In this issue of Blood, El-Mansi et al have used proximity biotinylation proteomics, together with a high-throughput dual loss-of-function screening, to find new factors involved in Weibel-Palade body (WPB) biology.1 Via the experimental setup designed to focus on the events that take place around WPBs during secretion, their study provides a new inventory of potential regulators of WPB exocytosis and identifies several new components that contribute to WPB cargo expulsion.
WPBs are endothelial cell–specific secretory organelles that, together with a long list of inflammatory and angiogenic mediators, store the hemostatic protein von Willebrand factor (VWF) as their main cargo.2 In response to vascular injury, WPBs are rapidly deployed to the vascular lumen, where they deliver their cocktail of procoagulant and vasoactive mediators, including the release of long platelet-adhesive strings of VWF multimers. Balanced VWF release is important, as shown by the pivotal role of VWF: low levels of VWF can lead to a bleeding tendency, such as in von Willebrand disease, and elevated levels of VWF are associated with an increased risk for thrombosis and associated cardiovascular disease.3
In the past, interactomic screens based on affinity purification mass spectrometry have been used to identify new components of the WPB exocytic machinery. But an important limitation of earlier work was the focus on only interactors of previously identified regulators and the requirement that the interactions survive the pull-down purification procedure; thus, weak and transient partnerships were missed. Proximity biotinylation does not depend on protein–protein interactions but rather uses the enzymatic activity of a biotin ligase-tag to biotinylate proteins surrounding the tagged protein of interest. The list of biotinylated proteins thus obtained is a log list of all the proteins that have been in the immediate vicinity, not all of which were in physical contact but are nevertheless potentially relevant for the biological process of interest.
For their screen for new exocytotic regulators, El-Mansi et al tagged the biotin ligase APEX2 to Rab27A, a small Rab GTPase that is recruited to mature WPBs and thereby focuses APEX2 biotinylation activity in a small zone around these organelles. The same APEX2 Rab27A–based strategy has been used before by Holthenrich and coworkers in resting endothelial cells, which identified Munc13-2 as a novel WPB-associated regulator of secretion.4 Notably, that study identified many proteins that are present on organelles other than WPBs—which probably reflects the rubbing up of WPBs against other organelles as they make their way through the cell—but lacked many of the known late-phase mediators of WPB exocytosis, such as SNARE proteins.5
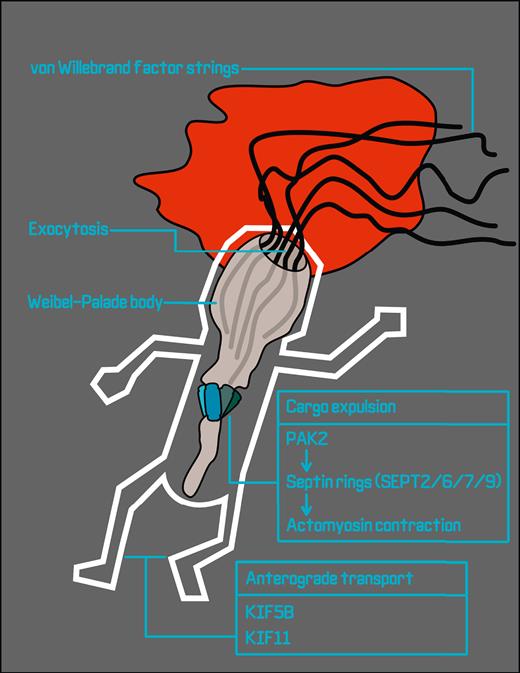
To specifically capture exocytotic regulators, El-Mansi et al took a forensic approach that combined APEX2–Rab27A proximity proteomes from endothelial cells in resting conditions with those from cells stimulated with agonists that induce WPB exocytosis. This cunning plan generates a snapshot of biotinylated proteins around WPBs during exocytosis, akin to a recording of a crime scene with perpetrators, accomplices, witnesses, and other bystanders all on tape. The gathered evidence implicates proteins and pathways caught in the act of exocytosis, such as microtubule motors that are involved in moving WPBs into the crime scene, SNARE proteins that pull the trigger on membrane fusion, and regulators of the actin cytoskeleton that keep WPBs in a headlock during release (see figure). The resulting list represents a major leap toward an understanding of the regulation of distinct phases of WPB exocytosis.
Proximity biotinylation proteomics in stimulated endothelial cells generates a snapshot of the proteins that take part in WPB exocytosis and VWF string release. Candidate plus-end directed motor proteins for anterograde transport of WPBs over microtubules that are identified in this study are kinesins KIF5B and KIF11. Following WPB fusion, p21-activated kinase PAK2 initiates the formation of septin rings consisting of heteroduplexes of SEPT2, SEPT6, SEPT7, and SEPT9, leading to the formation of contractile actomyosin rings that squeeze VWF out of the postfusion WPB.
Proximity biotinylation proteomics in stimulated endothelial cells generates a snapshot of the proteins that take part in WPB exocytosis and VWF string release. Candidate plus-end directed motor proteins for anterograde transport of WPBs over microtubules that are identified in this study are kinesins KIF5B and KIF11. Following WPB fusion, p21-activated kinase PAK2 initiates the formation of septin rings consisting of heteroduplexes of SEPT2, SEPT6, SEPT7, and SEPT9, leading to the formation of contractile actomyosin rings that squeeze VWF out of the postfusion WPB.
A class of proteins that so far had remained elusive are the plus-end directed motor proteins that are responsible for anterograde transport of WPBs along the microtubule cytoskeleton. From their screen in resting endothelial cells, the authors identify the kinesin heavy chain KIF5B, a novel component of WPBs that appears remarkably concentrated at one end of the organelle at contact points with microtubule filaments. A particularly interesting observation from their data sets is that the kinesins that were identified differed in the unstimulated (KIF5B) vs the agonist-induced conditions (KIF11), suggesting that during the final stages of the WPB lifecycle, a dynamic exchange of distinct kinesins occurs that may have specialized contributions to the exocytotic process. Whether, and exactly how, these or other kinesins participate in steady-state WPB transport or fast mobilization of WPBs during exocytosis cannot yet be determined, but the proteomic data sets reported here will help in directing future studies on this topic.
VWF is densely packed in tubules that are arranged in parallel along the length of the WPB; the VWF tubules unwind into long strings as they emerge from the fusing WPB. The high state of condensation and large size of VWF (in the range of tens of MDa per multimer) represent significant hurdles to efficient release and may require an extra push to get out. Contractile rings consisting of actomyosin can form around fused WPBs and contribute to the expulsion of VWF into strings,6 but an actomyosin-independent mechanism for VWF expulsion also operates on a subsecond timescale well before the appearance of actomyosin rings.7 The relative contributions of each of the 2 pathways to VWF release have yet to be determined, and conceivably, they could work in tandem on the same fusing granule. El-Mansi and coworkers identify several new components of the actomyosin ring pathway that put the squeeze on VWF, including p21-activated kinase 21 (PAK2) and members of the septin protein family. They show that upon activation by PAK2, heteroduplexes consisting of 4 different septins (SEPT2, SEPT6, SEPT7, and SEPT9) can arrange themselves in a ring around WPBs. This ring forms the starting point for the formation of a contractile actomyosin ring that, as it tightens its grip on the granule, wrings out the remaining VWF. Genetic as well as pharmacologic silencing of this pathway does not affect the rate of WPB exocytosis, but it does result in diminished VWF secretion, as well as fewer and shorter VWF strings. The data of El-Mansi et al further emphasize the relevance of the actomyosin ring pathway for VWF expulsion. These data also raise the question of whether platelet alpha-granules, which similarly contain tubules of highly multimeric VWF,8,9 also require septins and actomyosin contractile rings for efficient release of VWF upon platelet activation.10
In conclusion, the elegant crime scene investigations by El-Mansi et al have very successfully identified a number of (usual and unusual) suspects that beyond any reasonable doubt are contributing to the intriguing regulation and content-expulsion of a crucial bio-reservoir for inflammatory and hemostatic cargo in the vasculature.
Conflict-of-interest disclosure: J.V. is listed as an inventor on a patent application describing novel ADAMTS13 variants. R.B. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal