In this issue of Blood, Abdullahi et al1 provide evidence that both fixed low- and moderate-dose daily hydroxyurea regimens, given instead of chronic transfusion therapy, are effective for secondary stroke prevention for patients with sickle cell anemia living in a low-resource setting.
The natural history of untreated sickle cell anemia is well known. Without screening programs and disease-modifying therapy, approximately 11% of children and young adults with sickle cell anemia suffer a clinically overt stroke by the age of 20 years.2 This greatly increased risk continues into the adult years.3 Without secondary prevention, overt stroke will recur in 50% to 90% of patients,4 mostly in the first 3 years after the initial stroke. In high-resource settings, the standard of care for secondary stroke prevention is exchange transfusion at the time of overt stroke followed by ongoing transfusion therapy.5
In much of sub-Saharan Africa, however, where sickle cell anemia is most prevalent, access to ongoing transfusion therapy is severely constrained by cost, safety, availability of chelation therapy, and infrastructure and further limited by the lack of acceptability of long-term use of blood.6 To identify a feasible alternative therapy for children with sickle cell anemia in the state of Kano, Nigeria, Abdullahi et al tested hydroxyurea for secondary stroke prevention. They performed a randomized, double-blind superiority trial comparing 2 fixed-dose regimens of hydroxyurea: low dose (10 mg/kg per day) and moderate dose (20 mg/kg per day). A single exchange transfusion was performed at the time of first overt stroke; no ongoing transfusion therapy was planned. Hydroxyurea was initiated within 30 days of the stroke. The investigators hypothesized that moderate-dose therapy would confer an 80% reduction in the primary outcome compared with low-dose therapy.
The investigators rightly deemed a placebo control to be unethical. They also chose not to study a regimen of hydroxyurea given at maximum tolerated dose, because they believed this regimen would not be sustainable after cessation of the trial owing to the financial burden on families. Again, owing to high cost and lack of availability, neuroimaging was not done. Instead, the investigators performed standardized examinations using the Pediatric National Institutes of Health Stroke Scale that were recorded on video and reviewed by central neurologists for adjudication.
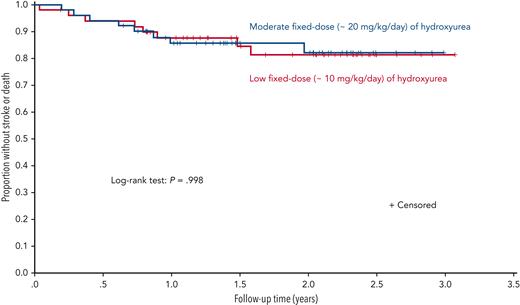
As initially designed, 60 participants (30 per arm) were to be randomized, with a planned minimum follow-up of 3 years. The sample size was increased to a maximum of 120 after the trial began when additional funding became available. The primary outcome was a composite measure of recurrent overt stroke, transient ischemic attack (TIA), and death. After randomization of 101 participants, the trial was stopped early for futility following an unplanned interim analysis. With a median follow-up of 1.6 years, the incidence rate ratio for the primary outcome measure was 0.98 (95% confidence interval, 0.32 to 3.00; P = .97; see figure). When considered separately, the rates of stroke and death were also not different. Adherence was high in both groups, and no participant had treatment interrupted for myelosuppression.
Survival analysis comparing fixed low-dose and moderate-dose regimens of hydroxyurea for secondary stroke prevention. The proportion of participants without recurrent stroke, TIA, or death was not different between groups at a median follow-up of 1.6 years (interquartile range, 1.0-2.3). See Figure 2 in the article by Abdullahi et al that begins on page 825.
Survival analysis comparing fixed low-dose and moderate-dose regimens of hydroxyurea for secondary stroke prevention. The proportion of participants without recurrent stroke, TIA, or death was not different between groups at a median follow-up of 1.6 years (interquartile range, 1.0-2.3). See Figure 2 in the article by Abdullahi et al that begins on page 825.
The most tenable conclusion from the trial is that moderate-dose hydroxyurea is not superior (risk reduction not ≥80%) to low-dose hydroxyurea for secondary prevention of neurologic events (stroke and TIA) and death in this short interval. This narrow interpretation is unsatisfying clinically, and we reflexively ask other questions: if moderate dose is not superior, is low dose just as good or at least not worse, and would the findings be different over a longer period of study? This superiority trial was not designed in advance to test equivalence or non-inferiority of the low-dose treatment. So, although the incidence of recurrent stroke was not different between groups, we cannot properly conclude that low-dose hydroxyurea is equivalent or non-inferior to moderate-dose hydroxyurea. However, the authors reasoned that the rates of recurrent stroke in both treatment arms were much lower than the expected rate without treatment obtained from pooled, historical data (6 to 7 vs 29 events per 100 person-years). Moreover, when additional data from a longitudinal follow-up study giving a total median follow-up of approximately 4 years were used, the main findings were unchanged. The investigators concluded that both regimens were efficacious for secondary stroke prevention and that a minimum known effective regimen is 10 mg/kg per day.
Commendably, Abdullahi et al also undertook 3 strategies to increase the likelihood of sustainability of their secondary stroke prevention programs after completion of the trial. First, they identified a Nigeria-based pharmaceutical corporation to provide hydroxyurea at a subsidized cost ($0.16 per day). Second, they successfully lobbied governmental authorities to pay for hydroxyurea produced in Nigeria for stroke prevention. Third, they partnered with local officials to establish state-sponsored stroke prevention teams at 3 Kano hospitals that follow over 20 000 children with sickle cell anemia. Given the findings of the trial, the investigators argued that the low-dose regimen would be preferable in this context because it could treat twice as many children for the same cost as the moderate-dose regimen.
Thinking broadly: what about other outcomes? This trial tested a composite of clinically overt acute neurologic events (stroke and TIA) and death but not other acute and chronic complications of sickle cell anemia. In a separate trial, the same core investigative team also studied low- and moderate-dose hydroxyurea therapy for primary stroke prophylaxis.7 Similarly, the rates of first stroke were not different between groups; however, there were lower rates of inpatient vaso-occlusive episodes and outpatient acute painful events with moderate-dose therapy. Would a cost-effectiveness argument for low-dose therapy still hold if moderate-dose therapy additionally ameliorated the disease in other ways (eg, decreasing pain, hospitalizations, and organ damage)—especially when considered over a much longer period than studied here? This deserves ongoing study.
As a global sickle cell community, we all aspire to provide all patients the most broadly effective therapy given at the best doses, ideally starting early in life for primary prevention against all complications of the disease. This is not an easy task in sub-Saharan Africa, of course, as resources are still lacking, but very similar barriers have already been overcome in the global treatment of HIV, malaria, and tuberculosis. People with sickle cell anemia deserve the same ongoing coordinated efforts and successes.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal