In this issue of Blood, Caiado et al1 present a novel irradiation- and transplantation-free model of Tet2-mutant clonal hematopoiesis (CH) and demonstrate age-associated interleukin-1 (IL-1)/IL-1 receptor (IL-1R) signaling as a therapeutically targetable driver of clonal expansion.
CH is a common, age-associated phenomenon where somatic mutations in hematopoietic stem and progenitor cells (HSPCs) impart a selective advantage and expansion of mutant progeny in blood.2 When the mutant allele fraction reaches at least 2%, the condition is defined as CH of indeterminate potential (CHIP). The main drivers of CH are loss-of-function heterozygous mutations in epigenetic regulators, such as TET2 and DNMT3A, and these alter the immune effector functions of cells like monocytes/macrophages and lymphocytes.3 Understanding and targeting the factors that drive clonal expansion in CH offers the tremendous potential to mitigate the risk of hematological cancers and a growing list of infectious and inflammatory diseases.3
Although mutation of the CH driver gene, TET2, imparts HSPC with cell-intrinsic advantages of increased self-renewal, repopulation capacity, and myelomonocytic proliferation, the cell-extrinsic influence of the “inflammaging” milieu on CH clones has been increasingly recognized.2 Given the role of IL-1 in these processes, Caiado et al tested the hypothesis that an age-associated increase in IL-1 and signaling through one of its key signaling receptors, IL-1R, were critical in driving TET2-mutant CH cell expansion.
Caiado et al employed an ingenious inducible Tet2+/− murine model of unperturbed aging. Their “triple transgenic” system restricted inducible Cre recombinase expression to HSPC using the Scl gene enhancer and an estrogen receptor ligand (tamoxifen [TAM]). To best mimic human CH, in which TET2 mutations are usually heterozygous, the transgenic system excised a single murine Tet2 allele (Tet2+/−) and activated a fluorescent reporter (ZsG). Control mice had intact wild-type (WT) Tet2 but inducible ZsG. The authors confirmed that this new system leads to a TAM dose-dependent, hematopoietic-inducible, and traceable chimeric model to study Tet2-mutant CH. This model circumvents the requirement for irradiation, which may also induce transient confounding inflammation, or niche limitations imposed by adoptive transfer of HSPC to nonirradiated mice and presents an opportunity to advance mechanistic and interventional studies of CH expansion by modulating genetic drivers and cell-extrinsic factors.
With a single TAM injection, the investigators induced 10% Tet2+/− clonal HSPC fractions in mice. These Tet2+/− HSPC demonstrated significantly higher expansion and multilineage differentiation, starting at ∼8 months post-TAM administration and continuing up to 28 months (about a twofold increase overall), vs WT. Consistent with previous studies, they found increased proinflammatory cytokine expression in the Tet2+/− total bone marrow (BM) and myeloid cells from aged mice, including IL-1α/β.
Given the age-dependent competitive fitness of Tet2+/− hematopoiesis over WT, the authors tested whether IL-1 signaling contributed to preferential mutant expansion using complementary approaches. First, they generated younger mice with 10% WT ZsG+ control or 10% Tet2+/− ZsG+ hematopoiesis and found that a 14-day exposure to IL-1α led to significant expansion only for Tet2+/−, occurring among multiple HSPC types and in mature myeloid, T, and B cells. Second, the results were not dependent on their novel triple transgenic system, as similar selective expansion of Tet2+/− over WT cells was observed in a standard model of lethally irradiated and chimeric BM-transplanted mice and after 14 days of IL-1α exposure. Third, using the same BM transplantation model with or without homozygous genetic deletion of Il1r1−/−, they observed no expansion of Tet2+/−; Il1r1−/− double-mutant cells after IL-1α exposure. Fourth, using the triple transgenic system, IL-1R antagonist treatment led to a statistically significant, ∼40% relative reduction of Tet2+/− expansion in peripheral blood and BM cells. Taken together, these results indicate that the IL-1α/IL-1R axis directly contributes to the clonal fitness advantage of Tet2+/− hematopoietic cells.
Mechanistic studies revealed increased proliferative activity of Tet2+/− HSPC over WT under chronic IL-1α exposure, associated with increased self-renewal capacity and differentiating divisions. These functional differences were reflected in the transcriptome of Tet2+/− HSPC, where IL-1α exposure resulted in an enrichment of gene expression related to DNA replication/repair and cell cycle progression, and greater resistance of Tet2+/− to the suppressive effects of IL-1α on self-renewal than WT.
In summary, this elegant murine study advances the understanding of CH-genotype specific regulation and dependency on the inflammatory environment of aging. These findings align with those of Bick et al,4 in which increased circulating IL-1b levels were associated with TET2-mutant human CHIP, and a recently published study5 also showed that genetic Il-1r1 loss could rescue mutant Tet2-associated HSPC abnormalities and immune perturbations. Resistance to chronic inflammation may also underpin the fitness advantage of other genetic drivers of CH. Age-associated tumor necrosis factor α (TNFα) and interferon gamma signaling promote the selective expansion of murine Dnmt3a-mutant HSPC, mediated by TNF-receptor 16 and Tnxip,7 respective candidate therapeutic targets to modulate DNMT3A-mutant CH expansion. Similarly, nr4a1 mediates resistance to inflammation and enhances the fitness of asxl1-mutant zebrafish HSPC.8
It remains to be determined whether breaking the cycle of CH and inflammation (see figure) will prove to be effective in controlling CH clones and clinical outcomes in humans. Patients with previous myocardial infarction and TET2-mutant CHIP may experience less adverse cardiac events when treated with an anti–IL-1β antibody.9 Another study involving 3 macaque nonhuman primates with established TET2, DNMT3A, and ASXL1 CH clones treated with a monoclonal antibody that blocks IL-6 signaling found a specific slowing of TET2-mutant clones, although the growth rate rebounded following treatment discontinuation.10
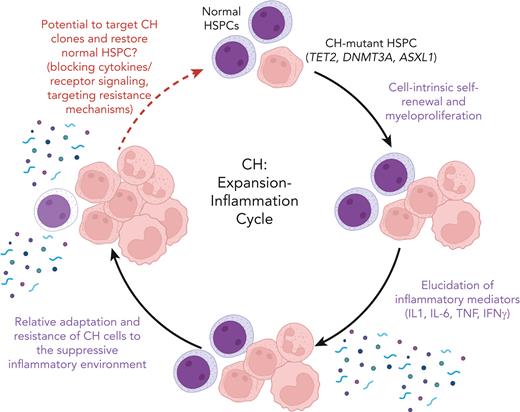
The CH expansion-inflammation cycle. Age-associated CH is most commonly driven by somatic mutations in HSPCs, involving the epigenetic regulator genes TET2, DNMT3A, and ASXL1 (top). Enhanced fitness imparted by increased self-renewal leads to CH-mutant HSPC expansion and myeloproliferation (right). In turn, and in the context of aging, this leads to increased and chronic expression of proinflammatory cytokines and other mediators that alter the hematopoietic milieu (bottom). While this environment suppresses normal HSPC, gene expression and other adaptations in CH-mutant HPSC lead to a vicious cycle of enhanced CH cell fitness and inflammation (left). This environment may increase the risk of cancer and exacerbate diseases associated with inflammation. The findings of Caiado et al in this issue of Blood (and other recent publications) suggest that targeting inflammatory cytokines, associated receptors, signaling, or other adaptive mechanisms may break this vicious CH cycle and decrease the risk of cancer and comorbid inflammatory diseases. Figure created with BioRender. IFN-γ, interferon gamma; TNF, tumor necrosis factor.
The CH expansion-inflammation cycle. Age-associated CH is most commonly driven by somatic mutations in HSPCs, involving the epigenetic regulator genes TET2, DNMT3A, and ASXL1 (top). Enhanced fitness imparted by increased self-renewal leads to CH-mutant HSPC expansion and myeloproliferation (right). In turn, and in the context of aging, this leads to increased and chronic expression of proinflammatory cytokines and other mediators that alter the hematopoietic milieu (bottom). While this environment suppresses normal HSPC, gene expression and other adaptations in CH-mutant HPSC lead to a vicious cycle of enhanced CH cell fitness and inflammation (left). This environment may increase the risk of cancer and exacerbate diseases associated with inflammation. The findings of Caiado et al in this issue of Blood (and other recent publications) suggest that targeting inflammatory cytokines, associated receptors, signaling, or other adaptive mechanisms may break this vicious CH cycle and decrease the risk of cancer and comorbid inflammatory diseases. Figure created with BioRender. IFN-γ, interferon gamma; TNF, tumor necrosis factor.
The next important steps are to examine the effect of anti-inflammatory therapy on CH clonal dynamics in humans. Longitudinal cohorts offered anti-inflammatory therapy as part of the standard of care for inflammatory diseases, with serial blood sampling, will permit the analysis of changes in CH clones. It is also unclear whether anti-inflammatory or other therapies will be effective in controlling established clones, better suited to prevent clone emergence, or if caution is necessary to prevent the outgrowth of other or resistant mutants. These are important considerations for future prospective CH studies and clinical trials, but the findings of Caiado et al and others offer promising targets for breaking the vicious inflammation-expansion cycle and decreasing the risk of cancer and CH-associated comorbidities.
Conflict-of-interest disclosure: The author declares no competing financial interest.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal