Key Points
Both low and moderate doses of hydroxyurea are efficacious for secondary stroke prevention in low-resource settings.
The median time between the initial stroke and death was less than a year, 0.8 years (interquartile range, 0.2-0.9).
Abstract
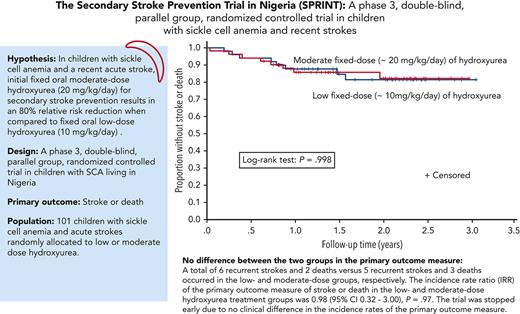
We tested the hypothesis that fixed oral moderate-dose hydroxyurea (20 mg/kg per day) for initial treatment of secondary stroke prevention results in an 80% relative risk reduction of stroke or death when compared with fixed oral low-dose hydroxyurea (10 mg/kg per day) in a phase 3 double-blind, parallel-group, randomized controlled trial in children with sickle cell anemia (SCA) living in Nigeria. A total of 101 participants were randomly allocated to low-dose (n = 49) and moderate-dose (n = 52) hydroxyurea treatment groups. The median participant follow-up was 1.6 years (interquartile range, 1.0-2.3), with a planned minimum follow-up of 3.0 years. A total of 6 recurrent strokes and 2 deaths vs 5 recurrent strokes and 3 deaths occurred in the low- and moderate-dose groups, respectively. The incidence rate ratio (IRR) of the primary outcome measure of stroke or death in the low- and moderate-dose hydroxyurea treatment groups was 0.98 (95% confidence interval [CI], 0.32-3.00; P = .97). The trial was stopped early owing to no clinical difference in the incidence rates of the primary outcome measure. The incidence rates of recurrent strokes were 7.1 and 6.0 per 100 person-years in the low- and moderate-dose groups, respectively, (IRR, 1.18; 95% CI, 0.30-4.88; P = .74). As a measure of adherence to the oral hydroxyurea therapy, the median percent of returned pills was 3.0% and 2.6% in the low- and moderate-dose groups, respectively. No participant had hydroxyurea therapy stopped for myelosuppression. For children with SCA in low-income settings without access to regular blood transfusion therapy, initial low-dose hydroxyurea is a minimum known efficacious dose for secondary stroke prevention.
Introduction
In high-income countries, the American Society of Hematology (ASH) 2020 guidelines for the prevention, diagnosis, and treatment of cerebrovascular disease in children and adults with sickle cell anemia (SCA) recommends regular blood transfusion therapy to keep the maximum hemoglobin S (HbS) level <30% and the total Hb level >9.0 g/dL for secondary stroke prevention.1 Unfortunately, blood transfusion therapy is not a feasible option for most children with SCA in sub-Saharan Africa because of the high cost of monthly blood transfusions, limited blood supply, cost of iron chelation therapy, and unsafe transfusion practices.2
To prevent secondary stroke, Nigerian pediatric investigators in the trial were at equipoise regarding the relative benefits of low- vs moderate-dose hydroxyurea therapy. Some pediatricians were in favor of the moderate-dose hydroxyurea (∼20 mg/kg per day) because this dose was used in the Stroke Prevention in Nigeria (SPIN) trial, an open-label, single-arm, primary stroke prevention feasibility trial (www.clinicaltrials.gov, #NCT01801423).3 In the SPIN trial, a total of 29 children with abnormal transcranial Doppler velocities were followed monthly with complete blood counts for 4.8 years (interquartile range [IQR], 3.7-5.6) without any myeloid toxicity that resulted in stopping therapy and its preliminary efficacy.3 Alternatively, other pediatricians believed that low-dose hydroxyurea might be beneficial because of the established benefits of low-dose hydroxyurea in decreasing vaso-occlusive events in low-income settings.4-6 No pediatrician participating in the trial was willing to consider the maximum tolerated dose (MTD) of hydroxyurea because of the associated laboratory costs for assessment of myelosuppression and the unsustainability of MTD of hydroxyurea after cessation of the randomized controlled trial due to the family financial burden. Hence, we tested the hypothesis that fixed oral moderate-dose hydroxyurea (20 mg/kg per day) for initial treatment of secondary stroke prevention results in an 80% relative risk reduction of stroke or death when compared with fixed oral low-dose hydroxyurea (10 mg/kg per day) in a phase 3, double-blind, parallel-group, randomized controlled trial in children with SCA living in Nigeria.
Study design
The SPRINT (hydroxyurea for secondary stroke prevention in children with sickle cell anemia in Nigeria) trial was conducted at Aminu Kano Teaching Hospital and Murtala Muhammed Specialist Hospital, both in Kano, Nigeria. The 2 hospitals manage over 16 000 children with SCA.7 Ethical approval for the study was obtained from the institutional review boards of Aminu Kano Teaching Hospital, the Kano State Ministry of Health, and Vanderbilt University, Nashville, TN. The National Agency for Food and Drug Administration and Control in Nigeria approved the trial. The Data and Safety Monitoring Board (DSMB) reviewed serious adverse events and study progress. All participants' guardians provided written informed consent before screening and enrollment. Children at least 7 years of age and older provided their assent.
Inclusion criteria included (1) ages 1 to 16 years, (2) high-performance liquid chromatography confirmed HbSS or HbSβ0 thalassemia, (3) acute strokes within 30 days before signing the informed consent, and (4) acceptance of hydroxyurea therapy for at least 2 years (but planned for at least 3 years). Exclusion criteria included (1) prior overt stroke (a focal neurological deficit of acute onset) occurring >30 days before study entry based on history, a focal neurological deficit on a standardized neurological examination using the Pediatric National Institutes of Health Stroke Scale (PedNIHSS) that was performed on all children at study entry,8 or concern for preexisting moderate or severe neurological deficit based on a positive "10 questions" screening;9 (2) confirmed pregnancy or considering discontinuing family planning; (3) children already on blood transfusion or hydroxyurea therapy; (4) significant baseline cytopenia (absolute neutrophil count [ANC] <1.0 × 103/μL, platelets <80 × 103/μL, Hb 8 g/dl when absolute reticulocytes <80 × 103/μL, other significant organ system dysfunction or other contraindications to hydroxyurea therapy, and history of seizures or diagnosis of epilepsy; (5) any other condition, such as malnutrition or chronic illness, which in the opinion of the site's principal investigator, makes study therapy not advisable or unsafe; (6) active infections: bacterial, viral, or fungal (tuberculosis, malaria, active hepatitis, and osteomyelitis); and (7) active chronic leg ulcers.
Research visits were scheduled monthly for participants and included a history, physical examination, and laboratory values. The trial pharmacist provided a maximum of 4 daily hydroxyurea capsules to reach the assigned mg/kg dosage of ∼10 mg/kg per day or ∼20 mg/kg per day. The hydroxyurea capsules were 500 mg, 250 mg, and 100 mg. A hydroxyurea suspension was unavailable in Nigeria at the start of the trial.
Based on the World Health Organization stroke definition, the primary end point was a recurrent clinical stroke,10 transient ischemic attacks, or death. After the Nigerian physicians decided that the participant's history and symptoms were suggestive of a stroke, the site study personnel performed and videotaped the baseline PedNIHSS. The neurology committee (L.C.J. and M.C.) reviewed the neurological examination video and case report forms of all suspected recurrent strokes, independent of group assignment.
Myelosuppression possibly related to hydroxyurea was defined as an ANC <1000 × 109/L or a platelet count <80 × 109/L and was assessed monthly. Adherence was measured with mean cell volume, hydroxyurea pill counts returned to the pharmacists monthly, and HbF levels assessed annually.
Medical monitors met weekly via videoconference with the site investigators and research team to discuss the laboratory values that fell outside the anticipated ranges based on the laboratory values of the SPRING (hydroxyurea for primary stroke prevention in children with sickle cell anaemia in Nigeria) trial.11
Except for the trial pharmacists, the statisticians, and, if needed, the medical monitor, all trial personnel, including study providers, were masked to the participant's treatment assignment. Two clinical trial pharmacists reviewed each hydroxyurea prescription as an internal quality check to ensure that the proper dose was assigned.
Sample size and statistical analysis
The original sample size goal was to randomly allocate 30 participants with an acute stroke to each treatment arm. Randomization was done by the project statistician using a permuted block allocation scheme with block sizes of 4, stratified by sex and site. Participants were assigned in a 1:1 ratio to low-dose (10 mg/kg per day) or moderate-dose (20 mg/kg per day) groups. We received research governance approval on 19 February 2018, to increase the enrollment from 60 to a maximum of 120 participants. The decision to double enrollment was not associated with increased research personnel. Thus, the focus shifted to identifying and capturing new neurological events with hospital source documentation and a video of the PedNIHSS for suspected stroke events rather than capturing all hospitalizations.
As is the case in most phase 3 clinical trials of a rare disease, estimates of the event rate required for the power analysis often have wide confidence intervals (CIs). After receiving additional funding, we elected to increase the probability that we would detect a difference in the stroke recurrence rates between the 2 treatment arms if a difference existed (power). The initial sample size of 60 corresponded to a power of 85% based on an 80% relative stroke recurrence event rate of 20.1 and 3.9 strokes per 100 person years in the low- and moderate-dose hydroxyurea therapy groups, respectively. The final planned sample size of 120 participants corresponded to 99% power with the same assumptions. The investigative team believed that low-dose hydroxyurea would benefit secondary stroke prevention because of the known association with a decrease in vaso-occlusive events.4-6 We used a conservative estimate of the stroke recurrence incidence rate in the low-dose hydroxyurea group of 20.1 strokes per 100 person-years, not 29.1 events per 100 person-years in untreated children with an initial stroke.1 We anticipated an 80% relative risk reduction (from 20.1 strokes to 3.9 strokes per 100 person-years). The DSMB ensured that stroke incidence rates were not excessive in either group compared with the expected rate throughout the trial.
The intention-to-treat principle was used to compare the primary outcome incidence rates between the 2 treatment groups. Descriptive statistics were the median and IQR for continuous data and counts and percentages for categorical data. The baseline and exit characteristics between the low- and moderate-dose groups were compared using the Mann-Whitney test for continuous variables and Pearson χ2 test for categorical variables. Cox regression models were constructed for stroke, or combined stroke or death, with predefined covariates of sex, age, total Hb, or HbF. Models included only a single covariate and treatment group because the small number of strokes or deaths precluded constructing a model with a set of covariates to predict either outcome based on baseline plausible risk factors. All tests used a 2-tailed probability, with P < .05 considered statistically significant. The SPRINT trial is registered on www.clinicaltrials.gov as #NCT02675790. To address the durability of the clinical trial findings, after completing the SPRINT trial, we formally invited all participants to an open-label stroke prevention registry (www.clinicaltrials.gov, #NCT04800809). We use data from the registry to refine estimates for the incidence rate of outcomes.
Results
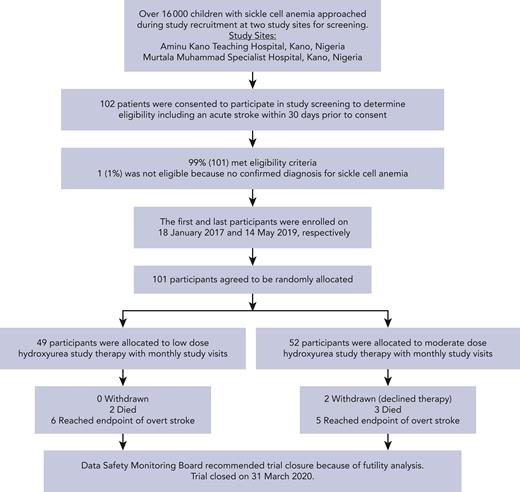
The first and last participants were enrolled on 18 January 2017 and 14 May 2019, respectively (Figure 1). None of the study participants were previously screened for primary stroke prevention with transcranial Doppler assessments.
Flow diagram for recruitment, screening, enrollment, and follow-up of fixed low- and moderate-dose hydroxyurea group participants in the SPRINT trial for secondary stroke prevention in children with SCA.
Flow diagram for recruitment, screening, enrollment, and follow-up of fixed low- and moderate-dose hydroxyurea group participants in the SPRINT trial for secondary stroke prevention in children with SCA.
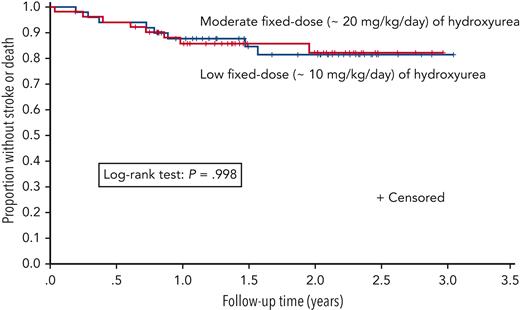
Kaplan-Meier plot of time to the primary outcome of stroke or death for fixed low-dose (n = 49) and moderate-dose (n = 52) hydroxyurea groups.
Kaplan-Meier plot of time to the primary outcome of stroke or death for fixed low-dose (n = 49) and moderate-dose (n = 52) hydroxyurea groups.
The trial was stopped early on 31 March 2020 because of the results of an analysis of the incidence rates of the primary outcome measures (stroke, transient ischemic attack, and death) in the 2 treatment arms, which was requested by the DSMB before the planned interim analysis. The median follow-up duration was 1.6 years at the end of March 2020, shortly before the time for the DSMB’s planned interim analysis, which was to include both an efficacy and, if necessary, a futility analysis. A Kaplan-Meier analysis showed no difference in the primary outcome measure of stroke or death (P = .998; Figure 2), and there was no difference in the incidence rates for the primary outcome measure in the low- and moderate-dose groups, which were 9.50 (95% CI, 4.10-18.72) and 9.68 (95% CI, 4.18-19.08) per 100 person-years, respectively, corresponding to an incidence rate ratio (IRR) of 0.98 (95% CI, 0.32-3.00; P = .97) (supplemental Table 1, available on the Blood website).
A total of 101 children were randomly assigned to low-dose (n = 49) or moderate-dose (n = 52) hydroxyurea (median age, 6.3 years [IQR, 4.1-8.0]; 45 [44.5%] females) (Table 1). The mean monthly visits were 22.4 and 21.5 in the low- and moderate-dose groups, respectively. The median time between the initial stroke and participant enrollment to start hydroxyurea was 9.0 days (IQR, 5.0-18.0).
Demographic, clinical, and laboratory features at baseline in the SPRINT trial
| Variable∗ . | Low-dose group (n = 49) . | Moderate-dose group (n = 52) . | P† . |
|---|---|---|---|
| Age at enrollment, y | 6.0 (4.1-8.0) | 6.6 (4.2-9.1) | .392 |
| Female sex | 23 (46.9) | 22 (42.3) | .640 |
| Follow-up time, y | 1.9 (1.1-2.4) | 1.5 (1.0-2.3) | .281 |
| Height, cm | 111.4 (98.3-120.6) | 112.2 (98.8-122.9) | .704 |
| Weight, kg | 16.0 (13.2-18.4) | 15.4 (12.1-19.0) | .868 |
| Body mass index, kg/m2 | 13.2 (12.1-14.1) | 13.0 (12.3-14.4) | .796 |
| Total Hb, g/dL | 7.9 (7.2-8.8) | 8.0 (6.9-8.6) | .403 |
| HbF (n = 100), % | 8.2 (4.4-15.8) | 7.7 (4.1-11.3) | .296 |
| White blood cell count, ×109/L | 14.4 (10.2-19.2) | 13.2 (10.2-17.6) | .497 |
| Platelets, ×103/μL | 469.0 (302.0-616.5) | 424.0 (351.2-536.8) | .439 |
| ANC per μL | 6594.0 (4593.2-9133.4) | 5790.7 (4429.6-8930.0) | .644 |
| Systolic blood pressure, mm Hg | 90.0 (80.0-95.0) | 90.0 (80.0-97.2) | .725 |
| MCV, fL | 84.0 (76.6-93.4) | 82.8 (77.0-87.9) | .331 |
| Variable∗ . | Low-dose group (n = 49) . | Moderate-dose group (n = 52) . | P† . |
|---|---|---|---|
| Age at enrollment, y | 6.0 (4.1-8.0) | 6.6 (4.2-9.1) | .392 |
| Female sex | 23 (46.9) | 22 (42.3) | .640 |
| Follow-up time, y | 1.9 (1.1-2.4) | 1.5 (1.0-2.3) | .281 |
| Height, cm | 111.4 (98.3-120.6) | 112.2 (98.8-122.9) | .704 |
| Weight, kg | 16.0 (13.2-18.4) | 15.4 (12.1-19.0) | .868 |
| Body mass index, kg/m2 | 13.2 (12.1-14.1) | 13.0 (12.3-14.4) | .796 |
| Total Hb, g/dL | 7.9 (7.2-8.8) | 8.0 (6.9-8.6) | .403 |
| HbF (n = 100), % | 8.2 (4.4-15.8) | 7.7 (4.1-11.3) | .296 |
| White blood cell count, ×109/L | 14.4 (10.2-19.2) | 13.2 (10.2-17.6) | .497 |
| Platelets, ×103/μL | 469.0 (302.0-616.5) | 424.0 (351.2-536.8) | .439 |
| ANC per μL | 6594.0 (4593.2-9133.4) | 5790.7 (4429.6-8930.0) | .644 |
| Systolic blood pressure, mm Hg | 90.0 (80.0-95.0) | 90.0 (80.0-97.2) | .725 |
| MCV, fL | 84.0 (76.6-93.4) | 82.8 (77.0-87.9) | .331 |
Based on the intention-to-treat analysis, participants with SCA with a previous stroke were randomly allocated to receive fixed low- (∼10 mg/kg per day) or moderate-dose (∼20 mg/kg per day) hydroxyurea and were followed for a median of 1.6 years (IQR, 1.0-2.3).
MCV, mean cell volume.
Count (percent) for categorical variables median (IQR) for continuous variables.
χ2 test for categorical variables; Mann-Whitney test for continuous variables.
The median follow-up of all participants was 1.6 years (IQR, 1.0-2.3), with a total of 166.8 person-years. During the trial, 2% (2/101) of participants withdrew; both participants were in the moderate-dose hydroxyurea group. Neither participant withdrew because of hydroxyurea-related myeloid suppression or hydroxyurea intolerance.
Strokes recurrence and mortality incidence rates were similar in low- and moderate-dose hydroxyurea treatment groups
A total of 6 and 5 strokes occurred in the low- and moderate-dose groups, respectively, with the incidence rates of recurrent strokes at 7.1 and 6.1 per 100 person-years (supplemental Table 1), respectively (IRR, 1.18; 95% CI, 0.30-4.88; P = .74). All children with stroke recurrence who presented with focal neurological deficits were managed in a hospital and received an exchange transfusion as standard care.2,12 The clinical findings associated with a stroke and laboratory values at the time of the stroke compared with baseline values are presented in Table 2.
Characteristics of therapy group participants who experienced a stroke recurrence (n = 11) or died (n = 5) during the trial
| Patient ID . | Age at enrollment, y . | Time from beginning hydroxyurea to end point, mo . | End point classification . | Description of stroke or death . | Mean dose of hydroxyurea, mg/kg per d . | Steady-state Hb, g/dL . | Hb at end point g/dL . |
|---|---|---|---|---|---|---|---|
| 1 | 5.6 | 9.5 | Stroke | Presented with acute worsening of mild left-sided weakness PedNIHSS 11. Adjudicated as a new stroke | 10.3 | 11.40 | 8.00 |
| 2 | 2.1 | 2.4 | Death | Fever with anemia | 10.0 | 7.1 | 7.1 |
| 3 | 5.5 | 3.4 | Stroke | Seizure and new left hemiparesis PedNIHSS increased from 3 to 28 | 12.1 | 7.90 | 8.00 |
| 4 | 6.0 | 19.0 | Stroke | Sudden onset of right hemiparesis and aphasia PedNIHSS increased from 3 to 14 | 11.6 | 7.00 | 7.70 |
| 5 | 4.0 | 17.8 | Stroke | Sudden worsening of baseline left hemiparesis, now requires a hand held to walk PedNIHSS increased from 4 to 6 | 13.6 | 8.30 | 9.00 |
| 6 | 5.4 | 8.8 | Stroke | Seizure followed by persistent worsening of left hemiparesis PedNIHSS increased from 5 to 8 | 11.3 | 7.48 | 7.00 |
| 7 | 6.6 | 4.5 | Stroke | Seizure followed by persistent worsening of left hemiparesis No longer has any use of left hand PedNIHSS increased from 3 to 8 | 13.2 | 10.30 | 7.00 |
| 8 | 7.4 | 10.7 | Death | Seizure Cause of death unclear | 10.1 | 6.1 | 6.9 |
| 9 | 3.8 | 7.4 | Stroke | Acute onset right hemiparesis, face, and arm with dysarthria PedNIHSS was 3 at baseline and 3 days after new event was 6 | 24.2 | 8.10 | 8.80 |
| 10 | 11.2 | 3.0 | Stroke | Presented with focal seizure and worsened right hemiparesis, PedNIHSS increased from 3 to 5 | 20.3 | 11.00 | 10.80 |
| 11 | 6.1 | 4.8 | Stroke | During a pain episode, sudden onset of new left-sided hemiparesis in a child with prior right hemiparesis PedNIHSS increased from 10 to 15 | 20.4 | 7.60 | 9.00 |
| 12 | 7.3 | 23.6 | Stroke | Fever with seizure and new left hemiparesis PedNIHSS increased from 7 to 24 | 19.8 | 8.60 | 7.90 |
| 13 | 3.0 | 8.7 | Death | Fever | 23.7 | 7.9 | 6.5 |
| 14 | 1.9 | 10.4 | Death | Acute chest syndrome | 25.0 | 6.1 | 6.0 |
| 15 | 5.8 | 11.9 | Stroke | Sudden, profound aphasia and weakness of all 4 extremities PedNIHSS was 6 at study entry due to right-sided weakness PedNIHSS worsened to 19 for right- and left-sided weakness, consistent with bilateral infarcts | 23.4 | 8.30 | 9.00 |
| 16 | 8.9 | 0.4 | Death | Acute chest syndrome | 17.7 | 7.8 | NA |
| Patient ID . | Age at enrollment, y . | Time from beginning hydroxyurea to end point, mo . | End point classification . | Description of stroke or death . | Mean dose of hydroxyurea, mg/kg per d . | Steady-state Hb, g/dL . | Hb at end point g/dL . |
|---|---|---|---|---|---|---|---|
| 1 | 5.6 | 9.5 | Stroke | Presented with acute worsening of mild left-sided weakness PedNIHSS 11. Adjudicated as a new stroke | 10.3 | 11.40 | 8.00 |
| 2 | 2.1 | 2.4 | Death | Fever with anemia | 10.0 | 7.1 | 7.1 |
| 3 | 5.5 | 3.4 | Stroke | Seizure and new left hemiparesis PedNIHSS increased from 3 to 28 | 12.1 | 7.90 | 8.00 |
| 4 | 6.0 | 19.0 | Stroke | Sudden onset of right hemiparesis and aphasia PedNIHSS increased from 3 to 14 | 11.6 | 7.00 | 7.70 |
| 5 | 4.0 | 17.8 | Stroke | Sudden worsening of baseline left hemiparesis, now requires a hand held to walk PedNIHSS increased from 4 to 6 | 13.6 | 8.30 | 9.00 |
| 6 | 5.4 | 8.8 | Stroke | Seizure followed by persistent worsening of left hemiparesis PedNIHSS increased from 5 to 8 | 11.3 | 7.48 | 7.00 |
| 7 | 6.6 | 4.5 | Stroke | Seizure followed by persistent worsening of left hemiparesis No longer has any use of left hand PedNIHSS increased from 3 to 8 | 13.2 | 10.30 | 7.00 |
| 8 | 7.4 | 10.7 | Death | Seizure Cause of death unclear | 10.1 | 6.1 | 6.9 |
| 9 | 3.8 | 7.4 | Stroke | Acute onset right hemiparesis, face, and arm with dysarthria PedNIHSS was 3 at baseline and 3 days after new event was 6 | 24.2 | 8.10 | 8.80 |
| 10 | 11.2 | 3.0 | Stroke | Presented with focal seizure and worsened right hemiparesis, PedNIHSS increased from 3 to 5 | 20.3 | 11.00 | 10.80 |
| 11 | 6.1 | 4.8 | Stroke | During a pain episode, sudden onset of new left-sided hemiparesis in a child with prior right hemiparesis PedNIHSS increased from 10 to 15 | 20.4 | 7.60 | 9.00 |
| 12 | 7.3 | 23.6 | Stroke | Fever with seizure and new left hemiparesis PedNIHSS increased from 7 to 24 | 19.8 | 8.60 | 7.90 |
| 13 | 3.0 | 8.7 | Death | Fever | 23.7 | 7.9 | 6.5 |
| 14 | 1.9 | 10.4 | Death | Acute chest syndrome | 25.0 | 6.1 | 6.0 |
| 15 | 5.8 | 11.9 | Stroke | Sudden, profound aphasia and weakness of all 4 extremities PedNIHSS was 6 at study entry due to right-sided weakness PedNIHSS worsened to 19 for right- and left-sided weakness, consistent with bilateral infarcts | 23.4 | 8.30 | 9.00 |
| 16 | 8.9 | 0.4 | Death | Acute chest syndrome | 17.7 | 7.8 | NA |
A total of 2 and 3 deaths occurred in the low- and moderate-dose groups, respectively. Death was associated with acute chest syndrome (n = 3), fever and pain (n = 1), and a seizure (n = 1). A stroke may have precipitated the participant’s death associated with a seizure; however, we could not access the child’s hospital records at a nonparticipating hospital. None of the 5 deaths were associated with hydroxyurea therapy based on the absence of prior myelosuppression. The median time between the initial stroke and death was 0.8 years (IQR, 0.2-0.9). The incidence rates of mortality in the low- and moderate-dose groups were 2.4 and 3.6 per 100 person-years, respectively (IRR, 0.66; 95% CI, 0.05-5.71; P = .49) (supplemental Table 1).
No postulated clinical risk factor was associated with stroke recurrence. Specifically, separate Cox regressions of sex, age at baseline, total baseline Hb level, HbF percentage at baseline, controlling for therapy group, showed no association (P > .05) with stroke recurrence (supplemental Table 2). A total of 36.4% and 63.6% of the strokes occurred within 6 and 12 months of the initial stroke, respectively.
A total of 94 of the 101 participants were eligible for the SPRINT follow-up registry, as 5 had died, and 2 withdrew during the trial. A total of 87.2% (82/94) of the original eligible cohort enrolled, with 41 participants from each dose group. The registry’s last day of continuous follow-up was 31 May 2022. The total follow-up time was 176.0 and 172.5 person-years in the low- and moderate-dose groups, respectively, corresponding to a median of 4.3 years (IQR, 3.5-4.6) and 3.8 years (IQR, 3.3-4.5) in the low- and moderate-dose groups, respectively. Including data from the trial and registry, 7 participants had strokes in the low- and moderate-dose groups, and 5 and 8 participants died in the low- and moderate-dose groups, respectively.
Based on the intention-to-treat principle and using the additional data from the registry, the incidence rates for the SPRINT trial’s primary outcome measure of death or stroke in the low- and moderate-dose groups are 6.82 per 100 person-years (95% CI, 3.52-11.91) and 8.70 per 100 person-years (95% CI, 4.87-14.34), respectively. The IRR of death and stroke between the 2 groups (moderate dose as the reference category) is 0.78 (95% CI, 0.34-1.79; P = .529).
Similarly, based on the additional data from the trial and registry, the incidence rates for stroke recurrence in the low- and moderate-dose group are 3.98 per 100 person-years (95% CI, 1.59-8.19) and 4.06 per 100 person years (95% CI, 1.63-8.36), with an IRR for stroke recurrence of 0.98 (95% CI, 0.33-4.12; P = .970).
Finally, based on the additional data from the registry, the incidence rates for death in the low- and moderate-dose groups are 2.84 per 100 person years (95% CI, 0.92-6.63) and 4.64 per 100 person years (95% CI, 2.00-9.14). The IRR for death is 0.61 (95% CI, 0.16-2.12; P = .390).
Hydroxyurea therapy–related myelosuppression did not occur in either treatment group
The mean prescribed doses for the low- and moderate-dose groups were 11.2 and 21.2 mg/kg per day, respectively. In the low-dose hydroxyurea group, the range of the prescribed mean dose was from 9.6 to 13.9 mg/kg per day; in the moderate-dose hydroxyurea group, the range of the prescribed mean dose was from 17.7 to 25.0 mg/kg per day. A total of 2217 monthly complete blood counts were obtained. No participant had hydroxyurea therapy stopped because of myelosuppression.
Hydroxyurea therapy adherence was high in both treatment groups
As a measure of adherence, we evaluated the magnitude, direction, and the complete blood count values. The median mean cell volume from baseline to end point increased by 4.1 and 13.0 fL in the low- and moderate-dose groups, respectively, with a higher end point value in the moderate-dose group (P = .010) (Table 3). The median mean white blood cell count decreased by 1.2 × 109/L and 3.8 × 109/L in the low- and moderate-dose groups, respectively, with a lower end point white blood cell count in the moderate-dose group (P < .001). The median ANC decreased by 576 × 109/L and 1673 × 109/L in the low- and moderate-dose groups, respectively, with a lower end point count in the moderate-dose group (P < .001). The median HbF level increased from baseline in absolute values of 2.4% and 8.1% in the low- and moderate-dose groups, respectively (P = .002). During the trial, the median percent of returned pills were 3.0% and 2.6% in the low- and moderate-dose groups, respectively (P = .76).
Laboratory measures of adherence and additional laboratory measures in the SPRINT trial
| . | Laboratory measures of hydroxyurea adherence∗ . | Additional laboratory measures∗ . | ||||
|---|---|---|---|---|---|---|
| MCV, fL . | Total Hb, g/dL . | White blood cell count, ×109/L . | ANC per μL . | Platelets, ×103/μL . | HbF (n = 93), % . | |
| Low-dose group (N = 49) | ||||||
| Baseline | 84.0 | 7.9 | 14.4 | 6594 | 469 | 8.2 |
| End point | 88.1 | 8.4 | 13.2 | 6018.7 | 376 | 10.6 |
| Moderate-dose group (N = 50) | ||||||
| Baseline | 82.8 | 8.0 | 13.2 | 5790.7 | 433 | 7.7 |
| End point | 95.8 | 8.8 | 9.4 | 4118.8 | 301.5 | 15.8 |
| P value for end points of low- vs moderate-dose groups† | .01 | .127 | <.001 | <.001 | .003 | .002 |
| . | Laboratory measures of hydroxyurea adherence∗ . | Additional laboratory measures∗ . | ||||
|---|---|---|---|---|---|---|
| MCV, fL . | Total Hb, g/dL . | White blood cell count, ×109/L . | ANC per μL . | Platelets, ×103/μL . | HbF (n = 93), % . | |
| Low-dose group (N = 49) | ||||||
| Baseline | 84.0 | 7.9 | 14.4 | 6594 | 469 | 8.2 |
| End point | 88.1 | 8.4 | 13.2 | 6018.7 | 376 | 10.6 |
| Moderate-dose group (N = 50) | ||||||
| Baseline | 82.8 | 8.0 | 13.2 | 5790.7 | 433 | 7.7 |
| End point | 95.8 | 8.8 | 9.4 | 4118.8 | 301.5 | 15.8 |
| P value for end points of low- vs moderate-dose groups† | .01 | .127 | <.001 | <.001 | .003 | .002 |
Based on the intention-to-treat analysis, participants were allocated to receive fixed low- (∼10 mg/kg per day) or moderate-dose (∼20 mg/kg per day) hydroxyurea and were followed for a median of 1.6 years (IQR, 1.0-2.3). Only those with entry and exit values are included (n = 99).
Median.
Mann-Whitney test.
Discussion
The SPRINT trial is the first randomized controlled trial demonstrating the benefits of initial hydroxyurea therapy for secondary stroke prevention in children with SCA. Compared with low-dose hydroxyurea, moderate-dose hydroxyurea had no difference in stroke recurrence rates or death. Both low- and moderate-dose treatment groups had stroke recurrence incidence rates significantly lower than the expected stroke recurrence rate from a prior pooled analysis of children with SCA not treated for secondary stroke (7.1 and 6.0 per 100 person years, respectively, vs 29.1 per 100 person-years without treatment).1
We selected a composite primary outcome measure of stroke recurrence, transient ischemic attack, and mortality because of the temporal association between strokes and death in children with SCA in Nigeria13 and the United States.14,15 In a previous secondary stroke prevention quality improvement study in Kano, we recorded 2 deaths in 29 children with SCA and overt strokes, for an overall mortality rate of 4.0 deaths per 100 person-years (95% CI, 0.7-13.3 deaths per 100 person-years).13 The short interval between the first stroke and subsequent death in the SPRINT trial confirms the results of the temporal relationship between the stroke and deaths in SCA.13,14
The temporal relationship between initial and recurrent strokes in children with SCA has been well documented. In the most extensive cohort study of children with SCA and strokes, Scothorn et al demonstrated that stroke recurrence rates were the highest in the first 24 months following regular blood transfusion therapy.16 In this retrospective cohort of 137 children with SCA followed for at least 5 years with regular blood transfusion, there was a median follow-up of 10 years and a 22% stroke recurrence prevalence. Among those with a second stroke, 35% (11/31) occurred within 24 months of the initial stroke.16 In our prospective quality improvement program at Aminu Kano Teaching Hospital, for children with an initial stroke treated with hydroxyurea to prevent a recurrence, 87% (7/8) of strokes occurred within 24 months of the initial stroke.13 Thus, regardless of treatment, a substantial proportion of children will have a stroke recurrence within 24 months of the initial stroke.
The SPRINT investigators were unwilling to propose a secondary stroke prevention trial with a placebo arm. The absence of any secondary stroke prevention was considered unethical owing to the high rate of stroke recurrence initially demonstrated in a pooled analysis17 and confirmed in the ASH central nervous system systematic review guidelines.1
In a pooled analysis, we showed a benefit of hydroxyurea when compared with no treatment (secondary stroke prevention rate, 3.8 vs 29.1 events per 100 person-years, respectively).1 Furthermore, in a quality improvement program, our team demonstrated the benefit of hydroxyurea for secondary stroke prevention compared with historical controls.13 The critical question to be addressed in a randomized controlled trial in a low-resource setting was the optimal hydroxyurea dose. Based on preliminary evidence that low-dose hydroxyurea is efficacious in preventing vaso-occlusive pain in low-to-middle–income settings,4-6 and evidence that moderate-dose hydroxyurea resulted in a significant drop in the transcranial Doppler velocity after 3 months,18 the Nigerian research team was at equipoise as to low- or moderate-dose hydroxyurea therapy for secondary stroke prevention.
The SPRINT trial has multiple strengths. To our knowledge, this is the first randomized controlled trial for secondary prevention of strokes conducted in sub-Saharan Africa, where almost 75% of all children with SCA in the world are born.19 Another strength is the evidence that low-dose hydroxyurea has clinical utility for secondary stroke prevention without the risk of myelosuppression or the requirement of laboratory surveillance beyond standard care. Furthermore, in the SPRING trial, a phase 3 randomized controlled primary stroke prevention trial in northern Nigeria, where 220 children with abnormal transcranial Doppler measurements were randomly allocated to fixed low- and moderate-dose hydroxyurea, no participant had their hydroxyurea stopped because of myelosuppression during a mean follow-up of 2.4 years.11 Based on the results of the controlled trials for primary and secondary stroke prevention, SPIN, SPRING, and SPRINT, hydroxyurea-associated myeloid suppression is unlikely to occur in children treated with low- or moderate-dose hydroxyurea. After completing all 3 trials, the Nigerian pediatricians participating in the trials now obtain complete blood counts every 6 months for children receiving low- or moderate-dose hydroxyurea therapy, as is the case for children with SCA followed in the region not treated with hydroxyurea.
The SPRINT trial has several limitations. Perhaps the most significant perceived limitation is the absence of brain imaging to confirm stroke recurrence. However, we applied the World Health Organization's stroke definition,10 which is clinical, generalizable to low-income settings, and used in National Institutes of Health–funded randomized controlled SCA stroke trials.11,20-22 To enhance the detection of strokes in the SPRINT trial, guardians were coached with written and verbal instructions to bring participants into the hospital for any acute neurological symptoms to increase the likelihood of detecting a stroke in the participants. Furthermore, the participants were evaluated with monthly clinic visits consisting of a standardized questionnaire to detect neurological impairment in children living in Africa9 and a standardized neurological examination to detect strokes.8 For suspected strokes, a video of the standardized neurological examination, the PedNIHSS, was completed and sent to Vanderbilt University Medical Center for the neurology committee to confirm a stroke recurrence. Together, these strategies significantly decreased the likelihood of an undetected and untreated stroke recurrence before every scheduled 30-day research visit during the trial. Another potential limitation is the premature cessation of the trial due to futility, with a median participant follow-up of 1.6 years compared to the planned minimum follow-up of 3.0 years for each participant.
To address the long-term efficacy of low- or moderate-dose hydroxyurea therapy for secondary stroke prevention, we initiated a stroke registry for SPRINT trial participants after completion of the trial. After a median continuous follow-up of ∼4 years, the participants in both the SPRINT trial and the prospective stroke registry continued to have an expected low stroke recurrence rate in both the low- and moderate-dose hydroxyurea groups of 3.98 and 4.06 events per 100 person-years, with an IRR of 0.98 (95% CI, 0.33-4.12; P = .970). In the ASH systematic central nervous system review, the pooled analysis of published studies revealed a stroke recurrence rate with hydroxyurea of 3.8 events per 100 peson-years.1
An additional limitation of the trial is the absence of MTD of hydroxyurea as a treatment option. However, Nigerian pediatricians deemed MTD of hydroxyurea unsustainable for most families before starting the clinical trial because of their lack of pediatric hematology expertise and the resource constraints required for MTD of hydroxyurea. In Kano, the cost of a complete blood count is $5.00 (as of March 2022), 5 times the cost of daily discretionary income of approximately $1.00 per individual in 40% of the population, roughly 83 million people in Nigeria.23 The magnitude of the poverty is accentuated because most medical expenses are self-pay in Nigeria and elsewhere in sub-Saharan Africa. Another significant challenge when initiating MTD of hydroxyurea is the observation that most children with SCA in the Kano state hospital system receive their health care from nurses without hematology training and medical officers (the equivalent of a medical student graduate without medical residency training).
After completing the trial, we undertook 3 overlapping strategies to increase the likelihood of sustainability of the secondary stroke prevention program. First, we identified a Nigerian-based pharmaceutical company (Bond Chemical, Ibadan, Nigeria) to provide hydroxyurea at a subsidized cost of $0.16 per day during and after the trial. Second, we met with the Kano state government health services leadership and the governing board. The government leaders agreed to pay for hydroxyurea produced in Nigeria for primary and secondary stroke prevention. Third, we partnered with the Kano state government officials to support state-sponsored stroke prevention teams at the 3 Kano state hospitals that follow over 20 000 children with SCA.24,25 The evidence that low-dose hydroxyurea was as protective as moderate-dose hydroxyurea directly translated into the state government, on a fixed budget, potentially preventing strokes in twice as many children with SCA for the same cost.
For children with SCA in low-income settings without access to regular blood transfusion therapy, initial low-dose hydroxyurea of ∼10 mg/kg per day is a demonstrated minimum efficacious dose for secondary stroke prevention. Low-dose hydroxyurea is a new evidence-based strategy to prevent strokes and minimize complete blood count testing for the assessment of myelosuppression.
Acknowledgments
The authors are thankful to their radiologist, Shehi Ali (now deceased), who tirelessly worked to certify physicians and nurses to conduct transcranial Doppler assessments in Nigeria. Without his effort, this trial would not have been completed. The authors are grateful to the research coordinators and study personnel who tirelessly coordinated the trial in Kano, Nigeria, and Kaduna, Nigeria. The authors appreciate Mustafa Nateqi, Khadijah Bulama, Mohammed Sani, Murtala Umar, Charity Dooshima Agba, Fahad Usman, Abdulrasheed Sani, Jamila Ibrahim, Gloria Bahago, Aisha Musa, Abdu Dambatta, Jamil Galadanci, and Jennifer Beck-Smith for facilitating the administrative tasks required for the successful conduct of this study.
This work was supported by the Thrasher Foundation and an Afolabi Family donation to Vanderbilt University Medical Center, grants from the National Institutes of Health (NIH), National Center for Advancing Translational Sciences (NCATS) (National Heart, Lung, and Blood Institute grant NHLBI K24HL147017 and NCATS grant UL1 TR000445, respectively), and the generous donation of the Phillips family.
The sponsors did not have any role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the National Institute of Health.
Authorship
Contribution: M.R.D. and M.R. had full access to all the data in the study and take responsibility for the integrity of the data and the data analyses' accuracy; M.R.D., S.U.A., M.H.A., L.C.J., and M.R. designed the trial; M.R. performed the analyses; M.R.D., S.U.A., L.C.J., and M.H.A. interpreted the results; S.U.A., L.C.J., M.H.A., M.R., and M.R.D. wrote the manuscript; and all authors reviewed the manuscript before submission.
Conflict-of-interest disclosure: M.R.D. and his institution are the sponsors of 2 externally funded research investigator-initiated projects; Global Blood Therapeutics will provide funding for these clinical studies but will not be a cosponsor of either study; he is not receiving any compensation for the conduct of these 2 investigator-initiated observational studies; he is a member of the Global Blood Therapeutics advisory board for a proposed randomized controlled trial, for which he receives compensation; he is on the steering committee for a Novartis-sponsored phase 2 trial to prevent priapism in men; he was a medical adviser for the development of the CTX001 Early Economic Model; he provided medical input on the economic model as part of an expert reference group for Vertex/CRISPR CTX001 Early Economic Model in 2020; and he provided a consultation to the Forma Pharmaceutical company about sickle cell disease from 2021 to 2022. The remaining authors declare no competing financial interests.
Correspondence: Michael R. DeBaun, Department of Pediatrics, Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Vanderbilt University Medical Center, 2525 West End Ave, Suite 750, Nashville, TN 37203-1738; e-mail: m.debaun@vumc.org.
References
Author notes
The trial’s protocol and deidentified individual participant data for the primary analysis will be available upon request until 2028, approximately 5 years after publication. After 2028, institutional resources may not be available to provide the data. Requests for data should be directed to m.debaun@vumc.org, and requestors will need to prepare and sign a data transfer agreement between Vanderbilt University Medical Center and their respective institutions.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal