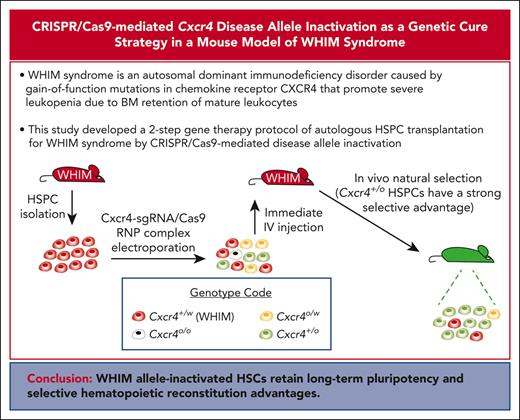
Key Points
A 2-step gene therapy protocol of autologous HSPC transplantation for WHIM syndrome by CRISPR/Cas9-mediated disease allele inactivation.
WHIM disease allele–inactivated HSPCs have a strong selective advantage for durable hematopoietic reconstitution over WHIM cells.
Abstract
WHIM syndrome is an autosomal dominant immunodeficiency disorder caused by gain-of-function mutations in chemokine receptor CXCR4 that promote severe panleukopenia because of retention of mature leukocytes in the bone marrow (BM). We previously reported that Cxcr4-haploinsufficient (Cxcr4+/o) hematopoietic stem cells (HSCs) have a strong selective advantage for durable hematopoietic reconstitution over wild-type (Cxcr4+/+) and WHIM (Cxcr4+/w) HSCs and that a patient with WHIM was spontaneously cured by chromothriptic deletion of the disease allele in an HSC, suggesting that WHIM allele inactivation through gene editing may be a safe genetic cure strategy for the disease. We have developed a 2-step preclinical protocol of autologous hematopoietic stem and progenitor cell (HSPC) transplantation to achieve this goal. First, 1 copy of Cxcr4 in HSPCs was inactivated in vitro by CRISPR/Cas9 editing with a single guide RNA (sgRNA) that does not discriminate between Cxcr4+/w and Cxcr4+/+ alleles. Then, through in vivo natural selection, WHIM allele–inactivated cells were enriched over wild-type allele–inactivated cells. The WHIM allele–inactivated HSCs retained long-term pluripotency and selective hematopoietic reconstitution advantages. To our knowledge, this is the first example of gene therapy for an autosomal dominant gain-of-function disease using a disease allele inactivation strategy in place of the less efficient disease allele repair approach.
Introduction
WHIM syndrome is a rare combined primary immunodeficiency disease named as an acronym based on its clinical features: warts, hypogammaglobulinemia, recurrent infections and myelokathexis (neutropenia from impaired neutrophil egress from bone marrow [BM]). The genetic basis is autosomal dominant gain-of-function mutations of the C-terminal cytoplasmic domain of CXCR4, a G protein-coupled leukocyte chemoattractant receptor specific for the chemokine CXCL12.1,2 WHIM mutations impair receptor downregulation, enhancing signaling. CXCR4 is constitutively expressed on many cell types, including hematopoietic stem and progenitor cells (HSPCs), most mature leukocyte subtypes, many nonhematopoietic cells, and some cancer cells. Among diverse hematologic functions, CXCR4 regulates homing, retention, and quiescence of hematopoietic stem cells (HSCs) in the BM3-7 as well as homing and retention of mature neutrophils in BM.8-10 These effects are exaggerated by gain-of-function WHIM mutations, causing myelokathectic neutropenia.11
Treatment is aimed at reducing the frequency and severity of infection and includes prophylactic antibiotics, immunoglobulin replacement, and granulocyte-colony stimulating factor (G-CSF), which selectively increases the neutrophil count in part by inducing the degradation of CXCL12, thereby reducing CXCR4 signaling. However, these treatments are nonspecific and have undefined efficacy in WHIM syndrome.2 Several patients with WHIM have been cured by allogeneic BM transplantation12-14; however, compared with the relatively benign clinical course of the disease, this approach has potential drawbacks, including graft-versus-host disease and a heightened risk of infection related to the genotoxic BM conditioning used to promote engraftment as well as the immunosuppressive agents needed to prevent acute rejection. In terms of CXCR4-targeted therapy, promising results have been published for 2 specific CXCR4 antagonists, plerixafor (Mozobil, AMD3100; Sanofi-Genzyme) and mavorixafor (X4P-001, AMD11070; X4-Pharma), which are currently in phase 3 clinical trials.11,15,16
Gene therapy is an ideal cure strategy for the disease. However, success is contingent upon engraftment of a sufficient amount of correctly edited HSCs to robustly and durably reprogram the hematopoietic system to maintain long-term benefits. In this regard, we previously described a patient with WHIM who was spontaneously cured by chromothripsis (chromosome shattering), presumably in a single HSC, in which the WHIM allele on chromosome 2 was deleted in toto rather than corrected, leaving the cell hemizygous for wild-type CXCR4 (CXCR4+/o). One copy of 163 other genes on chromosome 2 was also deleted by the event. Remarkably, the chromothriptic HSC acquired a selective engraftment advantage, leading to ∼100% chimerism with CXCR4+/o myeloid cells, which reversed severe neutropenia and monocytopenia for ∼25 years until the patient’s death from COVID-19.17 For unclear reasons, the lymphoid lineage was unaffected by the chromothriptic event. In searching for factors advantaging engraftment of the chromothriptic HSC in this patient, we previously found that donor HSCs from Cxcr4+/o mice have a strong selective advantage for durable hematopoietic reconstitution in competitive transplantation experiments against both Cxcr4+/+ and Cxcr4+/w donor HSCs.18,19 Importantly, Cxcr4+/o mice have a lifespan and hemogram similar to those of healthy mice and no overt phenotypes. These findings suggested that WHIM allele inactivation may be a preferred strategy for genetic cure of WHIM syndrome.
Methods
Mice
The generation and description of Cxcr4+/w mice with C57BL/6 background, in which a human S338X WHIM mutation was introduced into the mouse Cxcr4 locus, have been previously reported. The mice were generously provided by Doctors Balabanian and Bachelerie and were subsequently crossed on a CD45.1, CD45.2, or heterozygous CD45.1/CD45.2 background.17,18,20 The mice were kept in a specific-pathogen-free facility at the National Institutes of Health and were aged from 6 to 8 weeks at the time of transplantation. All animal experiments were performed using a National Institute of Allergy and Infectious Diseases Animal Care and Use Committee–approved protocol.
Mouse HSPC isolation
Murine BM cells were isolated by flushing tibias and femurs in Hanks' Balanced Salt Solution supplemented with 2% heat-inactivated bovine serum (Gibco, Grand Island, NY). HSPCs (cKit+ or CD117+) were isolated using a kit from Miltenyi Biotec (Gaithersburg, MD), following the manufacturer’s instructions.
Gene editing
The gene-editing procedure was followed by modifying that given by Gundry et al.21 Freshly isolated HSPCs were cultured in X-Vivo 15 media (Lonza, Bend, OR), supplemented with 2% fetal bovine serum (FBS), 50 ng/mL stem cell factor (SCF), 50 ng/mL thrombopoietin, 10 ng/mL IL-3, and 10 ng/mL IL-6. Cytokines were obtained from Peprotech (Cranbury, NJ). After 2-hour culture at 37°C with 5% CO2, cells were resuspended into buffer T from the Neon Transfection Kit (Invitrogen, Waltham, MA) at 5 × 105 cells per 10 μL. The Cxcr4-sgRNA/Cas9 ribonucleoprotein (RNP) complexes were prepared by mixing equal volumes of sgRNA (0.5 μg/μL; custom-made from Invitrogen) and Cas9 protein (1 μg/μL; PNA Bio, Thousand Oaks, CA) for 10 or 15 minutes at room temperature. Cells and RNP complexes were then mixed with a ratio of 10 μL cells and 2 μL RNP complexes and electroporated with the neon transfection system under preoptimized conditions (1700 V, 20 ms, 1 pulse). Electroporated cells were resuspended in X-Vivo 15 media supplemented with 1X PenStrep (Invitrogen) and used immediately for transplantation. For DNA isolation, electroporated cells were cultured 2 days in X-Vivo 15 with 2% FBS and the aforementioned cytokines. For mock-transfected controls, the conditions were the same except for the absence of single guide RNA (sgRNA).
Genomic DNA isolation
Genomic DNA from cell line L1.2 and mouse cells was isolated using the DNeasy Blood & Tissue Kit (Qiagen, Germantown, MD).
PCR cloning and sequencing
A target sequence of 1086 base pairs (bp) that includes both the Cxcr4 target site with protospacer adjacent motif (PAM) site used for CRISPR/Cas9 editing, and the WHIM mutation site was amplified using the primers Fw2300 (5′- CTTTGCAGATATACACTTCTGATAAC-3′) and Rew3386 (5′-ATATGTCTTTGCATAAGTGTTAGCTG-3′). The polymerase chain reaction (PCR) product was isolated using the PCR purification kit (Qiagen), cloned with a PCR Cloning Kit (New England Biolabs), and sequenced using Eurofins (Eurofins Scientific, Louisville, KY). Sequence analysis was performed with Sequencher (Gene Codes Corp, Ann Arbor, MI).
T7E1 assay
A Cxcr4 amplicon of 769 bp was generated using the primers EditF (5′-GTGACGTTGTCTGTCCCTGT-3′) and NestR (5′-TAGGATGAGGATGACTGTCG-3′). The PCR products were diluted 1:4 in 1 part NEBuffer 2 and hybridized slowly in a thermal cycler. Twenty microliters of hybridized fragments were then digested with 1.25 U of T7 endonuclease 1 (New England Biolabs) for 10 minutes at 37°C. Digested fragments were separated via agarose gel electrophoresis.
Transplantation experiments
Cxcr4-sgRNA/Cas9 RNP–transfected and mock-transfected HSPCs, 5 × 106 each, in 0.5 mL X-Vivo 15 media were injected via tail vein into sex-matched recipient WHIM mice that had undergone lethal irradiation (900 rads) 8 hours before transplantation. Mice were given neomycin in drinking water for 4-weeks after irradiation. Results were similar in both male and female mice.
Flow cytometry analysis
For leukocyte subset analysis, 100 μL of mouse blood was collected and incubated for 10 minutes with 2 μL of Fc block (BioLegend, San Diego, CA) before incubation with the following monoclonal antibodies at 4°C for 30 minutes: CD45.1-PECy7 and CD45.2-eFluor450 (eBioscience, San Diego, CA) and Ly6G-APC-Cy7, CD11b-PerCP-Cy5.5, CD19-FITC, and CD3-APC (Biolegend). Erythrocytes were lysed with 3 mL ammonium chloride–potassium lysis buffer (Quality Biologicals, Gaithersburg, MD) for 3 minutes at room temperature. Cells were then washed once with fluorescence-activated cell sorting (FACS) buffer and analyzed using a Fortessa FACS cytometer (BD Biosciences, San Jose, CA) and FlowJo software (TreeStar Inc, Ashland, OR). A similar procedure was followed for staining BM cells. Additional monoclonal antibodies used for HSPC analysis were biotinylated CD34, streptavidin-PE, Lin-FITC (FITC-conjugated antibodies against lineage markers including B220, CD3, CD4, CD8, CD11b, Gr1, and Ter119), Sca1-APC-Cy7, cKit-APC, and Flt3-PE-Cy5 (all from BioLegend, except CD34 from eBioscience).
Cell isolation for Cxcr4 genomic analysis
CD11b+ cells derived from donor HSPCs were isolated from the blood of mice that received transplantation by positive sorting (FACS Aria II, BD Biosciences) with antibodies directed against CD45.1-PECy7, CD45.2-eFluor450, and CD11b-PerCP-Cy5.5. From the BM of mice that underwent transplantation, LSK (lineage–Sca1+cKit+) cells derived from donor HSPCs were isolated via lineage depletion with the Lineage Depletion Kit (Miltenyi Biotec) and then via FACS (FACS Aria II, BD Biosciences) with antibodies directed against Sca1-APC-Cy7, cKit-APC, streptavidin-PE (to target residual lineage-positive cells after lineage depletion), CD45.1-PECy7, and CD45.2-eFluor450.
Statistical analysis
A two-tailed Student t test for single comparisons and the two-way analysis of variance for multiple comparisons were used. P < .05 was considered significant.
Results
WHIM allele inactivation is a safe cure strategy for WHIM syndrome
Because WHIM syndrome is caused by autosomal dominant gain-of-function mutations of the C-terminus of CXCR4, genetic cure might be accomplished by either in situ correction of the disease allele or complete inactivation of the gene (Figure 1A). We previously reported that donor Cxcr4+/o BM cells have an engraftment advantage over donor Cxcr4+/+ and Cxcr4+/w BM cells in mice that underwent competitive transplantation;17-19 therefore, we favored a disease allele inactivation strategy using CRISPR/Cas9 technology, which is technically more efficient than allele correction and does not require modification for the individual WHIM mutation. To increase the editing efficiency and avoid in vitro screening that might reduce the pluripotency of gene-edited HSPCs, we inactivated Cxcr4 using an sgRNA that does not distinguish between the disease allele and the wild-type allele, with the reasoning that selection for HSCs in which only the disease allele was inactivated would proceed naturally in vivo because of the engraftment advantage of Cxcr4+/o HSCs.
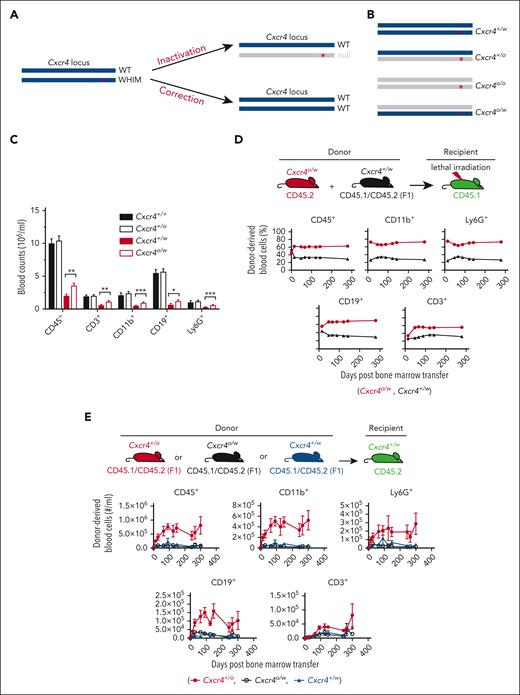
Feasibility of an allele-nonselective Cxcr4 inactivation cure strategy for WHIM syndrome. (A) Possible gene-editing cure strategies for the gain-of-function mutations causing WHIM syndrome. ∗ denotes WHIM mutation location in Cxcr4. (B) The 4 possible genotypes produced by allele-nonselective Cxcr4 inactivation. (C) Absolute leukocyte subtype counts in mouse blood stratified by Cxcr4 genotype. N > 19 mice in each group. (D-E) Hematopoietic reconstituting activity of Cxcr4o/w vs Cxcr4+/w BM cells during competitive transplantation of lethally irradiated Cxcr4+/w recipient mice (D) and after single genotype BM transplantation of unconditioned Cxcr4+/w recipient mice (E). The experimental schemes are shown at the top of panels D and E. Donor and recipient cells were marked by different CD45 isoforms for detection and quantitation via flow cytometry. n = 5 mice in each group in panels D and E. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Data in panels D and E are from a single experiment and are representative of 2 experiments for panel D.
Feasibility of an allele-nonselective Cxcr4 inactivation cure strategy for WHIM syndrome. (A) Possible gene-editing cure strategies for the gain-of-function mutations causing WHIM syndrome. ∗ denotes WHIM mutation location in Cxcr4. (B) The 4 possible genotypes produced by allele-nonselective Cxcr4 inactivation. (C) Absolute leukocyte subtype counts in mouse blood stratified by Cxcr4 genotype. N > 19 mice in each group. (D-E) Hematopoietic reconstituting activity of Cxcr4o/w vs Cxcr4+/w BM cells during competitive transplantation of lethally irradiated Cxcr4+/w recipient mice (D) and after single genotype BM transplantation of unconditioned Cxcr4+/w recipient mice (E). The experimental schemes are shown at the top of panels D and E. Donor and recipient cells were marked by different CD45 isoforms for detection and quantitation via flow cytometry. n = 5 mice in each group in panels D and E. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Data in panels D and E are from a single experiment and are representative of 2 experiments for panel D.
Because this strategy may also generate Cxcr4o/o and Cxcr4o/w HSCs (Figure 1B), we first considered the efficiency and safety of reconstituting a mouse with HSCs having these undesired genotypes. Cxcr4o/o HSCs home poorly to BM niches and are unable to reconstitute hematopoiesis.6,7 To assess Cxcr4o/w HSCs, we generated Cxcr4o/w mice by crossing Cxcr4+/o and Cxcr4+/w mice. Like Cxcr4+/w mice, Cxcr4o/w mice had a normal lifespan and were panleukopenic with circulating leukocyte counts slightly greater than those of Cxcr4+/w mice (Figure 1C). Cxcr4o/w and Cxcr4+/w BM cells also had similar hematopoietic reconstitution activity in competitive transplantation experiments in both lethally irradiated recipient mice (Figure 1D) and unconditioned WHIM recipient mice (Figure 1E). Thus, any Cxcr4o/w HSCs generated by gene editing that might engraft would be unlikely to introduce a new safety concern, but complete exclusion of new safety concerns will require extensive studies of Cxcr4o/w mice, including infection and tumor models.
Development of a CRISPR/Cas9-mediated Cxcr4 inactivation protocol
We designed 2 sgRNAs, designated as left and right, that start 104 and 132 bp downstream, respectively, from the 5′ end of exon 2 of Cxcr4 (Figure 2A). We first attempted to use plasmid vectors for Cxcr4-sgRNA/Cas9-mediated gene editing. However, although they were efficient in cultured cell lines, they were inefficient in primary mouse HSPCs (data not shown). Therefore, we tested preformed recombinant Cas9 protein and Cxcr4-sgRNAs as a RNP delivery system, as described by Gundry et al.21 This system significantly reduces cytotoxicity and the probability of off-target activities because it is transient with no risk of genomic integration or stable expression of the RNP components.22
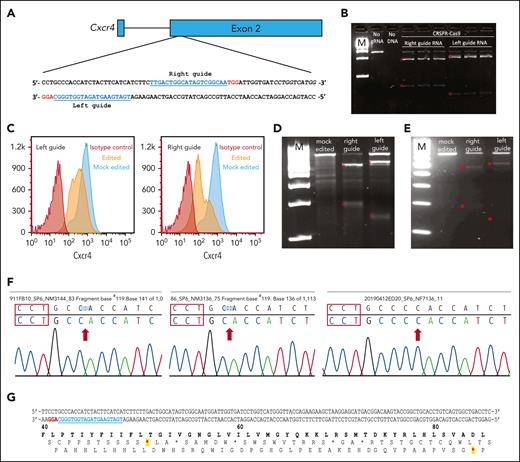
CRISPR/Cas9-mediated Cxcr4 inactivation. (A) Sequences and gene locations of 2 sgRNAs that nonspecifically target both the wild-type and WHIM Cxcr4 alleles. sgRNA sequences are in blue and the PAM sequence is in red. (B) Cell-free system. Cxcr4 amplicons generated via PCR were incubated with Cxcr4-sgRNA/Cas9 RNP complex in vitro and the cleavage products were detected by agarose gel electrophoresis. Experimental conditions are indicated at the top of each lane: M, 1 kb DNA ladder markers; no sgRNA, control Cxcr4-amplicon incubated with Cas9 but without sgRNA; Right guide RNA and left guide RNA refer to Cxcr4 amplicons incubated with RNPs containing the indicated sgRNA, each performed in triplicate. ∗ denotes expected fragment sizes based on the sgRNA location within the amplicon. (C-F) Cell-based transfection system using Cxcr4-sgRNA/Cas9 RNP complex. (C-D) Editing of the pre–B-cell lymphoma cell line L1.2. (C) FACS analysis of nonpermeabilized L1.2 cells with Cxcr4 antibody. The sgRNA is specified at the upper left of each panel. Red and orange histograms, Cxcr4-sgRNA/Cas9 RNP-transfected cells tested with isotype control antibody (red) or anti-Cxcr4 antibody (orange). Blue histograms, mock-transfected cells tested with anti-Cxcr4 antibody. (D) T7 endonuclease 1 (T7E1) editing assay of L1.2 cells. Cxcr4 amplicons from mock-transfected cells or cells transfected with either the left or right sgRNA/Cas9 RNP-transfected, as specified at the top of each lane, were treated with T7E1 and the products were revealed via agarose gel electrophoresis. M, 100 bp DNA ladder. Red stars indicate the DNA fragments cleaved by T7E1. (E-G) Editing of primary cKit+ HSPCs from BM of C57BL/6 mice. (E) T7E1 assay. (F) Identification of cleavage site indels. Sequences of 3 Cxcr4 amplicons from edited HSPCs are shown. The PAM sequence is located within the red boxes. Red arrows, deletions of 1 (left) or 2 nucleotides (middle), and insertion of 1 nucleotide (right). (G) Location of premature stop codons in Cxcr4 introduced by CRISPR/Cas9-generated indels. In the DNA sequence, the PAM site is highlighted in red and the sgRNA sequence is in blue. Protein sequence numbers at the top demarcate amino acid positions; red stars show positions of chain termination introduced by the 1 deletion (first star) and 2 deletions or 1 addition (second star).
CRISPR/Cas9-mediated Cxcr4 inactivation. (A) Sequences and gene locations of 2 sgRNAs that nonspecifically target both the wild-type and WHIM Cxcr4 alleles. sgRNA sequences are in blue and the PAM sequence is in red. (B) Cell-free system. Cxcr4 amplicons generated via PCR were incubated with Cxcr4-sgRNA/Cas9 RNP complex in vitro and the cleavage products were detected by agarose gel electrophoresis. Experimental conditions are indicated at the top of each lane: M, 1 kb DNA ladder markers; no sgRNA, control Cxcr4-amplicon incubated with Cas9 but without sgRNA; Right guide RNA and left guide RNA refer to Cxcr4 amplicons incubated with RNPs containing the indicated sgRNA, each performed in triplicate. ∗ denotes expected fragment sizes based on the sgRNA location within the amplicon. (C-F) Cell-based transfection system using Cxcr4-sgRNA/Cas9 RNP complex. (C-D) Editing of the pre–B-cell lymphoma cell line L1.2. (C) FACS analysis of nonpermeabilized L1.2 cells with Cxcr4 antibody. The sgRNA is specified at the upper left of each panel. Red and orange histograms, Cxcr4-sgRNA/Cas9 RNP-transfected cells tested with isotype control antibody (red) or anti-Cxcr4 antibody (orange). Blue histograms, mock-transfected cells tested with anti-Cxcr4 antibody. (D) T7 endonuclease 1 (T7E1) editing assay of L1.2 cells. Cxcr4 amplicons from mock-transfected cells or cells transfected with either the left or right sgRNA/Cas9 RNP-transfected, as specified at the top of each lane, were treated with T7E1 and the products were revealed via agarose gel electrophoresis. M, 100 bp DNA ladder. Red stars indicate the DNA fragments cleaved by T7E1. (E-G) Editing of primary cKit+ HSPCs from BM of C57BL/6 mice. (E) T7E1 assay. (F) Identification of cleavage site indels. Sequences of 3 Cxcr4 amplicons from edited HSPCs are shown. The PAM sequence is located within the red boxes. Red arrows, deletions of 1 (left) or 2 nucleotides (middle), and insertion of 1 nucleotide (right). (G) Location of premature stop codons in Cxcr4 introduced by CRISPR/Cas9-generated indels. In the DNA sequence, the PAM site is highlighted in red and the sgRNA sequence is in blue. Protein sequence numbers at the top demarcate amino acid positions; red stars show positions of chain termination introduced by the 1 deletion (first star) and 2 deletions or 1 addition (second star).
We first tested the Cxcr4-sgRNA/Cas9 RNP complex using an in vitro cell-free DNA cleavage assay. Both the left and right sgRNAs targeted the correct location in Cxcr4 and allowed Cas9 to cleave a 769 bp Cxcr4 amplicon to generate the expected DNA fragments (589 bp and 180 bp for the right sgRNA and 628 bp and 141 bp for the left sgRNA; Figure 2B). Next, we electroporated the RNP complex into the murine pre–B-cell lymphoma cell line L1.2 to test whether it would reduce cell surface Cxcr4 expression in intact cells.23 Flow cytometry analysis showed that RNP-transfected cells had significantly reduced cell surface Cxcr4 expression compared with that of mock-transfected cells (Figure 2C). To directly assess editing at the genomic level, we performed T7E1 assays and found that a significant fraction of L1.2 cells that had been edited by RNPs accrued Cxcr4 indels at the targeted location, revealed by the appearance of Cxcr4 T7E1 digestion products of the expected size; no Cxcr4 indels were detected in mock-transfected cells (Figure 2D).
We then tested whether this gene-editing method would be successful in primary mouse HSPCs. Because Cxcr4 expression on the HSPC surface is dynamic and heterogeneous at different stages of stem and progenitor cell differentiation,24 it is difficult to analyze Cxcr4 gene-editing efficiency based on the Cxcr4 cell surface expression by flow cytometry. Therefore, we used T7E1 assays again to evaluate editing efficiency directly at the genomic level. After electroporation of RNP complexes containing either left or right sgRNAs, a large fraction of the HSPCs had Cxcr4 indels (Figure 2E). No indels were detected in mock-transfected HSPCs.
To define the indels, we next sequenced the Cxcr4 amplicons generated from edited HSPCs via PCR. We sequenced 10 PCR clones from HSPCs transfected using the left sgRNA and found that 3 were edited by CRISPR/Cas9. The cleavage site was located 3 nucleotides upstream of the PAM site, resulting in 3 types of indels: 2 with 1 or 2 nucleotides deleted and 1 with a single nucleotide insertion (Figure 2F). The cleaved site and indels are consistent with those in reported CRISPR/Cas9 activity.25 All 3 indels caused frameshifts leading to premature Cxcr4 gene termination at the 5′-end of the open reading frame (Figure 2G).
In vivo selection of WHIM allele–inactivated donor-derived hematopoietic cells
We then tested whether Cxcr4-sgRNA/Cas9 RNP–transfected HSPCs (abbreviated as Cxcr4-RNP–transfected HSPCs) from Cxcr4+/w donor mice are viable for hematopoietic reconstitution and whether Cxcr4+/o cells generated by editing might be selected in vivo over Cxcr4+/w cells. For this, we conducted competitive transplantation experiments using Cxcr4+/w mice that received lethal irradiation as recipients and Cxcr4-RNP–transfected and mock-transfected Cxcr4+/w HSPCs as competitive donor cells. To track the transplanted donor cell fate in vivo, the Cxcr4-RNP–transfected, mock-transfected Cxcr4+/w donor mice, and the Cxcr4+/w recipient mice had distinct CD45 congenic markers: CD45.2, CD45.1/CD45.2, and CD45.1, respectively (Figure 3A).
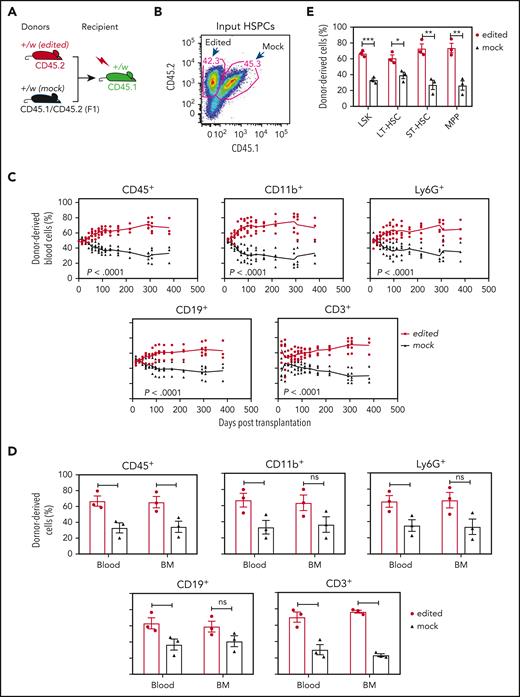
Cxcr4-sgRNA/Cas9 RNP-transfected WHIM HSPCs have a selective advantage for durable hematopoietic reconstitution over mock-transfected cells in lethally irradiated Cxcr4+/w recipient mice. (A) Competitive gene therapy experimental design. The editing status and CD45 isoforms used for distinguishing donor and recipient cells are given at the tops and bottoms of the mouse icons, respectively. Left-Cxcr4-sgRNA/Cas9 RNP-transfected (edited) and mock-transfected (mock) HSPCs were mixed 1:1 and transplanted into lethally irradiated Cxcr4+/w recipients. (B) Cxcr4-sgRNA/Cas9 RNP–transfected (edited) and mock-transfected (mock) donor HSPCs were transplanted in approximately equal numbers. Numbers indicate the percentage of total cells in each gate before transplantation. (C) Hematopoietic reconstitution of the blood. The leukocyte subsets are indicated above each panel. Each data point represents an individual mouse and the solid lines trace the means across time for the experimental conditions coded at the right of the panel. (D-E) Hematopoietic reconstitution of the BM. Mature BM cells (D) and HSPCs (E) were analyzed on day 381 after transplantation when the experiment was arbitrarily terminated. In panel D, data for the same leukocyte subsets in the blood are regraphed from the same day from panel C to facilitate comparison. Data are presented as a scatter plot (n = 3) and as the mean ± SEM of the percent of total donor-derived cells for each subset. Data are from a single experiment, representative of 3 independent experiments. LT-HSC, long-term hematopoietic stem cell (CD34-Flt3−LSK); MPP, multipotential progenitor (CD34+Flt3+LSK); ST-HSC, short-term hematopoietic stem cell (CD34+Flt3−LSK).
Cxcr4-sgRNA/Cas9 RNP-transfected WHIM HSPCs have a selective advantage for durable hematopoietic reconstitution over mock-transfected cells in lethally irradiated Cxcr4+/w recipient mice. (A) Competitive gene therapy experimental design. The editing status and CD45 isoforms used for distinguishing donor and recipient cells are given at the tops and bottoms of the mouse icons, respectively. Left-Cxcr4-sgRNA/Cas9 RNP-transfected (edited) and mock-transfected (mock) HSPCs were mixed 1:1 and transplanted into lethally irradiated Cxcr4+/w recipients. (B) Cxcr4-sgRNA/Cas9 RNP–transfected (edited) and mock-transfected (mock) donor HSPCs were transplanted in approximately equal numbers. Numbers indicate the percentage of total cells in each gate before transplantation. (C) Hematopoietic reconstitution of the blood. The leukocyte subsets are indicated above each panel. Each data point represents an individual mouse and the solid lines trace the means across time for the experimental conditions coded at the right of the panel. (D-E) Hematopoietic reconstitution of the BM. Mature BM cells (D) and HSPCs (E) were analyzed on day 381 after transplantation when the experiment was arbitrarily terminated. In panel D, data for the same leukocyte subsets in the blood are regraphed from the same day from panel C to facilitate comparison. Data are presented as a scatter plot (n = 3) and as the mean ± SEM of the percent of total donor-derived cells for each subset. Data are from a single experiment, representative of 3 independent experiments. LT-HSC, long-term hematopoietic stem cell (CD34-Flt3−LSK); MPP, multipotential progenitor (CD34+Flt3+LSK); ST-HSC, short-term hematopoietic stem cell (CD34+Flt3−LSK).
Once the Cxcr4-RNP–transfected and mock-transfected HSPCs were electroporated and mixed (Figure 3B), they were immediately transplanted into lethally irradiated WHIM mice without in vitro culture. We predicted that in vivo conditions would be superior to in vitro expansion for cell recovery and were concerned that in vitro culture might reduce the expression of Cxcr4, which is required for HSPC homing to the BM.18 In addition, in vitro culture might reduce HSPC stemness, resulting in potential cell loss. After transplantation, blood cells were analyzed via FACS over time for durable hematopoietic reconstitution.
The mice that underwent transplantation did not have any overt clinical abnormalities (increased mortality, decreased body weight, spontaneous infections, or tumors). The results showed that Cxcr4-RNP–transfected HSPCs had a strong selective advantage over mock-transfected cells to reconstitute blood leukocytes in recipient mice. The frequencies of Cxcr4-RNP–transfected donor-derived leukocytes in the blood were significantly higher for all subsets tested than the frequencies of Cxcr4-RNP–transfected HSPCs in the input donor cells. Myeloid cells reconstituted in recipient blood from the Cxcr4-RNP–transfected donor peaked at 85% of total recipient cells (Figure 3C). The strong selective advantage for Cxcr4-RNP–transfected donor HSPC-derived cells was stable up to experiment termination (day 381 after transplantation). When the mice were euthanized, we found that the frequency of Cxcr4-RNP–transfected donor-derived mature leukocytes also significantly increased in the BM (but to a lesser extent than in the blood) compared with the input donor HSPC frequency (Figure 3D). Consistent with this, the frequencies of Cxcr4-RNP–transfected donor-derived HSPCs were also significantly increased in the BM compared with the input donor HSPC frequency (Figure 3E).
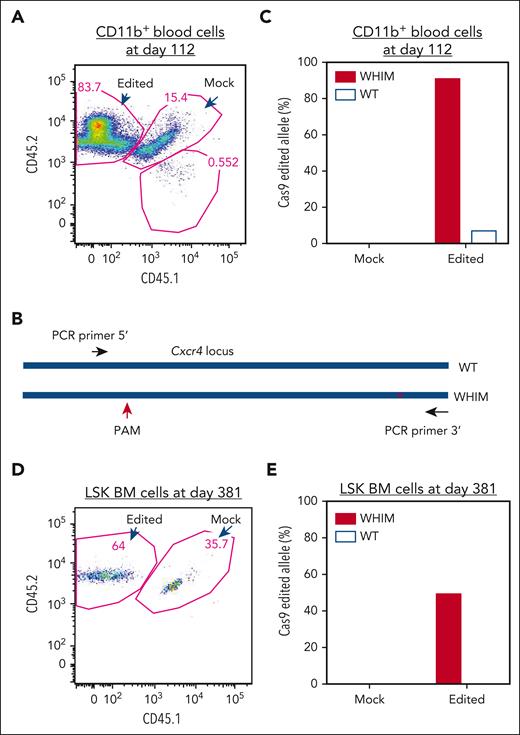
We next examined whether WHIM allele–inactivated donor-derived cells were selectively enriched in vivo by analyzing CD11b+ cells sorted from the blood at day 112 after transplantation that were derived from either Cxcr4-RNP–transfected or mock-transfected donor HSPCs (Figure 4A). DNA purified from the cells was analyzed via T7E1 and DNA sequencing of cloned Cxcr4 amplicons that included both the PAM site used for CRISPR/Cas9 targeting and the WHIM mutation site, thereby, enabling precise assignment to the wild-type vs the WHIM allele of any indels found (Figure 4B). Of the 25 PCR clones derived from the Cxcr4-RNP–transfected donor cells from 1 mouse that underwent transplantation, 12 clones were from the WHIM allele and 13 were from the wild-type allele, and 11 of the 12 WHIM sequences had indels at the expected CRISPR/Cas9 targeting site, whereas only 1 wild-type sequence had an indel; none of 18 clones derived from the mock-transfected donor cells had indels (Figure 4C). The sequencing results were confirmed via T7E1 analysis of DNA from 5 mice (data not shown).
WHIM allele–inactivated HSPCs have a selective advantage for engraftment to reconstitute hematopoietic cells. (A) Flow cytometry plot of sorted CD11b+ cells in blood from a recipient mouse 112 days after transplantation. DNA was isolated for analysis of allele-specific edits from the sorted CD11b+ cells, which were derived from RNP-transfected and mock-transfected HSPCs post transplantation, as described in Figure 3. (B) PCR cloning design for sequencing Cxcr4 that covers both the CRISPR/Cas9 PAM site and the WHIM mutation site. (C) Edit analysis for the sorted CD11b+ cells shown in panel A. Edits were scored on the wild-type and WHIM alleles for CD11b+ cells derived from either the mock-transfected or Cxcr4-sgRNA/Cas9 RNP–transfected donor HSPCs. Eighteen and 25 clones were sequenced for the CD11b+ cells derived from mock-transfected and Cxcr4-sgRNA/Cas9 RNP–transfected HSPCs, respectively. (D) Flow cytometry plot of LSK cells sorted from BM of the same recipient mouse shown in panel A 381 days after transplantation. (E) Edit analysis for the sorted LSK cell populations shown in panel D. A total of 6 and 10 clones were sequenced for the cells derived from the mock-transfected and Cxcr4-sgRNA/Cas9 RNP–transfected HSPCs, respectively.
WHIM allele–inactivated HSPCs have a selective advantage for engraftment to reconstitute hematopoietic cells. (A) Flow cytometry plot of sorted CD11b+ cells in blood from a recipient mouse 112 days after transplantation. DNA was isolated for analysis of allele-specific edits from the sorted CD11b+ cells, which were derived from RNP-transfected and mock-transfected HSPCs post transplantation, as described in Figure 3. (B) PCR cloning design for sequencing Cxcr4 that covers both the CRISPR/Cas9 PAM site and the WHIM mutation site. (C) Edit analysis for the sorted CD11b+ cells shown in panel A. Edits were scored on the wild-type and WHIM alleles for CD11b+ cells derived from either the mock-transfected or Cxcr4-sgRNA/Cas9 RNP–transfected donor HSPCs. Eighteen and 25 clones were sequenced for the CD11b+ cells derived from mock-transfected and Cxcr4-sgRNA/Cas9 RNP–transfected HSPCs, respectively. (D) Flow cytometry plot of LSK cells sorted from BM of the same recipient mouse shown in panel A 381 days after transplantation. (E) Edit analysis for the sorted LSK cell populations shown in panel D. A total of 6 and 10 clones were sequenced for the cells derived from the mock-transfected and Cxcr4-sgRNA/Cas9 RNP–transfected HSPCs, respectively.
Assuming that none of the sorted cells was derived from Cxcr4o/o HSPCs, the data indicate that the WHIM allele was inactivated in ∼92% of CD11b+ cells derived from the Cxcr4-RNP–transfected donor, whereas the wild-type allele was inactivated in only ∼8% of these cells. Because the Cxcr4-RNP–transfected donor-derived CD11b+ cell fraction in recipient blood was 84% (Figure 4A), the final percentage of WHIM allele–inactivated CD11b+ cells was ∼77% (92% of 84%).
We next estimated the frequencies of WHIM and wild-type allele–inactivated cells in the BM. On day 381 after transplantation, we euthanized the mice that underwent transplantation and FACS-purified LSK BM cells derived from the Cxcr4-RNP–transfected and mock-transfected donor HSPCs (Figure 4D). Cxcr4 PCR amplicons were generated from the sorted cells and sequenced to identify the frequency of indels on the wild-type vs the WHIM allele. Of the 10 PCR clones examined from Cxcr4-RNP–transfected cells, 4 were generated from a WHIM allele and 6 from a wild-type allele, of which 2 WHIM alleles had indels, and no wild-type alleles had indels; as expected, none of the 6 clones derived from mock-transfected donor HSPCs had indels (Figure 4E). In a separate experiment, indels appeared in the input Cxcr4-RNP–transfected donor HSPCs before transplantation with a similar frequency on the wild-type and the WHIM allele, supporting a selective advantage in vivo of Cxcr4+/o-derived cells produced through CRISPR/Cas9 editing.
Discussion
Here, we describe the development of a two-step preclinical gene therapy protocol for WHIM syndrome: CRISPR/Cas9-mediated, allele-nonselective inactivation of 1 copy of Cxcr4 in Cxcr4+/w HSPCs in vitro followed by in vivo natural selection to enrich for WHIM allele–inactivated nonpathogenic Cxcr4+/o hematopoietic cells. CRISPR technology has been used previously as a cure strategy in several inherited diseases including a mouse model of progeria using a viral vector delivery system,26 clinical trials using Cas9-sgRNA RNP as a delivery system to treat sickle cell disease,27,28 and a clinical trial to treat transthyretin amyloidosis using lipid nanoparticles encapsulating Cas9 mRNA and sgRNA.29 Compared with current gene therapy approaches involving viral or plasmid vectors, our approach does not have the risk of viral vector integration and the concerns of immunologic surveillance. Moreover, our strategy confers an intrinsic selective advantage mechanism for disease allele–inactivated cells to engraft in the BM and release mature donor-derived hematopoietic cells from the BM and involves the immediate transplantation of the edited HSPC IV, allowing the editing to occur in vivo after the cells have homed to the BM niches.
Nonselective Cxcr4 inactivation potentially generates HSCs having the 3 other genotypes in the population of edited cells, Cxcr4o/o, Cxcr4+/o and Cxcr4o/w, depending on which alleles are edited. Cxcr4o/o HSCs have been reported to inefficiently home from the blood to BM and are, therefore, unlikely to be a safety concern.6,7 We previously reported that the desired Cxcr4+/o HSCs have a selective advantage over Cxcr4+/w HSCs for BM engraftment and hematopoietic reconstitution, based on competitive transplantation experiments both in lethally irradiated recipients and in unconditioned WHIM mice.17-19 The proposed mechanism is enhanced proliferation of Cxcr4+/o HSCs in BM and preferential egress of mature Cxcr4+/o leukocytes from BM.17,18 Enhanced Cxcr4+/o HSC proliferation did not result in myelodysplasia or other untoward effects, because chimerism may be limited by the total number of specific niches available.6,30 Importantly, reconstitution of recipient mice with mature Cxcr4+/o leukocytes in the blood was durable and stable. Moreover, we have previously reported that Cxcr4+/w mice fully reconstituted with Cxcr4+/o BM are healthy and have a normal lifespan.18 In addition, our finding of a patient with WHIM cured by WHIM allele deletion in HSCs17 further supports the safety of the strategy to treat WHIM syndrome via disease allele inactivation.
Cxcr4o/w mice have slightly higher blood counts than Cxcr4+/w mice (Figure 1C). In competitive transplantation experiments with Cxcr4-edited and mock-edited HSPCs (Figure 3 and 4), Cxcr4o/w cells were significantly less competitive than Cxcr4+/o cells. This result is consistent with our previous finding of the genotype rank order of Cxcr4+/o > Cxcr4+/+ > Cxcr4+/w for hematopoietic reconstitution in competitive BM transplantation; thus, the stronger the Cxcr4 signaling activity, the weaker the ability to compete for blood reconstitution in competitive transplantation experiments.18,19 The Cxcr4o/w genotype has no wild-type allele; therefore, total Cxcr4 signaling activity in cells with this genotype should be substantially lower than that for Cxcr4+/w cells. Importantly, Cxcr4o/w mice had a normal lifespan without any clinical or hematologic abnormalities compared with that of Cxcr4+/w mice. Overall, the data suggest that Cxcr4o/w cells generated by Cxcr4 editing do not impose a safety concern.
Unlike knocking out gene function, which requires high editing yields, our goal was to silence only 1 copy of the Cxcr4 gene, therefore, optimal conditions to create a high percentage of cells with only 1 copy of Cxcr4 edited are critical. The RNP-based method, owing to its transient “hit-and-run” approach via electroporation of RNPs, the short lifetime of the sgRNA, and the controllable RNP concentration, allows optimization of the gene-editing conditions to edit only 1 copy of the Cxcr4 gene in each cell. Because expression of Cxcr4 on the cell surface of HSPCs is dynamic and heterogeneous at different stages of stem and progenitor cells,24 we do not have data to quantitively assess the editing yield of 1 or both alleles in cells by FACS. However, direct sequencing data indicated that ∼30% of Cxcr4 alleles were edited under our experimental conditions. If the number of cells with 2 alleles edited is small, then the percentage of cells with only 1 allele edited will be ∼60%, in which about half should be cells with the edited WHIM allele. After being equally mixed with mock-edited HSPCs, the WHIM allele–edited (Cxcr4+/o) donor cells in our experiment should have therefore been ∼15%, which allowed stable reconstitution of the recipient blood compartment with ∼77% of myeloid cells from the edited donor. This figure is close to that in our previous report that showed ∼10% of Cxcr4+/o donor BM cells could compete with 90% of Cxcr4+/w BM cells to reconstitute up to 80% of myeloid cells in the blood of lethally irradiated recipients.19 Thus, under our gene-editing conditions, a majority of the edited cells were edited on only 1 Cxcr4 allele. Consistent with this, our 60% editing efficiency of mouse HSPCs is similar to results reported for green fluorescent protein–transgenic mouse HSPCs, in which the authors could analyze both genomic sequence and green fluorescent protein expression.21
Because the cells we edited were HSPCs, which contain various progenitors besides HSCs, and because it is difficult to isolate small numbers of edited HSCs for genetic analysis, we do not have direct evidence to show whether HSCs themselves underwent successful Cxcr4 gene editing upon the transfection of Cas9/sgRNAs, and whether WHIM allele–inactivated HSCs were enriched after in vivo selection. However, we did detect enriched WHIM allele–inactivated LSK cells (∼32%) in the BM 381 days after transplantation, when the experiment ended. It has been reported that the BM residence time for multipotential progenitors is ∼70 days, whereas more committed progenitors, such as common myeloid progenitors and common lymphoid progenitors, have even shorter residence times. Short-term HSCs have a residence time of ∼330 days, whereas the residence time for long-term HSCs is the lifetime of the mouse.30 In our experiment, the high percentage of Cxcr4-edited, HSC-derived peripheral blood cells (77%) maintained for >381 days after transplantation indicates that these cells must be derived from HSCs. Thus, under our nonselective Cxcr4-editing conditions, Cxcr4 in HSCs from WHIM mice was successfully edited ex vivo in a subset of cells and those HSCs in which the WHIM allele but not the wild-type allele was inactivated (Cxcr4+/o HSCs) were enriched in vivo. The net result was reconstitution of the blood with mature Cxcr4+/o leukocytes resulting from preferential engraftment of Cxcr4+/o HSCs and preferential release from BM of mature Cxcr4+/o leukocytes.
In conclusion, we have developed a preclinical, allele-nonselective HSC Cxcr4 inactivation gene therapy protocol for WHIM syndrome using CRISPR/Cas9 technology, in which sufficient numbers of appropriately edited Cxcr4+/o HSCs are selectively enriched in vivo to allow for selective hematopoietic reconstitution with mature Cxcr4+/o leukocytes. The edited HSCs retained their long-term pluripotency and selective engraftment advantages, replicating the function of our previously studied Cxcr4+/o HSCs obtained from Cxcr4+/o mice. Future development of this protocol will involve additional safety testing with regard to off-target activity of Cas9, infection susceptibility, and tumor development, with the ultimate goal of clinical translation in which patient conditioning to effect cure after transplantation of edited cells is limited or even unnecessary.
Acknowledgments
The authors thank Francoise Bachelerie and Karl Balabanian for the contribution of WHIM mice.
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Authorship
Contribution: J.-L.G., D.H.M., H.L.M., and P.M.M designed the experiments; J.-L.G., A.O.-A, A.Y., E.Y., P.J., S.M., U.C., and C.L.S. generated and analyzed the experiment data; J.-L.G. and P.M.M. supervised the process and were principally responsible for writing the manuscript.
Conflict-of-interest disclosure: J.-L.G., D.H.M., P.J., and P.M.M. are listed as inventors on a US patent (#:US20170196911 A1) disclosing a method for using CXCR4 haploinsufficient HSCs to enhance BM engraftment in transplantation. The remaining authors declare no competing financial interests.
Correspondence: Philip M. Murphy, Bldg 10, Room 11N113, National Institutes of Health, Bethesda, MD 20892; e-mail: pmm@nih.gov.
References
Author notes
Data are available on request from the corresponding author, Philip M. Murphy (pmm@nih.gov).
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal