reported an elegant observation of VWF under flow. After coating the surface of a flow chamber with recombinant VWF lacking the platelet-interacting A1 domain, the authors observed no platelet accumulation under moderate shear forces. However, when reference recombinant VWF was added to the ΔA1 VWF coated chamber, platelet accumulation was observed, suggesting that reference VWF was able to “self-associate,” adsorbing to the surface coated with the ΔA1 VWF.2 Subsequent studies have demonstrated that shear force on immobilized VWF exposes a cryptic A2 domain epitope, facilitating further self-association, balanced by ADAMTS13-mediated cleavage of the Tyr1605-Met1606 scissile bond (see figure).3 Therefore, the same forces that allow for VWF self-association (procoagulant) also allow for cleavage of VWF multimers (anticoagulant). The amount of VWF self-association increases with shear force but spontaneously dissociates as this force subsides, indicating a noncovalent mechanism that could dynamically change in circulating blood and allow for the embolization of VWF-platelet–rich fragments into the microcirculation. Kinetic studies of this tension-dependent association suggest a self-limited process mediated by decreased tensile forces on the accumulating individual VWF concatemers.4
Schema for the procoagulant consequences of VWF self-association. In the arterial circulation, VWF circulates in an inactive globular form until it encounters areas of high shear forces or endothelial cell activation. High shear promotes VWF self-association to tethered VWF strands on endothelial cells. Self-associated VWF is active and binds platelets. This noncovalent VWF/platelet aggregation creates the potential for VWF/platelet-rich emboli into the distal arterial microcirculation. Figure created with Biorender.com.
Schema for the procoagulant consequences of VWF self-association. In the arterial circulation, VWF circulates in an inactive globular form until it encounters areas of high shear forces or endothelial cell activation. High shear promotes VWF self-association to tethered VWF strands on endothelial cells. Self-associated VWF is active and binds platelets. This noncovalent VWF/platelet aggregation creates the potential for VWF/platelet-rich emboli into the distal arterial microcirculation. Figure created with Biorender.com.
In 2016, Chung et al described the inhibitory effects of HDL cholesterol on VWF self-association.5 These studies were inspired in part by the observation that the presence of an unknown plasma factor reduced the adsorption of VWF to polyethylene tubes. Of the 3 plasma proteins that remained in solution after boiling, the HDL-associated apolipoprotein A-I (ApoA-I) effectively prevented VWF adsorption under static conditions, and HDL > ApoA-I reduced VWF self-association in shear conditions. A potential connection to thrombotic thrombocytopenic purpura (TTP) was observed in a mouse model, where infusions of purified HDL reduced thrombocytopenia after VWF injection into Adamts13-deficient mice.
In the current study, Chung et al extend their investigations of VWF self-association by demonstrating opposing actions of HDL and LDL. They first observed increased adsorption of VWF to tube walls in a vortex model when the ratio of LDL to HDL was increased with purified lipoproteins. This finding was directly observed in a microfluidic system, where greater accumulation of VWF was observed on flow chamber microposts when LDL concentrations were manipulated in either buffered solutions or citrated plasma. Labeled LDL particles interacted directly with the VWF forming around the microposts, suggesting a direct role in further VWF recruitment. Interestingly, greater VWF deposition was observed in plasma from patients with hypercholesterolemia compared with controls and was correlated with their LDL-to-HDL ratio.
In vivo studies were supportive of these findings. In a double Adamts13-deficient, Ldlr-deficient murine model (to generate hypercholesterolemia), ultrasonographic imaging studies detected increased platelet and VWF deposition in the microcirculation as well as mildly impaired left ventricular function in Adamts13/Ldlr > Adamts13-deficient mice compared with controls. These findings were consistent with intravital microscopy experiments in wild-type mice injected with LDL vs buffer. Here, ionophore-induced VWF release caused platelet-rich thrombi in greater amounts and for longer duration in mice with elevated LDL regardless of ADAMTS13 activity. Finally, although antibody-mediated inhibition of ADAMTS13 enhanced VWF self-association in flow chamber studies, ADAMTS13 activity was equivalent in plasma from patients with hypercholesterolemia compared with controls assessed by VWF multimer patterns. This suggests that ADAMTS13 activity competes with VWF self-association, but LDL does not inhibit ADAMTS13.
Although these studies nicely link LDL to increased VWF self-association and suggest a possible mechanism for increased myocardial ischemia and thrombotic microangiopathy in patients with elevated LDL-to-HDL ratios, it remains difficult to definitively link VWF self-association to disease risk. Direct observation of lateral VWF aggregates in vivo without genetic or chemical manipulation remains challenging. Human genomic studies provide some clues to the role of LDL and VWF in common vascular diseases. Mendelian randomization (MR) studies support the hypothesis that elevated VWF/factor VIII levels are not just associated with increased venous thromboembolism (VTE) and myocardial infarction risk but are actually causal.6 MR studies also demonstrate a causal relationship between LDL cholesterol levels and myocardial infarction risk, as expected, but do not show protection from elevated HDL levels, explaining why therapies that lower LDL are more effective than those that increase HDL.7 The current study suggests that LDL may play a causal role in myocardial infarction and TTP by increasing the propensity to form hypercoagulable forms of self-associated VWF. Although no studies have been published examining LDL and TTP risk, both epidemiologic and MR studies have failed to connect LDL levels and VTE risk, suggesting self-association is primarily an arterial phenomenon.8
Although questions remain about the extent of self-associated VWF in the pathogenesis of myocardial infarction and TTP, the current study provides an entirely plausible mechanism. Future studies will be required to make a causal connection of LDL to other endothelial cell–activating conditions, like malaria, sickle cell disease, and TTP, through a VWF self-association mechanism. The current study by Chung et al supports the hypothesis that propensity to form an alternate prothrombotic form of VWF is enhanced by plasma LDL and serves as a fascinating potential connection of VWF biology and cholesterol metabolism to arterial thrombotic disorders.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal