In this issue of Blood, Pecquet et al1 report that hematopoietic cells with a mutation in calreticulin (CALR) secrete a soluble form of the protein that acts in paracrine fashion to enhance the growth of surrounding tumor cells.
The BCR-ABL-negative myeloproliferative neoplasms (MPNs) are caused by mutations in the kinase JAK2, the thrombopoietin receptor MPL (also known as TpoR), and the endoplasmic reticulum (ER) chaperone CALR.2 Although it was readily apparent that JAK2 and MPL mutations promote cytokine independent growth through activation of the JAK/STAT signaling pathway, how mutations in CALR contribute to the MPNs was not obvious. CALR is involved in the quality control of newly synthesized proteins and glycoproteins that prevent misfolded proteins from leaving the ER prematurely. CALR is also involved in the regulation of intracellular calcium levels, integrin signaling, and loading of antigens onto the major histocompatibility complex. CALR can be found on the cell surface where it initiates prophagocytic signals and mediates immunostimulatory effects. Mutations of CALR in the MPNs result in an altered C terminus that selectively binds TpoR in the ER and leads to its activation independent of its ligand thrombopoietin.3 Mutant CALR is then transported to the cell surface along with TpoR, where it leads to JAK/STAT pathway activation and promotes malignant growth. Glycosylation of TpoR is necessary for the CALR binding through its lectin binding domain, and this former modification was recently shown to be a therapeutic vulnerability in CALR mutant MPNs.4 In addition to binding TpoR, previous reports showed that mutant CALR is also found as a soluble form.5-7
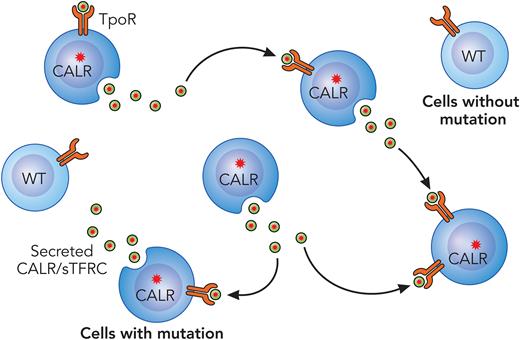
Pecquet et al sought to understand the biological function of soluble mutant CALR in MPNs. They began by demonstrating that MPN patients with CALR mutations have elevated levels of soluble mutant CALR in the plasma and that these levels correlate with the mutational burden. Next, by immunoelectron microscopy, they demonstrated that mutant CALR follows a secretory pathway, as it was detected in the Golgi apparatus and plasma membrane, whereas wild-type CALR was mainly localized to the ER. Further analysis demonstrated that the secretion of mutant CALR is independent of TpoR expression, since mice lacking TpoR had comparable levels of soluble mutant CALR in the plasma to that of control mice. The authors then generated recombinant mutant CALR protein to investigate whether it could act as a stimulatory factor in hematopoietic cells. They discovered that the recombinant protein has a half-life 10-fold shorter compared with mutant CALR isolated from the plasma of MPN patients. This led them to investigate the factors that increased mutant CALR stability in the plasma and to their findings that transferrin receptor 1 (TFRC) forms a complex with mutant CALR and that adding recombinant TFRC increases its half-life in vitro. Furthermore, the authors observed that mutant CALR and TFRC colocalize in intracellular vesicles and that mutant CALR increases the cleavage of TFRC, the soluble form. Next, by using Nano-BRET, they showed that exogenous soluble mutant CALR can bind to TpoR on the cell surface and induce JAK/STAT pathway activation. The major advance in the Pecquet et al study is the finding that secreted mutant CALR acts as a “rogue cytokine” to enhance the megakaryocytic differentiation of MPN progenitors (see figure). Intriguingly, the exogenous mutant CALR had a selective effect on hematopoietic cells also harboring the CALR mutation.
CALR acts as a rogue cytokine in the MPNs. Mutant CALR protein is secreted from CALR mutant MPN cells where it binds the TpoR of surrounding cells to enhance downstream JAK/STAT activation. The mutant protein has a much lesser effect on wild-type (WT) hematopoietic cells. Note that it is also possible that this secreted CALR rogue cytokine may also act in an autocrine fashion. sTFRC, soluble TFRC. Professional illustration by Patrick Lane, ScEYEnce Studios.
CALR acts as a rogue cytokine in the MPNs. Mutant CALR protein is secreted from CALR mutant MPN cells where it binds the TpoR of surrounding cells to enhance downstream JAK/STAT activation. The mutant protein has a much lesser effect on wild-type (WT) hematopoietic cells. Note that it is also possible that this secreted CALR rogue cytokine may also act in an autocrine fashion. sTFRC, soluble TFRC. Professional illustration by Patrick Lane, ScEYEnce Studios.
An important unresolved question is the degree to which the secreted CALR protein contributes to the disease. Although CALR in the plasma is readily detectable and correlates with allele burden, how much this drives expansion of the tumor cell population is not clear. Studies to impair its secretion in an in vivo setting would shed light on this point. Another question is why the CALR cytokine selectively promotes growth and megakaryocytic differentiation of CALR mutant progenitors but not healthy hematopoietic cells that express TpoR. The authors found that mutant CALR binds the transferrin receptor, which mediates iron uptake. Previous reports have shown that low iron levels promote megakaryopoiesis,8 raising the possibility that modulation of iron homeostasis, together with TpoR activation, contributes to the prominent effect of the CALR mutant on megakaryocytes. Finally, how much of the activity involves autocrine signaling where the CALR mutant cytokine reinforces enhanced JAK/STAT activation in the secreting cell itself is unclear.
The observation that secreted mutant CALR has a positive effect on growth of MPN cells raises the possibility that targeting this factor in the microenvironment may ameliorate the disease. Consistent with this prediction, a recent study demonstrated that treatment of Calr mutant mice with an antibody targeting CALR mutant protein decreased circulating platelet counts.9 Another recent study reported that secreted CALR has an immunosuppressive effect, providing further rationale for targeting this protein.10
In summary, the Pecquet et al study provides exciting new insights into the pathogenesis of CALR mutant MPNs and supports targeting CALR as a therapeutic axis in this malignancy. This discovery therefore represents a major advance in our understanding of how mutations in CALR contribute to the MPNs.
Conflict-of-interest disclosure: J.D.C. is a member of the SAB of Alethiomics and a consultant for Cellarity. J.M.-C. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal