TO THE EDITOR:
The risks of therapy-related myelodysplastic syndromes and acute myeloid leukemia (t-MDS/AML) are well-studied1-3; however, few studies have quantified risks of less common therapy-related hematopoietic neoplasms (t-HNs) in chemotherapy-exposed populations. Hindering the assessment of these t-HNs is their rarity, which requires the study of large numbers of patient populations who are at risk. We therefore sought to systematically quantify the risks for several t-HNs, including chronic myeloid leukemia (t-CML), classical myeloproliferative neoplasms (t-cMPNs), chronic myelomonocytic leukemia (t-CMML), and acute lymphoblastic leukemia/lymphoma (tr-ALL) (supplemental Table 1; available on Blood website). We evaluated these t-HNs after a broad spectrum of first primary cancers among adults treated with initial chemotherapy leveraging US population-based cancer registries.
In the 17 Surveillance, Epidemiology, and End Results (SEER-17) program areas covering ∼28% of the US population, we identified a cohort of adults (20-84 years) diagnosed with a first primary malignant neoplasm during 2000 to 2017 and initially treated with chemotherapy (with/without radiotherapy). Our primary analysis focused on 1-year survivors of a first primary solid or lymphoid neoplasm. The SEER program collects information on patient demographics, tumor characteristics, stage at initial diagnosis, initial therapy in broad categories (eg, any chemotherapy or any radiotherapy), and vital status. Detailed initial treatment data and subsequent therapy for disease progression/relapse are not available. We examined t-HN risks after first primary solid tumors overall and by site-specific cancers4 and after first primary lymphoid neoplasms overall and by major subtypes5 (supplemental Table 2).
Person-years of follow-up were accumulated beginning one year after the diagnosis of a first primary solid or lymphoid neoplasm until the second primary neoplasm diagnosis date, attainment of 85 years of age, loss to follow-up, death date, or study end (31 December 2018), whichever occurred earliest. We calculated standardized incidence ratios (SIRs) and associated 95% confidence intervals (CIs) to quantify t-HN risks after chemotherapy for a first primary malignancy compared to the specified HN risk in the general population using SEER∗Stat (version 8.3.9). Cumulative incidence of t-HNs was calculated considering other neoplasms (excluding specified t-HN) and death as competing risks.6
The distribution of t-HNs varied from that of primary HNs (supplemental Table 3). Among 1 038 828 solid tumor (mean follow-up time was 4.5 person-years) and 186 503 lymphoid neoplasm (mean follow-up time was 5.3 person-years ) 1-year survivors who received chemotherapy for their first primary neoplasm, we identified 4964 t-HNs. t-MDS/AML and t-CMML were associated with the shortest median time-to-onset after a solid tumor and lymphoid neoplasm, respectively (Table 1); t-cMPNs were associated with the longest time-to-onset after solid and lymphoid neoplasms. The poorest median survival after t-HN diagnosis was noted for t-MDS/AML, t-CMML, and tr-ALL (supplemental Table 4).
Risk of therapy-related hematopoietic neoplasms among adult 1-year survivors of first primary neoplasms treated with initial chemotherapy (with or without radiotherapy) and diagnosed between 2000 and 2017 (followed through 2018), SEER-17
| First primary neoplasm . | Subsequent therapy-related hematologic neoplasm . | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t-MDS/AML . | t-CML . | t-cMPNs . | t-CMML . | tr-ALL∗ . | ||||||||||||||||
| Solid tumors | ||||||||||||||||||||
| Median time to t-HN (y) | 3.7 | 4.8 | 5.6 | 4.1 | 4.7 | |||||||||||||||
| Lymphoid neoplasms | ||||||||||||||||||||
| Median time to t-HN (y) | 4.2 | 5.0 | 5.6 | 2.9 | 5.0 | |||||||||||||||
| Obs | % | SIR | 95%CI | Obs | % | SIR | 95%CI | Obs | % | SIR | 95%CI | Obs | % | SIR | 95%CI | Obs | % | SIR | 95%CI | |
| All solid tumors | 2616 | 63.6 | 2.76 | 2.65-2.86 | 175 | 77.4 | 1.43 | 1.23-1.66 | 234 | 82.7 | 0.72 | 0.63-0.81 | 64 | 57.7 | 1.24 | 0.96-1.58 | 144 | 62.1 | 2.43 | 2.05-2.86 |
| Oral cavity and pharynx | 105 | 2.6 | 2.22 | 1.81-2.68 | 11 | 4.9 | 1.72 | 0.86-3.07 | 16 | 5.7 | 1.05 | 0.60-1.70 | <3 | ∼ | ∼ | ∼ | 5 | 2.2 | 1.71 | 0.56-4.00 |
| Stomach | 48 | 1.2 | 2.62 | 1.93-3.48 | 3 | 1.3 | 1.50 | 0.31-4.38 | <3 | ∼ | ∼ | ∼ | <3 | ∼ | ∼ | ∼ | <3 | ∼ | ∼ | ∼ |
| Colon, excluding rectum | 123 | 3.0 | 1.03 | 0.86-1.23 | 20 | 8.8 | 1.52 | 0.93-2.35 | 35 | 12.4 | 0.99 | 0.69-1.38 | 8 | 7.2 | 1.13 | 0.49-2.22 | 7 | 3.0 | 1.20 | 0.48-2.48 |
| Rectum | 93 | 2.3 | 1.37 | 1.11-1.68 | 9 | 4.0 | 1.15 | 0.53-2.18 | 5 | 1.8 | 0.25 | 0.08-0.58 | <3 | ∼ | ∼ | ∼ | 10 | 4.3 | 2.84 | 1.36-5.22 |
| Lung/bronchus | 360 | 8.8 | 4.11 | 3.70-4.56 | 14 | 6.2 | 1.47 | 0.80-2.46 | 20 | 7.1 | 0.76 | 0.47-1.18 | 10 | 9.0 | 1.91 | 0.91-3.50 | 7 | 3.0 | 1.67 | 0.67-3.44 |
| Breast | 964 | 23.4 | 3.10 | 2.91-3.30 | 71 | 31.4 | 1.50 | 1.17-1.90 | 89 | 31.4 | 0.68 | 0.55-0.84 | 22 | 19.8 | 1.64 | 1.02-2.48 | 74 | 31.9 | 2.98 | 2.34-3.74 |
| Cervix uteri | 43 | 1.0 | 3.81 | 2.76-5.14 | <3 | ∼ | ∼ | ∼ | 6 | 2.1 | 1.24 | 0.45-2.69 | <3 | ∼ | ∼ | ∼ | 3 | 1.3 | 2.88 | 0.59-8.42 |
| Corpus uteri | 88 | 2.1 | 4.67 | 3.75-5.76 | 3 | 1.3 | 1.27 | 0.26-3.71 | 5 | 1.8 | 0.66 | 0.22-1.55 | <3 | ∼ | ∼ | ∼ | 5 | 2.2 | 4.25 | 1.38-9.92 |
| Ovary | 189 | 4.6 | 6.14 | 5.30-7.09 | 4 | 1.8 | 1.00 | 0.27-2.55 | 8 | 2.8 | 0.66 | 0.29-1.31 | <3 | ∼ | ∼ | ∼ | 5 | 2.2 | 2.39 | 0.78-5.58 |
| Urinary bladder | 125 | 3.0 | 1.37 | 1.14-1.63 | 10 | 4.4 | 1.19 | 0.57-2.18 | 22 | 7.8 | 0.98 | 0.62-1.49 | 9 | 8.1 | 1.47 | 0.67-2.80 | 4 | 1.7 | 1.15 | 0.31-2.95 |
| Lymphoid neoplasms | 1496 | 36.4 | 6.40 | 6.08-6.74 | 51 | 22.6 | 1.82 | 1.36-2.40 | 49 | 17.3 | 0.69 | 0.51-0.91 | 47 | 42.3 | 3.36 | 2.47-4.47 | 88 | 37.9 | 6.79 | 5.45-8.37 |
| Hodgkin lymphoma | 101 | 2.5 | 6.89 | 5.61-8.37 | 4 | 1.8 | 1.33 | 0.36-3.41 | 6 | 2.1 | 1.06 | 0.39-2.30 | 3 | 2.7 | 4.11 | 0.85-12.02 | 6 | 2.6 | 3.23 | 1.19-7.04 |
| NHL, excluding PCN | 1165 | 28.3 | 6.64 | 6.26-7.03 | 41 | 18.1 | 2.05 | 1.47-2.78 | 40 | 14.1 | 0.76 | 0.55-1.04 | 37 | 33.3 | 3.49 | 2.46-4.81 | 47 | 20.3 | 5.24 | 3.85-6.97 |
| DLBCL | 365 | 8.9 | 5.26 | 4.74-5.83 | 20 | 8.8 | 2.56 | 1.56-3.95 | 12 | 4.2 | 0.58 | 0.30-1.02 | 12 | 10.8 | 2.87 | 1.48-5.01 | 15 | 6.5 | 4.14 | 2.32-6.83 |
| Follicular lymphoma | 241 | 5.9 | 6.68 | 5.87-7.58 | 8 | 3.5 | 1.89 | 0.82-3.73 | 10 | 3.5 | 0.89 | 0.43-1.63 | 3 | 2.7 | 1.40 | 0.29-4.10 | 10 | 4.3 | 5.11 | 2.45-9.39 |
| PCN | 213 | 5.2 | 5.40 | 4.70-6.18 | 5 | 2.2 | 1.11 | 0.36-2.59 | 3 | 1.1 | 0.25 | 0.05-0.74 | 7 | 6.3 | 2.93 | 1.18-6.03 | 35 | 15.1 | 18.09 | 12.60-25.15 |
| First primary neoplasm . | Subsequent therapy-related hematologic neoplasm . | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t-MDS/AML . | t-CML . | t-cMPNs . | t-CMML . | tr-ALL∗ . | ||||||||||||||||
| Solid tumors | ||||||||||||||||||||
| Median time to t-HN (y) | 3.7 | 4.8 | 5.6 | 4.1 | 4.7 | |||||||||||||||
| Lymphoid neoplasms | ||||||||||||||||||||
| Median time to t-HN (y) | 4.2 | 5.0 | 5.6 | 2.9 | 5.0 | |||||||||||||||
| Obs | % | SIR | 95%CI | Obs | % | SIR | 95%CI | Obs | % | SIR | 95%CI | Obs | % | SIR | 95%CI | Obs | % | SIR | 95%CI | |
| All solid tumors | 2616 | 63.6 | 2.76 | 2.65-2.86 | 175 | 77.4 | 1.43 | 1.23-1.66 | 234 | 82.7 | 0.72 | 0.63-0.81 | 64 | 57.7 | 1.24 | 0.96-1.58 | 144 | 62.1 | 2.43 | 2.05-2.86 |
| Oral cavity and pharynx | 105 | 2.6 | 2.22 | 1.81-2.68 | 11 | 4.9 | 1.72 | 0.86-3.07 | 16 | 5.7 | 1.05 | 0.60-1.70 | <3 | ∼ | ∼ | ∼ | 5 | 2.2 | 1.71 | 0.56-4.00 |
| Stomach | 48 | 1.2 | 2.62 | 1.93-3.48 | 3 | 1.3 | 1.50 | 0.31-4.38 | <3 | ∼ | ∼ | ∼ | <3 | ∼ | ∼ | ∼ | <3 | ∼ | ∼ | ∼ |
| Colon, excluding rectum | 123 | 3.0 | 1.03 | 0.86-1.23 | 20 | 8.8 | 1.52 | 0.93-2.35 | 35 | 12.4 | 0.99 | 0.69-1.38 | 8 | 7.2 | 1.13 | 0.49-2.22 | 7 | 3.0 | 1.20 | 0.48-2.48 |
| Rectum | 93 | 2.3 | 1.37 | 1.11-1.68 | 9 | 4.0 | 1.15 | 0.53-2.18 | 5 | 1.8 | 0.25 | 0.08-0.58 | <3 | ∼ | ∼ | ∼ | 10 | 4.3 | 2.84 | 1.36-5.22 |
| Lung/bronchus | 360 | 8.8 | 4.11 | 3.70-4.56 | 14 | 6.2 | 1.47 | 0.80-2.46 | 20 | 7.1 | 0.76 | 0.47-1.18 | 10 | 9.0 | 1.91 | 0.91-3.50 | 7 | 3.0 | 1.67 | 0.67-3.44 |
| Breast | 964 | 23.4 | 3.10 | 2.91-3.30 | 71 | 31.4 | 1.50 | 1.17-1.90 | 89 | 31.4 | 0.68 | 0.55-0.84 | 22 | 19.8 | 1.64 | 1.02-2.48 | 74 | 31.9 | 2.98 | 2.34-3.74 |
| Cervix uteri | 43 | 1.0 | 3.81 | 2.76-5.14 | <3 | ∼ | ∼ | ∼ | 6 | 2.1 | 1.24 | 0.45-2.69 | <3 | ∼ | ∼ | ∼ | 3 | 1.3 | 2.88 | 0.59-8.42 |
| Corpus uteri | 88 | 2.1 | 4.67 | 3.75-5.76 | 3 | 1.3 | 1.27 | 0.26-3.71 | 5 | 1.8 | 0.66 | 0.22-1.55 | <3 | ∼ | ∼ | ∼ | 5 | 2.2 | 4.25 | 1.38-9.92 |
| Ovary | 189 | 4.6 | 6.14 | 5.30-7.09 | 4 | 1.8 | 1.00 | 0.27-2.55 | 8 | 2.8 | 0.66 | 0.29-1.31 | <3 | ∼ | ∼ | ∼ | 5 | 2.2 | 2.39 | 0.78-5.58 |
| Urinary bladder | 125 | 3.0 | 1.37 | 1.14-1.63 | 10 | 4.4 | 1.19 | 0.57-2.18 | 22 | 7.8 | 0.98 | 0.62-1.49 | 9 | 8.1 | 1.47 | 0.67-2.80 | 4 | 1.7 | 1.15 | 0.31-2.95 |
| Lymphoid neoplasms | 1496 | 36.4 | 6.40 | 6.08-6.74 | 51 | 22.6 | 1.82 | 1.36-2.40 | 49 | 17.3 | 0.69 | 0.51-0.91 | 47 | 42.3 | 3.36 | 2.47-4.47 | 88 | 37.9 | 6.79 | 5.45-8.37 |
| Hodgkin lymphoma | 101 | 2.5 | 6.89 | 5.61-8.37 | 4 | 1.8 | 1.33 | 0.36-3.41 | 6 | 2.1 | 1.06 | 0.39-2.30 | 3 | 2.7 | 4.11 | 0.85-12.02 | 6 | 2.6 | 3.23 | 1.19-7.04 |
| NHL, excluding PCN | 1165 | 28.3 | 6.64 | 6.26-7.03 | 41 | 18.1 | 2.05 | 1.47-2.78 | 40 | 14.1 | 0.76 | 0.55-1.04 | 37 | 33.3 | 3.49 | 2.46-4.81 | 47 | 20.3 | 5.24 | 3.85-6.97 |
| DLBCL | 365 | 8.9 | 5.26 | 4.74-5.83 | 20 | 8.8 | 2.56 | 1.56-3.95 | 12 | 4.2 | 0.58 | 0.30-1.02 | 12 | 10.8 | 2.87 | 1.48-5.01 | 15 | 6.5 | 4.14 | 2.32-6.83 |
| Follicular lymphoma | 241 | 5.9 | 6.68 | 5.87-7.58 | 8 | 3.5 | 1.89 | 0.82-3.73 | 10 | 3.5 | 0.89 | 0.43-1.63 | 3 | 2.7 | 1.40 | 0.29-4.10 | 10 | 4.3 | 5.11 | 2.45-9.39 |
| PCN | 213 | 5.2 | 5.40 | 4.70-6.18 | 5 | 2.2 | 1.11 | 0.36-2.59 | 3 | 1.1 | 0.25 | 0.05-0.74 | 7 | 6.3 | 2.93 | 1.18-6.03 | 35 | 15.1 | 18.09 | 12.60-25.15 |
SEER-17 includes the population-based cancer registry coverage of the areas of Atlanta, Detroit, Seattle-Puget Sound; the states of Connecticut, Hawaii, Iowa, New Mexico, California (San Francisco-Oakland, Los Angeles, San Jose-Monterey, Greater California), Georgia (Rural Georgia, Greater Georgia), Utah, Kentucky, Louisiana, and New Jersey. The table is limited to first primary sites with at least 20,000 patients who are at risk. Data for outcomes associated with <3 cases are suppressed to protect patient confidentiality. First primary pancreas and brain cancers were omitted from the table because there were fewer than 3 cases of t-CML, t-cMPN, t-CMML, and tr-ALL.
DLBCL, diffuse large B-cell lymphoma; ICD-O-3, International Classification of Diseases for Oncology, third edition; Obs, observed; NHL, non-Hodgkin lymphoma; PCN, plasma cell neoplasms; t- or tr-, therapy-related; ∼, value suppressed because the case count was <3.
For analyses of tr-ALL, all ICD-O-3 codes included in the ALL category (as specified in supplemental Table 1) and all cases of blastic plasmacytoid dendritic cell neoplasm (ICD-O-3 code 9727/3) were excluded from the first primary lymphoid neoplasms category and the respective subcategories.
Compared with the incidence of MDS/AML in the general population, the risk of t-MDS/AML was significantly increased after all solid tumors (SIR = 2.76; 95% CI, 2.65-2.86) and for all specified sites other than colon (SIR = 1.03), with substantial variation. Risk patterns were similar, albeit based on fewer cases, for tr-ALL, with 2.43-fold (95% CI, 2.05-2.86) increased risk after all solid tumors and >2-fold significantly increased SIRs after cancers of the rectum, breast, and uterine corpus. Notably, more than half of the tr-ALL cases occurred after breast cancer. The risks of t-CML and t-CMML were only modestly increased after all solid tumors (t-CML, SIR = 1.43; 95% CI, 1.23-1.66; t-CMML, SIR = 1.24; 95% CI, 0.96-1.58), with significant site-specific risks observed only after breast cancer (t-CML, SIR = 1.50; 95% CI, 1.17-1.90; t-CMML, SIR = 1.64; 95% CI, 1.02-2.48). In contrast, the risk of t-cMPN after all solid tumors was significantly decreased (SIR = 0.72; 95% CI, 0.63-0.81).
Survivors of lymphoid neoplasms had >5-fold increased risks of t-MDS/AML after Hodgkin lymphoma (HL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and plasma cell neoplasms (PCNs). Following all lymphoid neoplasms, we observed >6-fold increased risks of t-MDS/AML and tr-ALL, comparatively smaller risk increases for t-CML and t-CMML, and a deficit of t-cMPNs. In subtype-specific analyses, we identified a significantly increased risk of t-CML after DLBCL, t-CMML after both DLBCL and PCN, and tr-ALL after HL, DLBCL, FL, and most strikingly PCN (SIR = 18.09; 95% CI, 12.60-25.15).
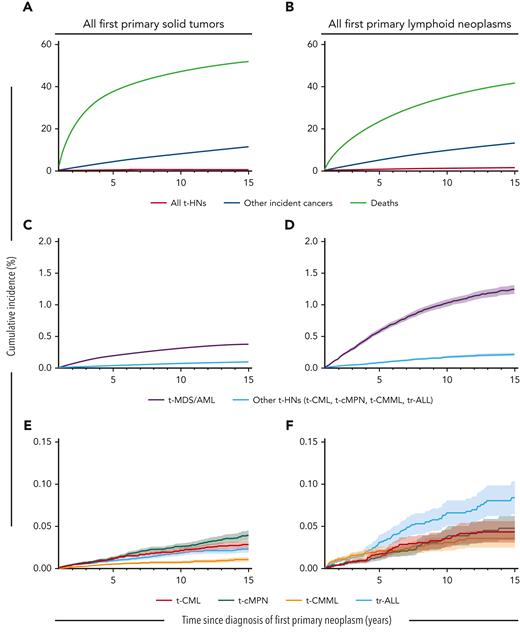
Ten years after a solid tumor or lymphoid neoplasm diagnosis, the cumulative incidence of t-MDS/AML was 0.31% and 1.02%, respectively, and for other t-HNs ranged from 0.01%-0.03% and 0.03%-0.07%, respectively. In contrast to the varied SIR patterns, absolute risks for t-CML, t-CMML, t-cMPN, and tr-ALL were all ∼10 orders of magnitude lower than those observed for t-MDS/AML, itself being a relatively rare complication of cancer chemotherapy (Figure 1; supplemental Table 5).
Cumulative incidence of therapy-related neoplasms, other incident cancers, and deaths following first primary solid tumors or lymphoid neoplasms. Cumulative incidence of (A,B) all t-HNs combined (t-MDS/AML, t-CML, t-cMPN, t-CMML, and tr-ALL), other incident cancers (excluding t-HNs), and deaths; (C,D) t-MDS/AML and other t-HNs combined (t-CML, t-cMPN, t-CMML, and tr-ALL); and (E,F) t-CML, t-cMPN, t-CMML, and tr-ALL. Number of individuals at risk at 1, 3, 5, 10, and 15 years with a first primary solid tumor: 1 038 829, 613 755, 428 193, 186 814, and 57 251, respectively; or with a first primary lymphoid neoplasm: 186 503, 129 157, 93 230, 39 532, and 11 910, respectively. Note the change in y-axis between figures.
Cumulative incidence of therapy-related neoplasms, other incident cancers, and deaths following first primary solid tumors or lymphoid neoplasms. Cumulative incidence of (A,B) all t-HNs combined (t-MDS/AML, t-CML, t-cMPN, t-CMML, and tr-ALL), other incident cancers (excluding t-HNs), and deaths; (C,D) t-MDS/AML and other t-HNs combined (t-CML, t-cMPN, t-CMML, and tr-ALL); and (E,F) t-CML, t-cMPN, t-CMML, and tr-ALL. Number of individuals at risk at 1, 3, 5, 10, and 15 years with a first primary solid tumor: 1 038 829, 613 755, 428 193, 186 814, and 57 251, respectively; or with a first primary lymphoid neoplasm: 186 503, 129 157, 93 230, 39 532, and 11 910, respectively. Note the change in y-axis between figures.
To our knowledge, this report is the first to concurrently quantify the relative and absolute risks of multiple t-HNs after first primary solid and lymphoid neoplasms overall and by age, latency, radiotherapy, and calendar year for select first primary sites with larger numbers of cases (supplemental Tables 6-9). SIRs of t-MDS/AML, t-CML, t-CMML, and tr-ALL were generally higher among younger (<50 years) than among older (≥50 years) individuals and were similarly elevated across latency periods, calendar years, and receipt of initial radiotherapy, after a first primary cancer diagnosis. t-cMPN deficits were noted across age, latency, calendar year, and radiotherapy groups.
tr-ALL has been reported as a rare complication of genotoxic therapy that is clinically and genomically distinct from de novo ALL.7-11 The risk pattern for tr-ALL after initial chemotherapy for solid and lymphoid neoplasms was generally similar to that for t-MDS/AML, although the burden of tr-ALL was substantially lower than that of t-MDS/AML. This similarity with t-MDS/AML suggests a role for chemotherapy in tr-ALL development after solid and lymphoid neoplasms, although a shared clonal relationship, leukemic transformation, and/or diagnostic misclassification may contribute as well.12,13 Notably, case reports of tr-ALL after the treatment of PCN and lymphoid neoplasms with maintenance lenalidomide require further investigation,12,14 particularly with lenalidomide implicated in promoting therapy-related myeloid neoplasms.15
t-CML studies have reported the presence of Philadelphia chromosome or BCR/ABL1 fusion protein, with a minority of cases having additional cytogenetic abnormalities.16-18 Epidemiologic studies quantifying t-CML risks are sparse, and several are based on <5 t-CML cases after chemotherapy exposure.19-21 There is also a paucity of data quantifying t-CMML risks after chemotherapy exposure. Possibly because of its rarity, t-CMML has largely been reported in case series.22,23 Compared with de novo CMML, a significantly higher proportion of intermediate- and high-risk cytogenetics has been suggested in some,23 but not all, reports of t-CMML.22 Nevertheless, our findings support a role for chemotherapy in t-CML and possibly t-CMML.
Risks of t-cMPN after chemotherapy exposure similarly have not been quantified previously. Our finding of significantly decreased t-cMPN risks suggests no association with prior chemotherapy, but a need for longer follow-up. Possible underreporting of cMPNs to cancer registries must also be considered.24
Despite more than 1 million patients exposed to chemotherapy and >4900 t-HNs, limitations include the relatively small numbers of cases by t-HN type in site-specific analyses. Additionally, SEER lacks a centralized pathology review, detailed information on cytogenetics or molecular studies, and data on chemotherapy agents or doses. Furthermore, we cannot exclude potential effects from radiotherapy, immunosuppression, clonal evolution or expansion of pre-existing malignant clones, genetic susceptibility, or other factors on t-HN risks.25
In summary, adult, chemotherapy-exposed, 1-year cancer survivors are at a risk for tr-ALL, t-CML, and t-CMML, although the absolute risk for each is orders of magnitude lower than that for t-MDS/AML. Ensuring the adequate capture of cMPNs and longer patient follow-up may shed light on the potential role of antecedent chemotherapy exposure. However, the risk patterns we observed across t-CML, tr-ALL, and possibly t-CMML, suggest that these are rare treatment-related events that should be considered as part of the spectrum of t-HNs among adult cancer survivors.
Acknowledgments
The authors thank Jeremy Miller (Information Management Services, Inc, Rockville, MD) for computing support. This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute, National Institutes of Health, Bethesda, MD.
Authorship
Contribution: G.M.D. drafted manuscript; L.M.M. financially supported the work; and all authors were responsible for conception and design, data analysis and interpretation, and the critical review, revision, and final approval of manuscript.
Conflict-of-interest disclosure:The authors declare no competing financial interests.
Correspondence: Graça M. Dores, Radiation Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, 9609 Medical Center Dr, Bethesda, MD 20852; e-mail: doresg@mail.nih.gov.
References
Author notes
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal