Key Points
Time-limited triple therapy leads to high rates of undetectable minimal residual disease and sustained remissions in high-risk CLL.
Adverse events occur primarily early during induction therapy and decrease over time.
Abstract
The final analysis of the open-label, multicenter phase 2 CLL2-GIVe trial shows response and tolerability of the triple combination of obinutuzumab, ibrutinib, and venetoclax (GIVe regimen) in 41 previously untreated patients with high-risk chronic lymphocytic leukemia (CLL) with del(17p) and/or TP53 mutation. Induction consisted of 6 cycles of GIVe; venetoclax and ibrutinib were continued up to cycle 12 as consolidation. Ibrutinib was given until cycle 15 or up to cycle 36 in patients not achieving a complete response and with detectable minimal residual disease. The primary end point was the complete remission rate at cycle 15, which was achieved at 58.5% (95% CI, 42.1-73.7; P < .001). The last patient reached the end of the study in January 2022. After a median observation time of 38.4 months (range, 3.7-44.9), the 36-month progression-free survival was 79.9%, and the 36-month overall survival was 92.6%. Only 6 patients continued ibrutinib maintenance. Adverse events of concern were neutropenia (48.8%, grade ≥3) and infections (19.5%, grade ≥3). Cardiovascular toxicity grade 3 occurred as atrial fibrillation at a rate of 2.4% between cycles 1 and 12, as well as hypertension (4.9%) between cycles 1 and 6. The incidence of adverse events of any grade and grade ≥3 was highest during induction and decreased over time. Progressive disease was observed in 7 patients between cycles 27 and 42. In conclusion, the CLL2-GIVe regimen is a promising fixed-duration, first-line treatment for patients with high-risk CLL with a manageable safety profile.
Introduction
Targeted therapies have changed the treatment landscape for patients with chronic lymphocytic leukemia (CLL). Nevertheless, there remains a need for improvement in the outcome of patients with high-risk CLL, defined as patients with del(17p) and/or TP53 mutation. Continuous single-agent Bruton tyrosine kinase inhibitor (BTKi) ibrutinib in patients with untreated CLL in long-term follow-up showed an estimated 5-year progression-free survival (PFS) of 70% and an estimated 5-year overall survival (OS) of 83%.1 The 6-year follow-up of the RESONATE trial with continuous ibrutinib monotherapy in relapsed/refractory CLL demonstrated a significantly lower PFS rate in the subgroup of patients with del(17p) and TP53 mutations than in patients without either of these abnormalities, whereas this difference was not significant at a follow-up of 19 months.2,3 In addition, second-generation BTKis, such as acalabrutinib and zanubrutinib, demonstrated a favorable outcome in subgroups with del(17p) and/or TP53 mutations with a reduced rate of cardiovascular side effects as compared with ibrutinib.4-6 In patients with del(17p) and/or TP53-mutated CLL, ibrutinib showed, after 5 years of continuous treatment, an estimated PFS and OS of 70% and 85%, respectively.7 But during continuous BTKi treatment, there is a risk of clonal evolution and acquired mutations in BTK or phospholipase Cγ2 (PLCγ2), especially in patients harboring a complex karyotype or with del(17p).8 Furthermore, in a relevant proportion of patients, ibrutinib treatment was discontinued because of intolerance due to off-target effects leading to cardiac arrhythmia, especially atrial fibrillation, hypertension, and bleeding events.9-12
Time-limited combination treatment of venetoclax and obinutuzumab is another standard treatment option, which induces deep remissions and high rates of undetectable measurable residual disease (uMRD),13 allowing for fixed-duration therapy. Nevertheless, del(17p) remains a prognostic factor for shorter PFS with venetoclax and obinutuzumab,14 with a 4-year PFS rate of 53.0% in patients with del(17p) vs 74.0% in other genetic subgroups.15 In patients with relapsed/refractory CLL with high-risk cytogenetics (del(17p)/TP53 mutation), continuous venetoclax monotherapy until disease progression or intolerance also achieves durable responses and is well tolerated.16 At 6-year follow-up, 48% of patients were alive, 24% had progression-free disease, and 16% remained on venetoclax, confirming the long-term activity of continuous venetoclax in this high-risk population.17
Time-limited combination therapy, whether oral or in combination with IV antibodies, is a promising approach in CLL treatment with the aim of reducing treatment-associated resistance and treatment-related toxicities. The combination of ibrutinib and venetoclax achieved high rates of complete remission (CR) and/or uMRD in previously untreated CLL.18,19 Furthermore, triplet regimens such as venetoclax and obinutuzumab in combination with a BTKi (ibrutinib, acalabrutinib, or zanubrutinib) showed promising efficacy and tolerability in single-arm trials of unselected frontline or relapsed CLL cohorts.20-24
The CLL2-GIVe trial selectively tested a time-limited, response-adapted triple combination treatment of obinutuzumab (GA-101, obinutuzumab), ibrutinib, and venetoclax (GIVe regimen) in patients with CLL with del(17p) and/or TP53 mutations, with the aim to induce deep and durable remissions.
Patients and methods
Oversight
CLL2-GIVe (NCT02758665) of the German CLL Study Group was an open-label, multicenter phase 2 trial at 11 German centers. The trial was approved by the institutional review board and conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. Written informed consent was obtained from all the patients. Here, we report the final analysis after the end of the study, with a data cutoff date of 14 June 2022.
Eligibility
Adults with previously untreated CLL with del(17p) and/or TP53 mutations detected via fluorescence in-situ hybridization and Sanger sequencing, respectively, were eligible when requiring treatment, according to the criteria of the 2008 International Workshop on Chronic Lymphocytic Leukemia (iwCLL).25 Other key inclusion criteria were age >18 years, measurable disease (lymphocytosis > 5 × 109 and/or palpable and measurable lymph nodes >1.5 cm), adequate renal function (creatinine clearance ≥ 50 mL/min), adequate liver function (a total bilirubin ≤ 2×, aspartate aminotransferase, and alanine transaminase ≤ 3× the institutional upper limit of normal [ULN]), and adequate bone marrow (BM) function (absolute neutrophil count [ANC] ≥ 1000/μL, platelets > 30 000/μL, and hemoglobin ≥ 8g/dl [unless directly attributable to the underlying CLL]). Complete inclusion and exclusion criteria are described in the data supplement, available on the Blood website (supplemental Table 1).
Study design and treatment
The treatment consisted of 3 parts (Figure 1A): 6 cycles of induction with a triple combination of GIVe treatment; 6 cycles of consolidation with ibrutinib and venetoclax up to cycle 12; followed by 3 cycles of ibrutinib monotherapy until final restaging at cycle 15; and response-adjusted (in case of no CR with uMRD) ibrutinib maintenance up to cycle 36. During the induction, obinutuzumab was administered IV in cycle 1 on day 1, 100 mg; day 2, 900 mg; and days 8 and 15, 1000 mg. From cycles 2 to 6, obinutuzumab was continued at 1000 mg on day 1 of each cycle. Ibrutinib was given orally at 420 mg per day (both from cycle 1, day 1). The weekly venetoclax ramp-up started on cycle 1, day 22 over 5 weeks from 20 mg, 50 mg, 100 mg, and 200 mg to 400 mg per day. Lymph nodes ≥10 cm or absolute lymphocyte count ≥ 50 × 109/L defined the risk categorization for tumor lysis syndrome (TLS) during screening. In cases of increased TLS risk, hospitalization was recommended. From cycles 13 to 15, ibrutinib was continued until final restaging at cycle 15. In case of detectable MRD at cycles 9 and/or 12 or the absence of a CR/ CR with incomplete regeneration (CRi) according to iwCLL criteria25 at cycle 15, ibrutinib maintenance was continued up to cycle 36. Beyond cycle 36, ibrutinib could be indefinitely continued (off study), at the investigator’s discretion.
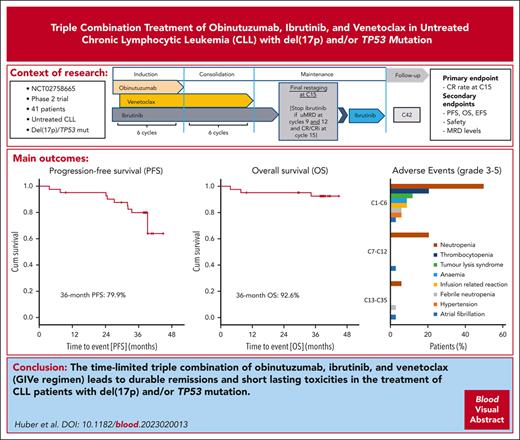
Study design and swimmerplot. (A) Study design with triple combination of GIVe from cycles 1 to 6, ibrutinib and venetoclax from cycles 7 to 12, and ibrutinib until cycle 15, in case of CR and uMRD at cycles 9 and 12. Otherwise, ibrutinib was scheduled to continue until cycle 36. (B) Treatment disposition of all patients according to genetics (del[17p] and TP53 mutation, sole del[17p], sole TP53 mutation). Gray lines indicate the duration of treatment, and blue lines indicate the follow-up period. Thirty-four of 41 patients reached final restaging. The symbols at the end of the gray lines define reasons for treatment discontinuation and time point of final restaging (between cycles 14 and 15 because of different timing of response assessment). The red X symbols indicate 7 disease progressions. Black crosses indicate 3 deaths.
Study design and swimmerplot. (A) Study design with triple combination of GIVe from cycles 1 to 6, ibrutinib and venetoclax from cycles 7 to 12, and ibrutinib until cycle 15, in case of CR and uMRD at cycles 9 and 12. Otherwise, ibrutinib was scheduled to continue until cycle 36. (B) Treatment disposition of all patients according to genetics (del[17p] and TP53 mutation, sole del[17p], sole TP53 mutation). Gray lines indicate the duration of treatment, and blue lines indicate the follow-up period. Thirty-four of 41 patients reached final restaging. The symbols at the end of the gray lines define reasons for treatment discontinuation and time point of final restaging (between cycles 14 and 15 because of different timing of response assessment). The red X symbols indicate 7 disease progressions. Black crosses indicate 3 deaths.
End points and assessments
Response was assessed at cycles 4 (day 1), 6, 9, 12 (day 28 for each of these), and 15 (day 1, final restaging) and every 3 cycles thereafter, up to 6 months after cycle 36. Peripheral blood (PB) MRD monitoring was performed at baseline and during cycles 9, 12, 15, and 36. MRD in BM was assessed during cycle 14 before the final restaging at cycle 15, day 1. The primary efficacy end point is the CR rate at cycle 15 (day 1; final restaging). Secondary end points included overall response rate (ORR), MRD in the PB and BM, time-to-event end points (PFS, OS, event-free survival [EFS], duration of response, treatment-free survival [TFS], and time to next CLL treatment [TTNT]), subsequent treatments, and safety parameters.
MRD was measured centrally via 4-color flow cytometry. uMRD in the PB and BM was defined as <10−4, that is, 1 CLL cell per 10 000 leukocytes. MRD intermediate was defined as ≥10−4 and <10−2, and MRD positive as ≥10−2.26,27 At baseline, PB samples were centrally tested for genetic profiles using fluorescence in-situ hybridization probes to detect abnormalities in chromosomes 13q, 12, 11q, and 17p (cutoff del[17p] > 7.45%) and TP53 mutational status via Sanger sequencing (exons 4-10), with a cutoff of variant allele frequency (VAF) of > 10% according to European Research Initiative on Chronic Lymphocytic Leukemia (ERIC) guidelines.28
Safety
Safety assessments were conducted during treatment in cycles 1 or 2 weekly, cycles 3 or 6 monthly, after that at least every 3 months, and up to cycle 42 via laboratory measurements (blood count and liver and renal function tests), history, and physical examination. Adverse events (AEs) and laboratory abnormalities were graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 and coded using the Medical Dictionary for Regulatory Activities classification system.
Statistical analysis
The data cutoff for this analysis was 14 June 2022 and included all patients in the study population. Safety analyses included all patients who received at least 1 dose of any component of the study drugs. The primary end point was the CR rate at cycle 15, with a null hypothesis of 25% tested using a 2-sided, 1-sample binomial test, including a 95% Clopper-Pearson confidence interval (95% CI). Secondary end points included time-to-event analyses (PFS, OS, TTNT, TFS, EFS, ORR, and ORR after the end of maintenance treatment), MRD levels, safety parameters, evaluation of subsequent treatment for CLL, and incidence of Richter transformation. Descriptive statistics were calculated, and Kaplan-Meier methods were used for time-to-event analyses. Exploratory subgroup analyses were performed using the Cox proportional hazards regression model and a 2-sided log-rank test with a test level at 5%. Analyses were conducted using IBM SPSS Statistics 27.
Inclusion and exclusion criteria, baseline characteristics, and secondary end points can be found in the supplemental Appendix.
Results
Patients
Between September 2016 and August 2018, 48 patients were screened for eligibility, and 41 patients were included. The last patient reached the end of the study time point in January 2022. The median observation time was 38.4 months (range, 3.7-44.9 months). The median age at baseline was 62 years (range, 35-85 years), 24 patients (58.5%) were male, 24 (58.5%) had a del(17p) and TP53 mutation, 15 (36.6%) had a TP53 mutation only, and 2 patients (4.9%) had a sole del(17p). Thirty-two patients (78.0%) had an unmutated immunoglobulin heavy-chain variable region gene (IGHV) status (Tables 1 and 2). Further baseline characteristics are included in the supplemental Table 2. Biological disease parameters are shown in supplemental Table 3.
Baseline characteristics: genetics per TP53 status
| TP53 status . | 41 . |
|---|---|
| None | 0 (0.0) |
| TP53 mutation and del(17p) | 24 (58.5) |
| TP53 mutation and no del(17p) | 15 (36.6) |
| No TP53 mutation and del(17p) | 2 (4.9) |
| TP53 status . | 41 . |
|---|---|
| None | 0 (0.0) |
| TP53 mutation and del(17p) | 24 (58.5) |
| TP53 mutation and no del(17p) | 15 (36.6) |
| No TP53 mutation and del(17p) | 2 (4.9) |
Baseline characteristics: genetics per the presence of del
| . | No del, n (%) . | del, n (%) . |
|---|---|---|
| Del(17p) | 15 (36.6) | 26 (63.4) |
| From >7.45%to 20% | 5 (12.2) | |
| >20% | — | 21 (51.2) |
| . | No del, n (%) . | del, n (%) . |
|---|---|---|
| Del(17p) | 15 (36.6) | 26 (63.4) |
| From >7.45%to 20% | 5 (12.2) | |
| >20% | — | 21 (51.2) |
| . | Unmutated, n (%) . | Mutated, n (%) . |
|---|---|---|
| TP53 | 2 (4.9) | 39 (95.1) |
| 1 | 35 (85.4) | |
| >1 | 4 (9.8) | |
| NOTCH1 | 38 (92.7) | 3 (7.3) |
| SF3B1 | 34 (82.9) | 7 (17.1) |
| IGHV∗ | 32 (78.0) | 6 (14.6) |
| . | Unmutated, n (%) . | Mutated, n (%) . |
|---|---|---|
| TP53 | 2 (4.9) | 39 (95.1) |
| 1 | 35 (85.4) | |
| >1 | 4 (9.8) | |
| NOTCH1 | 38 (92.7) | 3 (7.3) |
| SF3B1 | 34 (82.9) | 7 (17.1) |
| IGHV∗ | 32 (78.0) | 6 (14.6) |
| . | Noncomplex, n (%) . | Complex, n (%) . |
|---|---|---|
| Karyotype† | 15 (38.5) | 24 (61.5) |
| . | Noncomplex, n (%) . | Complex, n (%) . |
|---|---|---|
| Karyotype† | 15 (38.5) | 24 (61.5) |
IGHV: 3 patients were not evaluable.
Karyotype: 2 patients were not evaluable.
Treatment disposition
The disposition of the 41 treated patients is illustrated in a swimmer plot in Figure 1B. At cycle 3, 1 patient discontinued treatment because of an AE (ovarian cancer, retrospectively preexisting at enrollment), and 40 patients completed induction. Thirty-four of 40 patients completed consolidation and reached final restaging at cycle 15. Six of 40 patients discontinued treatment early (1 fatal AE of cardiac failure due to a mitral valve insufficiency at cycle 9; 1 withdrawal of informed consent at cycle 9 after diagnosis of progressive multifocal leukoencephalopathy; 2 patients discontinued because of AEs at cycle 12 [atrial fibrillation CTCAE grade 3 and cerebral aspergillosis CTCAE grade 4]; and 2 patients with uMRD not according to the protocol at cycle 12). In 21 of the 34 patients, treatment was stopped according to the protocol because of CR/CRi at final restaging and uMRD. Seven of 34 patients discontinued treatment unscheduled because of other reasons (5 of 7 patients because of uMRD) and partial remission (PR) not according to the protocol, 1 patient with CR and uMRD because of AE (arthralgia CTCAE grade 1), and 1 patient declined ibrutinib maintenance (reaching PR and uMRD). Overall, 6 of 34 patients reaching only PR and/or no uMRD continued ibrutinib maintenance until cycle 36 according to protocol. In sum, 14 of 41 patients stopped treatment unscheduled over the course of treatment for the aforementioned mentioned reasons. A detailed disposition is illustrated in Figure 2, and AE-related treatment discontinuation in supplemental Table 4. During treatment, no patient had disease progression.
Treatment disposition. Forty-one patients were enrolled and treated, and 34 patients completed induction and consolidation treatment. Twenty-one patients stopped according to protocol at the final restaging. Seven patients discontinued before C15, not according to protocol, because of the reasons described. Six patients continued ibrutinib maintenance until cycle 36.
Treatment disposition. Forty-one patients were enrolled and treated, and 34 patients completed induction and consolidation treatment. Twenty-one patients stopped according to protocol at the final restaging. Seven patients discontinued before C15, not according to protocol, because of the reasons described. Six patients continued ibrutinib maintenance until cycle 36.
Efficacy
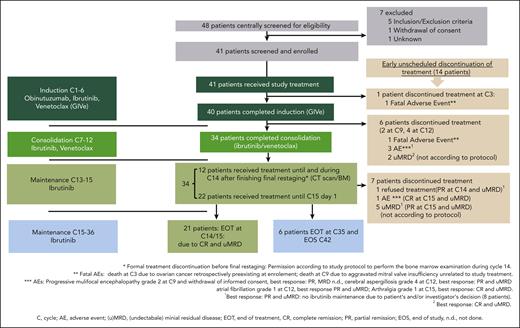
The CR rate was 58.5% (24 patients) at final restaging at cycle 15, meeting the primary end point (95% CI, 42.1-73.7; P < .001). The PR rate was 41.5% (17 patients), resulting in 100% ORR (Figure 3A). PB uMRD at cycles 9 and 12 was achieved in 36 patients (87.8%), and at cycle 15 in 32 (78%) of all 41 patients. At cycle 36, 18 patients (43.9%) had uMRD, and 12 patients (29.3%) had detectable MRD in the PB (8 patients had intermediate MRD [≥10−4 but <10−2; 19.5%], and 4 patients had positive MRD results [≥10−2; 9.8%]). In this analysis, for 11 patients (26.8%), an MRD analysis could not be performed because of death (n = 2), beginning of a new CLL therapy (n = 2), withdrawal of informed consent (n = 1), missed visits during the COVID19 pandemic (n = 3), and loss to follow-up (n = 3). MRD status in the course of treatment is shown in Figure 3B.
ORR, CR rate, and MRD results. (A) Clinical response at final restaging and (B) MRD response (patient numbers and percentages) at cycles 9, 12, 15, and 36, all in the PB and in BM at cycle 15.
ORR, CR rate, and MRD results. (A) Clinical response at final restaging and (B) MRD response (patient numbers and percentages) at cycles 9, 12, 15, and 36, all in the PB and in BM at cycle 15.
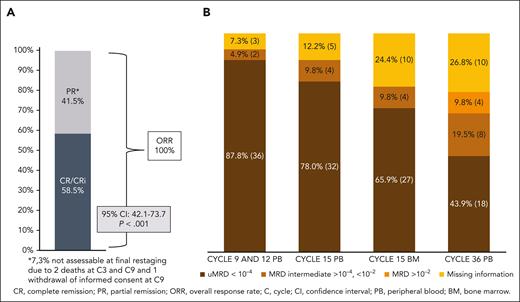
After a median observation time of 38.4 months (range, 3.7-44.9 months), 9 PFS events occurred. The PFS rate at 36 months was 79.9%, and the median PFS was not reached (Figure 4A). Three patients died (1 patient at cycle 3 because of retrospectively preexisting ovarian cancer, 1 patient at cycle 9 because of cardiac failure during an aggravated mitral valve insufficiency unrelated to study treatment, and 1 patient because of respiratory tract infection after Richter transformation under subsequent treatment). The 36-month OS rate was 92.6% (Figure 4D), and the median OS was not reached. Secondary end points EFS, TTNT, TFS, and ORR at the end of maintenance are shown in the supplement (supplemental Figures 1Ai-3Ai; supplemental Table 5).
Progression-free survival and overall survival. (A) PFS: 9 PFS events occurred after a median observation time of 38.4 months (range, 3.7-44.9 months). The 3-year PFS rate was 79.9%, and the median PFS was not reached. (B) PFS and del(17p): no PFS event occurred in the 15 patients with a sole TP53 mutation (blue). PFS after 36 months in patients with del(17p) and/or TP53 mutation (red) was 67.6% (P = .012). (C) PFS and IGHV: In patients with mutated IGHV (red), no disease progression occurred. In patients with unmutated IGHV (blue), the 36-month PFS was 74.0 % (P = .147). (D) OS: 3 OS events occurred, the 3-year OS rate was 92.6%, and the median OS was not reached.
Progression-free survival and overall survival. (A) PFS: 9 PFS events occurred after a median observation time of 38.4 months (range, 3.7-44.9 months). The 3-year PFS rate was 79.9%, and the median PFS was not reached. (B) PFS and del(17p): no PFS event occurred in the 15 patients with a sole TP53 mutation (blue). PFS after 36 months in patients with del(17p) and/or TP53 mutation (red) was 67.6% (P = .012). (C) PFS and IGHV: In patients with mutated IGHV (red), no disease progression occurred. In patients with unmutated IGHV (blue), the 36-month PFS was 74.0 % (P = .147). (D) OS: 3 OS events occurred, the 3-year OS rate was 92.6%, and the median OS was not reached.
Correlative analyses
Exploratory correlative analyses were performed for the following genetic markers: IGHV mutational status, del(17p), TP53 mutational status, del(11q), del(13q), trisomy 12, complex karyotype, mutations in NOTCH1, SF3B1, ATM, and RSP15 (supplemental Figures 4Ai-6Ai). In 15 patients without del(17p) (cutoff < 7.45%), no PFS event occurred, corresponding to a 36-month PFS rate of 100%, whereas patients with del(17p) showed a 36-month PFS rate of 67.6% (P = .012; Figure 4B). Among 20 patients harboring del(17p) < 20%, the 36-month PFS was 100%, whereas in 21 patients with del(17p) ≥20%, it was 61% (hazard ratio [HR], 9.491; 95% CI, 1.184-76.103; P = .034). None of the patients with mutated IGHV (n = 6) progressed, whereas the 36-month PFS rate in patients with unmutated IGHV was 74.0% (P = .147; Figure 4C). Among the 39 patients who had TP53 mutation, 4 had more than 1 mutation, whereas 35 had only 1 mutation. The 36-month PFS rates with 0, 1, and >1 TP53 mutations were 50%, 82.1%, and 75%, respectively (1 vs 0 mutation: HR, 0.277; 95% CI, 0.033-2.326; P = .237; >1 vs 1 mutation: HR,1.626; 95% CI, 0.196-13.521; P = .653). The 36-month PFS rate of the 24 patients with del(17p) and TP53 mutations was 69.1%, whereas none of the 15 patients with a sole TP53 mutation progressed (P = .03).
The presence of the NOTCH1 mutation was observed in a small subset (n = 3; 7.3%) and showed an impact on the PFS (HR, 7.053; 95% CI, 1.387-35.876; P = .019; supplemental Figure 4Ai). Seven patients with the SF3B1 mutation (17.1%; supplemental Figure 5Ai; HR, 0.365; 95% CI, 0.042-3.171; P = .361) had a 36-month PFS of 100%. The presence of a complex karyotype (≥3 aberrations) in 24 patients (61.5%; HR, 2.611; 95% CI, 0.541-12.602; P = .232; supplemental Figure 6Ai) correlated with a numerically shorter PFS without reaching statistical significance.
Ibrutinib maintenance
At the final restaging, 17 patients reached a PR, and ibrutinib maintenance was intended according to protocol in these patients. However, only 6 of 17 patients received ibrutinib maintenance, and among these, 3 had uMRD, whereas 3 had intermediate MRD at final restaging. At cycle 36, 1 of 6 patients (16.7%) remained with intermediate MRD, whereas 5 of 6 (83.3%) had uMRD. None of these patients had disease progression. Another 6 patients with PR and uMRD stopped ibrutinib treatment at cycle 15, not according to protocol: 5 because of uMRD and the physician’s decision, and 1 patient declined ibrutinib maintenance. Five patients had a PR and discontinued treatment: 1 because of MRD intermediate in PB and BM, 3 patients were not assessable (2 deaths, 1 at cycle 3 and the other at cycle 9; and 1 because of AE and retraction of informed consent.
PD
Progressive disease (PD) was observed in 7 patients between cycles 27 and 42. PD was nodal with an inconspicuous blood count (n = 3) or nodal with lymphocytosis (n = 3), and 1 case of Richter transformation occurred. Of the PD events, 5 patients had a CR and uMRD at final restaging, and 2 patients had a PR and uMRD in the PB (1 patient: BM analysis was not performed; 1 patient: uMRD in the BM). No PD was observed on ibrutinib maintenance treatment. Subsequent therapy was administered in 4 patients (1 patient with 3 regimens: venetoclax, idelalisib/rituximab, and ibrutinib; 1 patient with 2 lines of obinutuzumab; 2 patients received acalabrutinib/venetoclax/obinutuzumab). The patient treated with obinutuzumab reached a PR during his first treatment with obinutuzumab; response to the second treatment with obinutuzumab and response in the patients treated with the triple combination are pending. The patient with venetoclax and rituximab/idelalisib reached stable disease under each treatment and had PD under ibrutinib treatment (Table 3).
PD and subsequent treatment
| Pat . | Genetics baseline . | C15 remission . | MRD at C15 . | EOT date . | PFS date . | Time point of PD in mo, from registration date . | Period of subsequent therapy . | Name of the first subsequent therapy . |
|---|---|---|---|---|---|---|---|---|
| 1 | Del(17p) 88% TP53mut Exon 8 60% | CR | uMRD | February 2018 | September 2019 | 32.0 | ||
| 2 | Del(17p) 21.5% TP53mut Exon 8 20% | CR | uMRD | November 2017 | February 2019 | 27.2 | ||
| 3† | Del(17p) 85% TP53mut Exon 5 60% | CR | uMRD | December 2017 | September 2019 | 33.5 | From November 2018 to February 2019 | Obinutuzumab |
| 4 | Del(17p) 93.5% TP53mut Exon 8 70% | CR | uMRD | May 2018 | December 2019 | 32.1 | February 2020 | Acalabrutinib/obinutuzumab/venetoclax |
| 5 | Del(17p) 93% TP53 umut | PR | uMRD PB; no BM | November 2018 | September 2019 | 24.6 | February 2020 | Acalabrutinib/obinutuzumab/venetoclax |
| 6∗ | Del(17p) 93.5% TP53mut Exon 7 95% | PR | uMRD | December 2018 | January 2020 | 24.2 | from April 2020 to July 2020 | Venetoclax |
| 7 | Del(17p) 16.5% TP53mut Exon 6 70% | CR | uMRD PB; iMRD BM | April 2019 | June 2021 | 38.6 |
| Pat . | Genetics baseline . | C15 remission . | MRD at C15 . | EOT date . | PFS date . | Time point of PD in mo, from registration date . | Period of subsequent therapy . | Name of the first subsequent therapy . |
|---|---|---|---|---|---|---|---|---|
| 1 | Del(17p) 88% TP53mut Exon 8 60% | CR | uMRD | February 2018 | September 2019 | 32.0 | ||
| 2 | Del(17p) 21.5% TP53mut Exon 8 20% | CR | uMRD | November 2017 | February 2019 | 27.2 | ||
| 3† | Del(17p) 85% TP53mut Exon 5 60% | CR | uMRD | December 2017 | September 2019 | 33.5 | From November 2018 to February 2019 | Obinutuzumab |
| 4 | Del(17p) 93.5% TP53mut Exon 8 70% | CR | uMRD | May 2018 | December 2019 | 32.1 | February 2020 | Acalabrutinib/obinutuzumab/venetoclax |
| 5 | Del(17p) 93% TP53 umut | PR | uMRD PB; no BM | November 2018 | September 2019 | 24.6 | February 2020 | Acalabrutinib/obinutuzumab/venetoclax |
| 6∗ | Del(17p) 93.5% TP53mut Exon 7 95% | PR | uMRD | December 2018 | January 2020 | 24.2 | from April 2020 to July 2020 | Venetoclax |
| 7 | Del(17p) 16.5% TP53mut Exon 6 70% | CR | uMRD PB; iMRD BM | April 2019 | June 2021 | 38.6 |
TP53mut, TP53 mutation; umut, unmutated. Pat, patient; del, deletion; mut, mutated; umut, unmutated; C, cycle; CR, complete remission; PR, partial remission; uMRD, undectable minimal residual disease; iMRD, intermediate minimal residual disease; PB, peripheral blood; EOT, end of treatment; PFS, progression-free survival.
A Richter transformation (other: diffuse large B-cell lymphoma clonal relationship not analyzed) has been documented 12 months after EOT in May 2020, after treatment with venetoclax, rituximab/idelalisib, and ibrutinib monotherapy was administered.
Due to detectable MRD in November 2018, the patient receieved obinutuzmab. The patient had a disease progression in September 2019.
Safety
AEs were reported in all 41 patients.29 The most frequent AEs of any grade as well as the most frequent AEs grade ≥3 appeared during triple induction therapy and decreased during consolidation and maintenance after cycle 15 (Figure 5A,B). The most common hematologic AEs in the 3 treatment parts were neutropenia (all grades: cycles 1-6, 46.3%; cycles 7-12, 22.0%; and cycle >13, 2.4% vs grade ≥3: cycles 1-6, 41.5%; cycles 7-12, 17.1%; and cycle >13, 2.4%) and thrombocytopenia (all grades: cycles 1-6, 22.0% and cycle >7, 0% vs grade ≥3: cycles 1-6, 17.1% and cycle >7, 0). Nonhematologic grade ≥3 AEs between cycles 1 and 6 were laboratory TLS (9.8%) and infusion-related reactions (7.3%). Cardiovascular toxicity grade ≥3 occurred as atrial fibrillation at a rate of 2.4% each in cycles from 1 to 6 and cycles from 7 to 12, and hypertension (4.9%) occurred between cycles 1 and 6.
AEs (>10%) of any grade over the course of treatment. (A) The most frequent AEs appeared during induction therapy and were lower during consolidation and maintenance. (B) AEs of grade ≥3 over the course of treatment. The highest incidences of the most frequent grade ≥3 AEs appeared during induction therapy and decreased during treatment.
AEs (>10%) of any grade over the course of treatment. (A) The most frequent AEs appeared during induction therapy and were lower during consolidation and maintenance. (B) AEs of grade ≥3 over the course of treatment. The highest incidences of the most frequent grade ≥3 AEs appeared during induction therapy and decreased during treatment.
Most AEs were grade 1 or 2 (75.2%) and resolved (89.5%) or resolved with sequalae (1.5%). Adjustment of study drugs, including dose reductions (29.8%), dose interruptions (89.3%), and permanent discontinuation (6.0%), was performed in 15.4% of the AEs. In total, 63 serious AEs were reported, 33 of them related to the study drug. The most frequent grade 3 or 5 AEs during the whole treatment period and up to the end of the study (observation time median, 38.4 months) were blood and lymphatic system disorders (58.5%), with neutropenia (48.8%) and thrombocytopenia (17.1%), and infections (19.5%), especially urinary and respiratory tract infections. The most frequent AEs of any grade were gastrointestinal disorders in 85.4% of patients, most of which were low grade. The most common cardiac disorder graded from 1 to 5 was atrial fibrillation in 14.6% of patients.
Discussion
The CLL2GIVe trial illustrates a highly active, time-limited treatment approach for patients with CLL with del(17p) and/or TP53 mutations with manageable tolerability. Treatment outcome remains heterogeneous in this distinct subgroup because, for example, patients with a sole TP53 mutation remained without disease progression during follow-up.
The efficacy of the GIVe triple combination therapy in 17p-deleted and/or TP53-mutated patients with CLL shows a 36-month OS rate of 92.6%, a 36-month PFS rate of 79.9%, and high rates of uMRD during and at the end of treatment. In comparison, continuous ibrutinib monotherapy in an untreated cohort of del(17p)/TP53-mutated CLL (n = 34) led to PFS rates of 85% at 36 months and 79% at 48 months.7 Comparing the CLL2GIVe trial to studies that recruited patients with CLL with but not limited to the del(17p)/TP53 mutation, continuous ibrutinib (alone or combined with rituximab) as the first-line treatment in the A041202 ALLIANCE trial (including 9 of 181 [5%] patients with del(17p)- and 15 of 168 [9%] patients with TP53 mutation) showed a 48-month PFS of 72.8%.30 In contrast, time-limited treatment with 12 cycles of venetoclax and obinutuzumab in the CLL14 trial demonstrated a 48-month PFS of 53.0% in patients with CLL with del(17p) (11.1%; 19 of 171) and TP53 mutation (8.5%; 17 of 200).13,15 The fixed-duration cohort of the CAPTIVATE trial with 15 months of ibrutinib and venetoclax treatment, including patients with high-risk features of the del(17p)/TP53 mutation (n = 27 of 159; 17%), showed a similar 36-month PFS of 80%.31,32 Whether the addition of obinutuzumab enhances PFS over the doublet regimen is, therefore, unclear. The ongoing randomized phase 3 ACE-CL-311 trial (NCT03836261), excluding patients with the del(17p)/TP53 mutation, tests the role of obinutuzumab in addition to acalabrutinib and venetoclax. A comparison of the efficacy of continuous monotherapy and time-limited regimens such as venetoclax/obinutuzumab and this trial in patients with high-risk CLL is shown in supplemental Table 6.7,15 The GIVe regimen combines targeted therapeutic options in a time-limited regimen, with outcomes comparable with those of continuous BTKi therapy. Treatment discontinuation may theoretically lead to a reduced risk of BTKi-associated resistance mutations at relapse, which will be studied with longer follow-up and acquisition of more relapse samples than used in this study.
Patients with CLL carrying the sole TP53 mutation (n = 15) showed no disease progression. This observation may point to a negative prognostic role of del(17p) as compared with sole TP53 mutations and, if confirmed, may help to define a subgroup in need of more effective treatment.33,34 In patients without disease progression, the proportion of cells with del(17p) ranged from 0.5% to 99%, and the TP53 VAF ranged from 5% to 95%. Furthermore, 6 of these 15 patients with sole TP53 mutations had a mutated IGHV status. Therefore, the sole TP53 mutation (without the del[17p]) and mutated IGHV status appear to mitigate the adverse outcome in the population of this trial. Similarly, the ERIC consortium in HARMONY showed in untreated patients with CLL the presence of the TP53 mutation only in unmutated IGHV CLL Binet A as a high-risk marker. Time to the first treatment was an end point of this analysis.35
Disease progression (n = 7) was observed among patients, seemingly irrespective of response (CR with uMRD [n = 5] or PR with uMRD [n = 2]). Among these, 6 patients had a TP53 mutation and del(17p) with large clone sizes, and 1 patient had a sole del(17p) (Table 3). Therefore, CLL with del(17p) and/or TP53 mutation represents a heterogeneous group of patients, and the outcome appears to depend also on IGHV mutation status and coexisting factors, such as NOTCH1 mutation,36,37 presence of a complex karyotype, and del(17p) in addition to TP53 mutation as well as the clone size of del(17p) and VAF of TP53 mutation.38
Nevertheless, in conclusion, for the overall CLL population with del(17p) and/or TP53 mutations, time-limited triple combination therapy appears efficient and well tolerated, especially with the option of MRD monitoring and treatment modification/retreatment. However, it is a nonlicensed treatment option, and MRD-guided therapy remains an experimental approach reserved for the clinical trial setting.
The most common grade 3 or 5 AEs in our study are comparable with those in other triple combination studies.21,22 A high rate of infections was observed, as described for combination treatments with ibrutinib and venetoclax or venetoclax and obinutuzumab, which lead to reductions in healthy B cells, natural killer cells, and T cells, as observed in vitro.39 Immune system restoration could be shown for the combination of ibrutinib and venetoclax with normalization of T-cell counts (CD4/CD8/regulatory T cells/PD-1) after 6 months of treatment as well as sustained regeneration of normal B-cell counts.40 Characteristic nonhematologic AEs were also seen in the combination trials of ibrutinib and venetoclax, including atrial fibrillation, hypertension, and infections.32,41
Limitations of this study appear mostly because of the small sample size limiting subgroup comparisons and correlative analyses. In light of the high uMRD (MRD < 10−4) rate, better prognostic discrimination may be achieved with more sensitive assays reaching deeper uMRD thresholds (<10−5 or <10−6), such as high-sensitivity flow cytometry and next-generation sequencing.42,43 MRD guidance is relevant in time-limited treatment to determine disease kinetics during and after the end of therapy,15 for example every 3 months as in the triple combination trial of acalabrutinib, venetoclax, and obinutuzumab, including patients with high-risk CLL (NCT 03580928),22 and to evaluate treatment length and retreatment timing. Retreatment in cases of disease progression according to IWCLL criteria is tested in the ReVenG study (NCT04895436). As shown in this trial, the response of high-risk CLL is heterogeneous; future phase 3 trials could also address the appearance of del(17p) and TP53 mutations and the role of maintenance therapy in this subgroup vs time-limited treatment in patients with a sole TP53 mutation (with mutated IGHV). Possible subsequent treatments in the case of double-refractory CLL are noncovalent BTKis,44 phosphoinositide 3′-kinase inhibitors,45 chimeric antigen receptor T-cell therapy,46,47 or allogeneic stem cell transplantation.48
There remains a small number of clinical trials specifically addressing del(17p)/TP53-mutated CLL in first-line or relapsed/refractory settings. The ongoing phase 3 CLL16 (NCT 05197192) trial tests the triple combination of acalabrutinib, venetoclax, and obinutuzumab vs venetoclax and obinutuzumab only in high-risk CLL. More clinical trials are needed for CLL with del(17p)/TP53 to define an optimal treatment approach for each patient.
Acknowledgments
The authors thank the patients and their families, the investigators at the sites, and the German CLL Study Group study office. The authors thank the team of Ulm University Medical Center study office and the team of the German CLL Study Group central office at Cologne University Medical Center for their contribution to the conduct of the study. The trial design was developed by the German CLL Study Group, sponsor of the study was Ulm University Medical Center, and the study was supported by Roche Pharma AG and Janssen Cilag. Sponsor and German CLL Study Group representatives analyzed the data and wrote the first draft of the manuscript.
Authorship
Contribution: H.H., S.E., J.v.T., E.T., C.S., M.F., P.D., M.R., T.I., A.L.I., J.D., S.B., C.U.N., O.A.-S., A.-M.F., K.F., H.D., M.H., B.E., and S.S. recruited the patients; K.-A.K., E.T., C.S., and S.S. performed the genetic analyses; H.H. and S.S. wrote the first draft of the manuscript; H.H., S.E., J.v.T., B.E., and S.S. designed and managed the clinical trial and interpreted the clinical data; M.R. and M.K. analyzed the MRDs; S.R., A.G., and C.Z. performed the statistical analyses; and all authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: J.v.T. received research support from Janssen/Roche; received travel grants from AbbVie, Janssen, Roche, and Celgene; received honoraria from Roche, Janssen, AbbVie, and AstraZeneca; and is on the advisory boards of Janssen, Roche, and AbbVie. H.H. received travel grants from Novartis and BeiGene; received honoraria from AbbVie and Janssen; and is on the advisory board of Janssen. P.D. reports consultancy for AbbVie, AstraZeneca, bluebird bio, Gilead, Janssen, Novartis, Riemser, and Roche; and is on the speakers’ bureau for AbbVie, AstraZeneca, Gilead, Novartis, Riemser, and Roche. S.B. received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Roche, AbbVie, Novartis, Becton Dickinson, Janssen, AstraZeneca, and Sanofi; received grants or contracts from Janssen Cilag Neuss. O.A.-S. received honoraria from Janssen-Cilag, Roche, Gilead Sciences, AbbVie, AstraZeneca, Adaptive Biotechnologies, and BeiGene; reports consulting or advisory role in Roche, Janssen-Cilag, Gilead Sciences, and AbbVie; received research funding from BeiGene, Roche, AbbVie, and Janssen/Pharmacyclics; received travel, accommodations, and expenses from Roche, AbbVie, Gilead Sciences, and Janssen-Cilag. J.D. received advisory board honoraria, travel support, and speaker fees from AbbVie, Amgen, AstraZeneca, Celgene, Hoffmann-La Roche, Sanofi, Janssen, and BeiGene. K.-A.K. reports consultancy, speakers bureau fees, and research support from Janssen, Roche, and AbbVie. A.-M.F. received research funding from Celgene; is on the advisory board of Janssen; and travel grants from AbbVie. C.U.N. received grant research from AbbVie, Janssen, and AstraZeneca; is on the advisory board of AbbVie, Janssen, Gilead, Roche, AstraZeneca, Acerta, and Sunsis; received travel reimbursement from Gilead, Roche, Novartis; and reports consultancy for CSL Behring. K.F. received honoraria from AbbVie and Roche; received travel support from Roche; is on the advisory board of AstraZeneca. H.D. reports consultancy (AdBoard) for AbbVie, Agios, Amgen, Astellas, AstraZeneca, Berlin-Chemie, BMS, Celgene, Daiichi Sankyo, Gilead, Janssen, Jazz, Novartis, Servier, Stemline, and Syndax; received clinical research funding to institution from AbbVie, Agios, Amgen, Astellas, Bristol Myers Squibb, Celgene, Jazz Pharmaceuticals, Kronos Bio, Novartis, and Pfizer. M.H. received honoraria from Roche, Janssen, AbbVie, Gilead Sciences, and AstraZeneca; reports consulting or advisory role for Janssen, AbbVie, Gilead Sciences, Genentech/Roche, AstraZeneca; is on the speakers' bureau at Janssen, AbbVie, Gilead Sciences, Roche/Genentech, and AstraZeneca; received research funding from Roche, AbbVie, Janssen, Gilead Sciences, and AstraZeneca; received travel, accommodations, and expenses from Roche and Janssen. B.E. received grants and personal fees from F. Hoffmann-La Roche Ltd, AbbVie, AstraZeneca, BeiGene, and Janssen; personal fees from Celgene, Novartis, ArQule, Gilead, Oxford Biomedica (UK), Adaptive Biotechnologies, and Hexal. S.S. received advisory board honoraria, research support, travel support, and speaker fees from AbbVie, Amgen, AstraZeneca, Celgene, Gilead, GSK, Hoffmann-La Roche, Janssen, Novartis, and Sunesis. The remaining authors declare no competing financial interests.
Correspondence: Stephan Stilgenbauer, Division of CLL, Department of Internal Medicine III, Universitätsklinikum Ulm, Albert-Einstein-Allee 23, 89081 Ulm, Germany; e-mail: stephan.stilgenbauer@uniklinik-ulm.de.
References
Author notes
Presented in abstract form as an oral presentation at the 64th annual meeting of the American Society of Hematology, from 10 December to 13 December 2022, New Orleans, LA.
Original data are available on request from the corresponding author, Stephan Stilgenbauer (stephan.stilgenbauer@uniklinik-ulm.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Study design and swimmerplot. (A) Study design with triple combination of GIVe from cycles 1 to 6, ibrutinib and venetoclax from cycles 7 to 12, and ibrutinib until cycle 15, in case of CR and uMRD at cycles 9 and 12. Otherwise, ibrutinib was scheduled to continue until cycle 36. (B) Treatment disposition of all patients according to genetics (del[17p] and TP53 mutation, sole del[17p], sole TP53 mutation). Gray lines indicate the duration of treatment, and blue lines indicate the follow-up period. Thirty-four of 41 patients reached final restaging. The symbols at the end of the gray lines define reasons for treatment discontinuation and time point of final restaging (between cycles 14 and 15 because of different timing of response assessment). The red X symbols indicate 7 disease progressions. Black crosses indicate 3 deaths.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/11/10.1182_blood.2023020013/1/m_blood_bld-2023-020013-gr1.jpeg?Expires=1765902048&Signature=egipHCzmVTiLAZdvqJR~O2RzKJkWFggzhH1VOD5VsZFfFUsJM9~Psu3KXHzDKeJ3NyXm2jX3odjj~9BjKRb1Xo-UGLtQ8YImfJhN1sQVElzRzCJw6Ph9oICBk~mVvyi87Y78ze0G88GgyaTQZVXxlMnO0FWST3~preNiWsL8yiLfKUSCDKqnJkuy70Ver9VRn7jnT4NfjXTdyfrAD6zyTNnhmGPQjj-PUy5X5tdCyMovlwDgk32mBdYwZudY1HIRyU3wB2PjnsoP1kwAg0IWznE~qJkn8ZJP6SsyRByAJNyyhXccjO453M0ODhtuTbWXzxtFWkrGJAyRmokazhxTXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal