Key Points
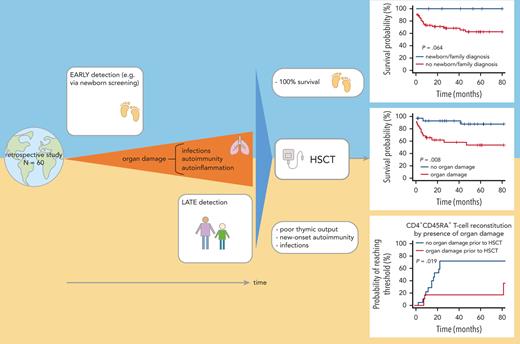
Infections, autoimmunity, and granuloma predispose to organ damage prior HSCT, thereby compromising survival and quality of immune reconstitution.
In patients with hypomorphic recombination-activating gene deficiency, HSCT with T-cell depleted grafts shows poor outcome.
Abstract
Patients with hypomorphic mutations in the RAG1 or RAG2 gene present with either Omenn syndrome or atypical combined immunodeficiency with a wide phenotypic range. Hematopoietic stem cell transplantation (HSCT) is potentially curative, but data are scarce. We report on a worldwide cohort of 60 patients with hypomorphic RAG variants who underwent HSCT, 78% of whom experienced infections (29% active at HSCT), 72% had autoimmunity, and 18% had granulomas pretransplant. These complications are frequently associated with organ damage. Eight individuals (13%) were diagnosed by newborn screening or family history. HSCT was performed at a median of 3.4 years (range 0.3-42.9 years) from matched unrelated donors, matched sibling or matched family donors, or mismatched donors in 48%, 22%, and 30% of the patients, respectively. Grafts were T-cell depleted in 15 cases (25%). Overall survival at 1 and 4 years was 77.5% and 67.5% (median follow-up of 39 months). Infection was the main cause of death. In univariable analysis, active infection, organ damage pre-HSCT, T-cell depletion of the graft, and transplant from a mismatched family donor were predictive of worse outcome, whereas organ damage and T-cell depletion remained significant in multivariable analysis (hazard ratio [HR] = 6.01, HR = 8.46, respectively). All patients diagnosed by newborn screening or family history survived. Cumulative incidences of acute and chronic graft-versus-host disease were 35% and 22%, respectively. Cumulative incidences of new-onset autoimmunity was 15%. Immune reconstitution, particularly recovery of naïve CD4+ T cells, was faster and more robust in patients transplanted before 3.5 years of age, and without organ damage. These findings support the indication for early transplantation.
Introduction
Variants in genes essential for V(D)J recombination lead to a developmental arrest of T and B lymphocytes. These genes encode components of the recombination activation complex, including the recombination-activating gene 1 (RAG1) and RAG2 proteins, and factors of the nonhomologous end-joining pathway of DNA double-strand break repair.1
Loss-of-function variants in RAG1 and RAG2 genes impede V(D)J recombination leading to severe combined immunodeficiency (SCID) characterized by absent or severely reduced T and B cells, but normal natural killer-cell numbers. In contrast, individuals harboring hypomorphic variants in the RAG genes usually present as either Omenn syndrome, or atypical combined immunodeficiency (CID) with residual T and B cells but decreased naïve T cells and a spectrum of clinical and immunological phenotypes. Patients with atypical CID may experience frequent and opportunistic infections, including severe infections with Herpesviridae and human papillomavirus, and a wide range of autoimmune manifestations, including cytopenias and granulomatous lesions of the skin.2 Autoimmune and autoinflammatory manifestations frequently predominate over infections. Patients with CID and hypomorphic RAG variants who undergo hematopoietic stem cell transplantation (HSCT) are often diagnosed in childhood or teenage years if not identified by low levels of T-cell receptor excision circles at newborn screening or by a positive family history. Although HSCT is potentially curative, data on clinical and immunological outcomes, including resolution of immune dysregulatory manifestations, are scarce. Patients with hypomorphic RAG variants often manifest infections, autoimmunity, and/or organ damage at the time of HSCT. Compared with patients with null RAG mutations presenting with SCID, they are at a higher risk of graft rejection because of their residual T-cell function. In addition, thymic abnormalities affecting mechanisms of immune tolerance have been described in patients with hypomorphic RAG variants. How these abnormalities affect the capacity of the thymus to sustain immune reconstitution and restore tolerance following HSCT is unknown.3-5 Here, we report on a worldwide cohort of 60 patients with hypomorphic RAG variants, focusing on the natural course before HSCT, HSCT characteristics and complications, as well as immunological outcomes following HSCT.
Methods
Inclusion criteria
Patients were eligible if they had received their first transplant between 2004 and 2019 for a confirmed RAG1/RAG2 deficiency, had >300 autologous T cells at diagnosis of immunodeficiency or <300 T cells but had received no HSCT before the age of 18 months. RAG1/RAG2 deficiency presenting as typical SCID or Omenn phenotype were excluded.
Data source
The retrospective study was approved by the scientific review board of the Inborn Errors Working Party of the European Society for Immunodeficiencies, the European Society for Blood and Marrow Transplantation (EBMT), the Primary Immune Deficiency Treatment Consortium Steering Committee, and the local IRB (TU Dresden BO-EK-372072021). A specific questionnaire for data collection and analysis was sent to the participating centers. Data of all patients registered at the EBMT office were in compliance with the General Data Protection Regulation (GDPR 2016/679). Data for patients previously enrolled in the Primary Immune Deficiency Treatment Consortium studies in the United States and Canada, obtained under approved Institutional Review Board protocols (NCT01346150) were retrieved and shared in irreversibly deidentified form. Data collected included clinical, genetic, and immunological characteristics before transplant; characteristics of HSCT; and outcome regarding engraftment, immunological reconstitution, and clinical status.
Definitions
Intensity of the conditioning regimen (CR) was categorized into 3 groups: myeloablative (MAC), reduced toxicity (RTC), and reduced intensity (RIC)/nonmyeloablative regimen, as defined in the last Inborn Errors Working Party guidelines6 and shown in supplemental Table 1, available on the Blood website.
Based on HLA compatibility, donors were grouped into 4 categories: matched unrelated donor (MUD) defined as 10/10 identical unrelated donor or 6/6 unrelated cord blood, matched sibling donor and matched family donor (MSD/MFD) as 10/10 or 6/6 HLA identical relatives, mismatched family donor (MMFD), and mismatched MUD (MMUD) as ≤9/10 HLA-matched. Engraftment definitions were in accordance with the EBMT handbook.7 Acute and chronic graft-versus-host disease (GvHD) were graded according to modified Seattle and National Institute of Health criteria, respectively.8 Organ damage was documented with respect to the affected organ: lung (eg, chronic bronchitis, bronchiectasis, and interstitial pneumonitis), liver (eg, viral hepatitis, cholestasis, and hepatic siderosis), kidney (eg, glomerular or tubular damage), and other (eg, colitis).
Immune reconstitution was assessed at different time points after transplant: ≥6 to ≤12 months, >12 to ≤18 months, >18 to ≤24 months, >2 to ≤5 years, >5 to ≤10 years, and >10 years after HSCT. For immune reconstitution, cumulative incidence function was used. An event was defined as having reached a cell count above a given threshold at a given time point. In case of censoring, the last follow-up time point of measurement of this parameter was used. Gray's test was used to compare the cumulative incidences by age.
Immunophenotyping included absolute numbers of CD3+, CD4+, CD8+, and CD19+ cells per μL. Percentages of naïve CD4+ T cells defined as CD3+CD4+CD45RA+/total CD3+CD4+ cells were also collected. Normal immune reconstitution was defined as CD3+ T cells >1000/μL, CD8+ T cells >300/μL, and CD4+ T cells age-adjusted between 400 and 1200/μL for 1 to >10 years of age. Normal percentages of naïve CD4+ T cells were also age-adjusted (between 43% and 72%). Chimerism, performed as per center protocols on whole blood or lineage-specific where available, was retrieved at engraftment and later during follow-up. Donor engraftment was documented as full donor chimerism (>90%), mixed chimerism (any value between 10% and 90% donor), or graft failure (<10% donor). B-cell subset phenotyping, ongoing immunoglobulin substitution, and T-cell functions were incomplete or unavailable for most of the individuals.
Measurement of V(D)J recombination activity
Measurement of V(D)J recombination activity of RAG1 or RAG2 variants was performed with an assay based on a v-Abl RAG1/RAG2−/− pro-B cell line containing a single pMX-INV integrated cassette, as described.9,10 The list of RAG variants identified and their respective levels of recombination activity are shown in supplemental Table 2.
Statistical analysis
End points were overall survival (OS) and quality of immune reconstitution with a focus on naïve CD4+ T cells. Statistical analysis was performed using the program “RStudio.” OS was estimated via Kaplan-Meier analysis. Survival analyses were censored as the last follow-up time point before the end of the study on 8 February 2021. Log-rank test was used to compare survival curves regarding certain variables. Risk factors for death were calculated via Cox regression with the time of death as end point. Hazard ratios (HR) were calculated by univariable Cox regression for pretransplant, transplant variables, and posttransplant variables. The maximum number of variables to insert in the Cox-regression model depended on the number of events (1 predictor per 10 events).11 For multivariable Cox regression, a stepwise selection was performed in a sense of forward selection owing to the small number of events (18 events). Only variables significant on the P = .05-significance level in the univariable model were added successively to the multivariable model and eliminated stepwise. The remaining variables with the highest impact were included in the final adjusted model. For Cox modeling, the proportional hazards assumption was checked via Schoenfeld and scaled Schoenfeld residuals test. Logistic regression was performed to find determinants for significant variables resulting from the Cox model.
To define the variable importance, a random forest algorithm was used. According to Breiman, random forests are a combination of tree predictors: each tree depends on the values of a random vector sampled independently and with the same distribution for all trees in the forest.12
By permuting data only for the variable of interest during the calculation of decision trees, the increase in prediction error can be used to estimate the importance of the variable.13
Missing data had been imputed by predictive mean matching.
Results
Population and HSCT characteristics
A total of 60 patients with homozygous or compound heterozygous variants in either RAG1 (n = 46) or RAG2 (n = 14) fulfilled the inclusion criteria and were included in the study. The median age at symptom onset was 1.4 years (range, 0-15.4 years). Median age at genetic diagnosis was 3.3 years (range, 0-40 years), with 8 patients (13% of the cohort) diagnosed neonatally via either newborn screening or positive family history, all free of symptoms at transplant. Median age at HSCT is shown in supplemental Figure 1. The characteristics of the cohort, including pre-HSCT complications, are detailed in Table 1. Infections were documented in 78% of patients (29% with active infection at HSCT), granulomas in 18% (all active at HSCT) and autoimmunity in 72%. Of all, 43 patients experienced 91 autoimmune manifestations (median of 2 manifestations per patient, range 1-5 for those with autoimmunity). Cytopenias occurred in 33 patients who had 50 episodes of autoimmune hemolytic anemia (n = 21), ITP (n = 15), neutropenia (n = 13), and pure red cell aplasia (n = 1). Twenty-one patients experienced 41 episodes of autoimmune organ involvement, the most frequent being skin disease (dermatitis, vitiligo, alopecia areata), followed by colitis, myositis, and myasthenia gravis. Autoimmunity was active at the time of HSCT in 30 of 37 patients for whom information was available (supplemental Table 3). Malignancies were a rare complication; 2 patients had developed lymphoma before HSCT: 1 EBV-associated lymphoma and 1 diffuse large B-cell lymphoma. Pre-HSCT complications resulted in organ damage in 57% of patients, mostly affecting the lungs (50%) or liver (17%) (supplemental Table 4).
Characteristics of cohort
| Population characteristics . | Total patient number n = 60 . |
|---|---|
| Sex, n (%) | |
| Male | 27 (45) |
| Female | 33 (55) |
| Median year of birth | 2008 (1976-2017) |
| Mutation type, n (%) | |
| RAG 1 | 46 (77) |
| RAG 2 | 14 (23) |
| Homozygous | 19 (32) |
| Compound heterozygous | 41 (68) |
| Median age at first symptoms, y (range) | 1.4 (0-15.4) |
| Median age at diagnosis, y (range) | 3.3 (0-39.9) |
| Diagnosed by newborn or family screening, n (%) | 8 (13) |
| Infection before HSCT, n (%) | 47 (78) |
| Active infection before HSCT, n (%) | 17 (29) out of 58 |
| Presence of autoimmunity and/or granuloma before HSCT, n (%) | 47 (78) |
| Autoimmunity | 43 (72) |
| Autoimmune cytopenia | 33 (55) |
| Other autoimmune disease | 24 (41) |
| Granuloma | 11 (18) |
| Active autoimmunity and/or granuloma before HSCT, n (%) | 32 (58) out of 55 |
| Malignancy/lymphoma, n (%) | 2 (3) |
| Organ damage, n (%) | 34 (57) |
| Lung | 30 (50) |
| Liver | 10 (17) |
| Kidney | 6 (10) |
| Other | 9 (15) |
| Population characteristics . | Total patient number n = 60 . |
|---|---|
| Sex, n (%) | |
| Male | 27 (45) |
| Female | 33 (55) |
| Median year of birth | 2008 (1976-2017) |
| Mutation type, n (%) | |
| RAG 1 | 46 (77) |
| RAG 2 | 14 (23) |
| Homozygous | 19 (32) |
| Compound heterozygous | 41 (68) |
| Median age at first symptoms, y (range) | 1.4 (0-15.4) |
| Median age at diagnosis, y (range) | 3.3 (0-39.9) |
| Diagnosed by newborn or family screening, n (%) | 8 (13) |
| Infection before HSCT, n (%) | 47 (78) |
| Active infection before HSCT, n (%) | 17 (29) out of 58 |
| Presence of autoimmunity and/or granuloma before HSCT, n (%) | 47 (78) |
| Autoimmunity | 43 (72) |
| Autoimmune cytopenia | 33 (55) |
| Other autoimmune disease | 24 (41) |
| Granuloma | 11 (18) |
| Active autoimmunity and/or granuloma before HSCT, n (%) | 32 (58) out of 55 |
| Malignancy/lymphoma, n (%) | 2 (3) |
| Organ damage, n (%) | 34 (57) |
| Lung | 30 (50) |
| Liver | 10 (17) |
| Kidney | 6 (10) |
| Other | 9 (15) |
“Other” organ damage refers to gastrointestinal complications (n = 7), steroid-induced diabetes (n = 1), and vasculitis-associated epilepsy (n = 1). For details of organ damage please refer to supplemental Table 6.
A total of 62 HSCTs were performed in this cohort of 60 patients between 2004 and 2019 at 31 different centers (40 patients in Europe, 18 in North America, and 2 in Australia). The characteristics of HSCT detailed in Table 2 are given for the first HSCTs. Median age was 3.4 (0-43) years. Donors were MUD, MSD/MFD, MMFD, and MMUD in 48%, 22%, 18%, and 12% of cases, respectively. Ex vivo T-cell depletion of the graft was performed in 15 cases (25%), with CD34+ positive selection (n = 5), TCRαβ/CD19 depletion (n = 6), or CD45RA+ depletion (CD34+ selection followed by CD45RA+ depletion of the negative fraction) (n = 4). Donors were MMFD (n = 7), MMUD (n = 2), MUD (n = 5), and MFD (n = 1). There was an equal distribution between MAC vs RTC vs RIC (Table 2). Nonmyeloablative conditioning with fludarabine was only applied to a single patient. The sources of stem cells for HSCT were bone marrow, PBSC, and cord blood, in 58%, 30%, and 12% of cases, respectively. All patients (98%) received GvHD prophylaxis with 1 agent (n = 19) or a combination of immunosuppressive drugs (n = 41). Serotherapy was given to 49 patients (rabbit ATG n = 32, alemtuzumab n = 17).
Characteristics of HSCT
| Transplant characteristics . | Total patient number n = 60 . |
|---|---|
| Median year of HSCT (range) | 2014 (2004-2019) |
| Median age at HSCT, y (range) | 3.4 (0.3-42.9) |
| Age < 3.5 | 31 (52) |
| Age ≥ 3.5 | 29 (48) |
| Donor, n (%) | |
| MSD or MFD | 13 (22) |
| MMFD | 11 (18) |
| MUD | 29 (48) |
| MMUD | 7 (12) |
| Graft, n (%) | |
| Bone marrow | 35 (58) |
| Cord blood | 7 (12) |
| PBSC | 18 (30) |
| In vitro T-cell depletion, n (%) | 15 (25) |
| CR, n (%) | |
| MAC | 19 (32) |
| RIC | 18 (30) |
| RTC | 22 (37) |
| Serotherapy, n (%) | |
| ATG | 32 (53) |
| Alemtuzumab | 17 (28) |
| Transplant characteristics . | Total patient number n = 60 . |
|---|---|
| Median year of HSCT (range) | 2014 (2004-2019) |
| Median age at HSCT, y (range) | 3.4 (0.3-42.9) |
| Age < 3.5 | 31 (52) |
| Age ≥ 3.5 | 29 (48) |
| Donor, n (%) | |
| MSD or MFD | 13 (22) |
| MMFD | 11 (18) |
| MUD | 29 (48) |
| MMUD | 7 (12) |
| Graft, n (%) | |
| Bone marrow | 35 (58) |
| Cord blood | 7 (12) |
| PBSC | 18 (30) |
| In vitro T-cell depletion, n (%) | 15 (25) |
| CR, n (%) | |
| MAC | 19 (32) |
| RIC | 18 (30) |
| RTC | 22 (37) |
| Serotherapy, n (%) | |
| ATG | 32 (53) |
| Alemtuzumab | 17 (28) |
ATG, anti-thymocyte globulin.
Survival analyses
The median follow-up was 39 months (57 months for survivors). Forty-two patients were alive at last follow-up. Most patient deaths occurred within the first 12 months, whereas 4 patients died between 12 and 48 months post-HSCT. Estimated OS at 1 and 4 years were 77.5% and 67.5%, respectively (Figure 1A). Eighteen patients (30%) died at a median interval of 5 months after HSCT (range, 0-46 months). The main causes of death were infections (n = 10), GvHD plus infections (n = 2), graft rejection (n = 2), and post-transplant autoimmunity (n = 1). Late deaths (>12 months posttransplant) were related to infection (n = 2), GvHD, and new-onset autoimmunity, each in 1 case.
Overall survival. Survival after HSCT (A) and according to age at HSCT (B) and newborn screening/family history (C) is depicted.
Overall survival. Survival after HSCT (A) and according to age at HSCT (B) and newborn screening/family history (C) is depicted.
In univariable analysis, age at HSCT (with a cutoff defined by the median age of 3.5 years) did not influence OS (Figure 1B). Survival of patients diagnosed by newborn screening or family history was 100% (Figure 1C).
In particular, the survival probability with or without organ damage prior HSCT was 65% vs 92% after 12 months and 55% vs 87% at 4 years post-HSCT (P = .008, Figure 2A). Any type of active infection at HSCT had a negative impact (P = .001, Figure 2B) whereas autoimmunity before HSCT had no impact on survival (Figure 3). In vitro T-cell depletion of the graft had a strong negative impact with an estimated OS at 12 months and 4 years, respectively, of 46% and 19% compared with 87% and 85% for patients transplanted with an unmanipulated graft (P < .001, Figure 2C; supplemental Table 5). Patients transplanted with MMFD donors had an inferior survival probability (45% at 12 months and 18% at 4 years post-HSCT, P < .001), compared with other donor sources (eg, MUD: 87% at 12 months and 76% at 4 years, P=.004, Figure 2D). Recipients of grafts from MMFD or T-cell depleted grafts did not differ for age at HSCT or morbidity pre-HSCT, and these variables did not confound each other. The choice of CR had no impact on survival (Figure 3). All variables with significance or borderline significance had the maximum drop in survival probability within the first 12 months after HSCT (Figure 3).
Overall survival according to organ damage before HSCT (A), active infections at HSCT (B), T-cell depleted grafts (C), and donor (D).
Overall survival according to organ damage before HSCT (A), active infections at HSCT (B), T-cell depleted grafts (C), and donor (D).
Multivariable analysis revealed that organ damage before HSCT and T-cell depletion of the graft were the major predictors for death (HR = 6.01, HR = 8.46, respectively), whereas age at HSCT, infections before HSCT, and active infectious burden at HSCT were not significant predictors. Random forest analysis showed the highest variable importance for T-cell depletion (2.39), followed by MMFD (1.7) and organ damage before HSCT (1.13) (Table 3 and supplemental Table 6).
OS - multivariable Cox regression
| Determinant . | n (%) . | Univariable model . | Stepwise multivariable model . | ||||
|---|---|---|---|---|---|---|---|
| HR . | (95% CI) . | P . | HR . | (95% CI) . | P . | ||
| Infection before HSCT | 47 (78.3) | 5.71 | (0.76-43.01) | .091 | |||
| Active infection at HSCT | 17 (29.3) | 4.57 | (1.77-11.79) | .002 | |||
| Organ damage | 34 (56.7) | 4.58 | (1.32-15.83) | .016 | 6.01 | (1.72-21.00) | .005 |
| MMFD | 11 (18.3) | 4.97 | (1.91-12.90) | .001 | |||
| T-cell depletion | 15 (25.0) | 6.79 | (2.61-17.64) | <.001 | 8.46 | (3.22-22.24) | <.001 |
| Determinant . | n (%) . | Univariable model . | Stepwise multivariable model . | ||||
|---|---|---|---|---|---|---|---|
| HR . | (95% CI) . | P . | HR . | (95% CI) . | P . | ||
| Infection before HSCT | 47 (78.3) | 5.71 | (0.76-43.01) | .091 | |||
| Active infection at HSCT | 17 (29.3) | 4.57 | (1.77-11.79) | .002 | |||
| Organ damage | 34 (56.7) | 4.58 | (1.32-15.83) | .016 | 6.01 | (1.72-21.00) | .005 |
| MMFD | 11 (18.3) | 4.97 | (1.91-12.90) | .001 | |||
| T-cell depletion | 15 (25.0) | 6.79 | (2.61-17.64) | <.001 | 8.46 | (3.22-22.24) | <.001 |
CI, confidence interval. Bold indicates significant determinants left following stepwise mutlivariable modelling for overall survival (or non-survival).
Owing to the strong negative impact of pre-HSCT organ damage on OS, we examined parameters that may be associated with its occurrence. Logistic regression revealed that autoimmunity and or/granulomas before HSCT (P = .003), age at HSCT ≥3.5 years (P = .005), infection before HSCT (P = .01), and a delay of >12 months between birth and diagnosis were significant determinants for organ damage before HSCT (Table 4).
Determinants for organ damage before HSCT
| Variable . | Organ damage before HSCT, n (%) . | ||
|---|---|---|---|
| No . | Yes . | OR (univariable)∗ . | |
| Autoimmunity and/or granuloma before HSCT | |||
| No | 11 (42.3) | 2 (5.9) | — |
| Yes | 15 (57.7) | 32 (94.1) | 11.73 (2.73-82.27, P = .003) |
| > 12 mo between birth and diagnosis | |||
| No | 7 (30.4) | 2 (6.2) | — |
| Yes | 16 (69.6) | 30 (93.8) | 6.56 (1.40-47.67, P = .029) |
| Infection before HSCT | |||
| No | 10 (38.5) | 3 (8.8) | — |
| Yes | 16 (61.5) | 31 (91.2) | 6.46 (1.70-31.95, P = .010) |
| Age at HSCT ≥ 3.5 y | |||
| No | 19 (73.1) | 12 (35.3) | — |
| Yes | 7 (26.9) | 22 (64.7) | 4.98 (1.69-16.04, P = .005) |
| Variable . | Organ damage before HSCT, n (%) . | ||
|---|---|---|---|
| No . | Yes . | OR (univariable)∗ . | |
| Autoimmunity and/or granuloma before HSCT | |||
| No | 11 (42.3) | 2 (5.9) | — |
| Yes | 15 (57.7) | 32 (94.1) | 11.73 (2.73-82.27, P = .003) |
| > 12 mo between birth and diagnosis | |||
| No | 7 (30.4) | 2 (6.2) | — |
| Yes | 16 (69.6) | 30 (93.8) | 6.56 (1.40-47.67, P = .029) |
| Infection before HSCT | |||
| No | 10 (38.5) | 3 (8.8) | — |
| Yes | 16 (61.5) | 31 (91.2) | 6.46 (1.70-31.95, P = .010) |
| Age at HSCT ≥ 3.5 y | |||
| No | 19 (73.1) | 12 (35.3) | — |
| Yes | 7 (26.9) | 22 (64.7) | 4.98 (1.69-16.04, P = .005) |
OR, odds ratio (95% CI).
Engraftment, chimerism, and posttransplant complications
Five patients died before engraftment. Graft failure after the first procedure occurred in 4 patients (7%), of whom 3 died. A second procedure was attempted in 2 individuals, but despite engraftment in both cases, 1 patient died of infection. Full chimerism (˃90% donor) was documented in 75% of patients who engrafted. Sinusoidal obstructive syndrome, mostly mild, occurred in 6 patients (10%) (Seattle grade 3 in 1 individual). Acute and chronic GvHD were documented in 28 and 11 patients, respectively, with a 12-month cumulative risk of 35% for grade II-IV aGvHD and 22% for cGVHD (Figure 4). Median onset of aGvHD was 1 month (range 0-2.4 months) after HSCT; it was mainly mild (grade ≤ 2), whereas grades 3 and 4 aGvHD were documented in 5 and 2 patients, respectively. Median onset of cGvHD was 4 months. Post-transplant infections, the main cause of death, occurred in 26 patients (43%), 20 of viral and 12 of nonviral origin, some in combination. Post-HSCT outcome of pre-existing autoimmunity, documented in 43 patients before HSCT, was available for 33 of them (supplemental Table 3). Autoimmune cytopenias, including pure red cell aplasia, resolved in all cases. Uveitis (n = 1) recurred in the early post-HSCT period, but then resolved. Myositis, evaluable in 1 patient, improved. Outcome of myasthenia gravis could be assessed in 1 long-term survivor in whom anticholinesterase treatment was slowly tapered and stopped 4 years later without a recurrence of symptoms, despite persistence of autoantibodies. Alopecia areata resolved in all evaluable patients; vitiligo had variable outcomes with resolution or improvement in 3 of 5 evaluable patients. Granuloma resolved in all survivors. In addition, 9 new-onset autoimmune manifestations occurred in 7 patients at a median of 4 months after HSCT (range, 1-6) (Figure 4). These were autoimmune hemolytic anemia (n = 3), autoimmune hyperthyroidism (n = 2), and myositis, myasthenia gravis, sclerosing cholangitis, and coeliac disease (in 1 patient each). None of the patients with new-onset autoimmune manifestations had cGvHD, and only 1 of them had mixed donor chimerism.
Cumulative incidence of aGvHD, cGvHD, and de novo/relapse autoimmunity.
Immune reconstitution
The probability of CD3+ T cell counts reaching >1000/μL within the first 12 months after HSCT was 55%, increasing to 79% at 4 years. CD8+ T cells >300/μL were reached in 60% of patients within 12 months after HSCT and in 90% at 4 years. The probability of CD4+ T cells reaching the age-adjusted reference ranges was 40% after 12 months and 80% within 4 years. Immune reconstitution defined by naïve CD4+ T cell counts above an age-adjusted threshold was seen in 18% at 12 months after HSCT and rose to 42% of all patients at 4 years.
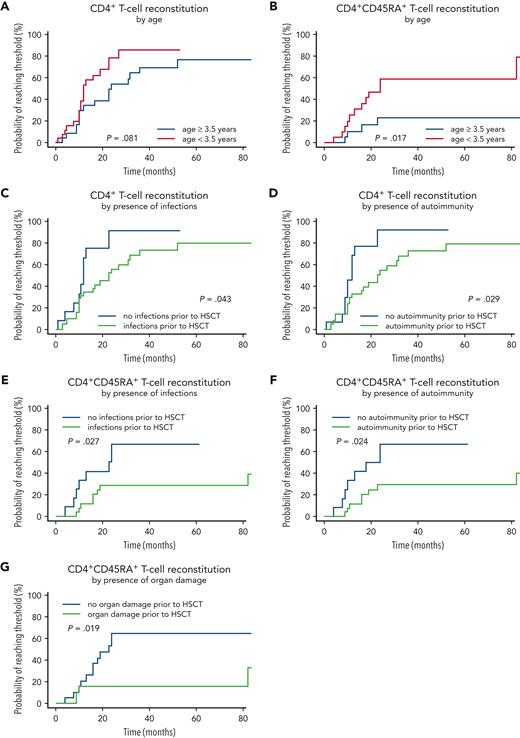
When analyzing immune reconstitution in this cohort, naïve CD4+ T-cell recovery was significantly different in patients younger or older than the median age of 3.5 years at HSCT. CD4+CD45RA+ T cell count rose faster in patients undergoing HSCT before age 3.5 years (P = .017). The probability of naïve CD4+ T cells reaching the age-adjusted reference range was 60% for the group transplanted <3.5 years, but only 20% in those ≥3.5 y/o at HSCT. Over the follow-up period, CD3+ and CD8+ T cell counts were lower in the older HSCT group (P = .01 and P = .03, respectively), whereas CD4+ T cells were not different (P = .081, Figure 5A-B).
T-helper and naive T-cell reconstitution. (A) CD4+ T-cell reconstitution by age at HSCT. (B) CD4+CD45RA+ T-cell reconstitution by age at HSCT. (C) CD4+ T-cell reconstitution by the presence of infections. (D) CD4+ T-cell reconstitution by the presence of autoimmunity. (E) CD4+CD45RA+ T-cell reconstitution by the presence of infections. (F) CD4+CD45RA+ T-cell reconstitution by the presence of autoimmunity. (G) CD4+CD45RA+ T-cell reconstitution by the presence of organ damage.
T-helper and naive T-cell reconstitution. (A) CD4+ T-cell reconstitution by age at HSCT. (B) CD4+CD45RA+ T-cell reconstitution by age at HSCT. (C) CD4+ T-cell reconstitution by the presence of infections. (D) CD4+ T-cell reconstitution by the presence of autoimmunity. (E) CD4+CD45RA+ T-cell reconstitution by the presence of infections. (F) CD4+CD45RA+ T-cell reconstitution by the presence of autoimmunity. (G) CD4+CD45RA+ T-cell reconstitution by the presence of organ damage.
The influence of the following variables on CD4+ T cells and CD4+CD45RA+ naïve T cell reconstitution were analyzed: pretransplant infections, autoimmunity and organ damage; conditioning (MAC, RT, RIC, supplemental Figure 2), donor type, T-cell depletion of grafts, GvHD, and new-onset or relapsed autoimmunity after HSCT. For CD4+ T cells, infections and autoimmunity before HSCT showed a significant adverse influence on immune reconstitution (Figure 5C-D). In patients without infections, autoimmunity, or organ damage before HSCT, immune reconstitution as measured by CD4+CD45RA+ T-cell numbers was faster and more robust (Figure 5E-G).
Finally, more than 80% of the patients had achieved independence from immunoglobulin replacement therapy by 5 years after HSCT (supplemental Figure 3). The type of CR and age at HSCT had no significant impact on the reconstitution of humoral immunity (supplemental Table 7).
Discussion
This is the largest retrospective study of outcomes of HSCT in patients with hypomorphic RAG1/RAG2 deficiencies. Only sporadic cases or small series have been published previously.14,15 Our study highlights the deleterious impact of pretransplant infections, autoimmunity, and organ damage on both survival and immune reconstitution post-HSCT. The cumulative disease burden pre-HSCT in individuals with hypomorphic RAG deficiencies observed beyond early childhood supports early diagnosis and intervention by HSCT.
The pretransplant characteristics of the cohort recapitulate the large spectrum of complications recognized in patients with hypomorphic RAG variants,16-18 including infection susceptibility, high rates of autoimmunity, and granulomatous inflammation predisposing to organ damage. Symptoms often manifested beyond infancy or early childhood up to 15 years of age, but molecular diagnosis was delayed with a surprisingly wide range up to midadulthood. This delay may both be related to the nonspecific nature of disease manifestations of hypomorphic RAG deficiency and to the retrospective nature of the study (including patients born in 1976-2017). In fact, the age of diagnosis was significantly lower in patients born from 2010 onwards, perhaps owing to the introduction of NBS but maybe also owing to better recognition of the disease and improved access to next-generation sequencing.
OS at 1 and 4 years after transplantation was comparable to previously published data for typical SCID because of RAG1/2 defects,19-21 and for primary immune regulatory disorders.22 The presence of active infection at HSCT was predictive of an unfavorable outcome in univariable analysis, as repeatedly shown for typical SCID in both the large North American and the European patient series.19,23 Organ damage at HSCT was a strong predictor of dismal outcome both in univariable and multivariable analyses, similar to the available large series of HSCT outcomes for other inborn errors of immunity (IEI).6,24 History of infection, autoimmunity, and granuloma that typically occurred early in the course of hypomorphic RAG deficiency were all predictive of organ damage. Young age at transplant has been correlated with favorable outcome in various IEIs6,24,25; however, median age at HSCT in this present cohort was not associated with OS. Interestingly, in the small group of 8 patients diagnosed very early by newborn screening (NBS) or owing to a positive family history, all were successfully transplanted before any symptoms from either MUD, MMFD, or MMUD. NBS by T-cell receptor excision circle measurement has been set up in several countries worldwide and offers the potential to identify patients with typical SCID early in life, permitting prompt definitive diagnosis and treatment if possible before the onset of symptoms.26 The ability of NBS to reliably identify patients with hypomorphic RAG deficiency needs to be verified.
In this series, HSCT performed following ex vivo T-cell depletion of the graft, including from a MMFD, had a significantly poorer outcome. HSCT from MMFD in SCID has commonly been associated with inferior OS and event-free survival.16,21,27 Large series of patients with CIDs also highlighted the inferior outcome with MMFD mainly following CD34+ selection of the graft, eg in CD40L or major histocompatibility complex class II deficiencies (24,28), the reasons for dismal outcome being mostly infections. Recent studies with new strategies of ex vivo depletion of the graft such as TCRαβ+/CD19+ depletion showed improved outcome in IEIs29 provided patients had not suffered from active infection at transplant.30,31 CD45RA+ depletion of the graft might be an alternative that could allow an early antiviral response with a limited risk of GvHD.32 Strategies using post-transplant cyclophosphamide as GvHD prophylaxis have also shown promising results.33,34 In our present series, various ex vivo manipulations of the graft were performed in 15 patients, not only in HLA-mismatched transplants but also in 5 MUD and 1 MSD, most likely to avoid the risk of GvHD in a nonmalignant setting. The outcome for T-cell depleted grafts in this cohort, however, was very poor, especially after CD34+ selection (0/5 survival) and TCRαβ+/CD19+ depletion (1/6 survival). Infections were the main cause of death documented in 9 of 11 patients who died, including 6 cases with active infections at the time of HSCT. These numbers are too small to draw firm conclusions, but they suggest that strategies allowing early immune reconstitution should be adopted when possible.
The burden of infection before and after HSCT results in significant morbidity and mortality, with infections being the leading cause of death after HSCT, frequently but not exclusively documented in patients who received T-cell depleted grafts. The high frequency of neutralizing anti–type I interferon antibodies in pretransplant patients with hypomorphic RAG1/2 deficiencies35 could contribute to this peritransplant risk factor, a hypothesis that could not be tested in this retrospective series.
Autoimmunity is one of the most frequent manifestations pre-HSCT in patients with hypomorphic RAG deficiencies. The pathophysiology is related to the loss of central and peripheral tolerance at various stages of development.36-39 Previous studies have shown that expression of AIRE, a transcriptional activator that allows expression of tissue-restricted antigens in the thymus enabling deletion of self-reactive T cells,40 is markedly reduced in the thymi of patients with RAG deficiency with Omenn syndrome and late-onset CID.3-5 In addition, a deficiency of FOXP3+ regulatory T cells was also documented in these conditions.5 Consistent with this, peripheral T cells from patients with hypomorphic RAG mutations have been shown to display molecular signatures of a self-reactive T-cell repertoire,41 indicating impaired purging of autoreactive T cells in the thymus. Furthermore, it has been demonstrated that patients with hypomorphic RAG mutations carry numerical and functional abnormalities of the Treg compartment,37 suggesting that defects of peripheral tolerance may also contribute to the immune dysregulation of this condition. New-onset autoimmunity was also observed early after transplant in 7 patients of our cohort at a median of 4 months. These were autoimmune cytopenias and autoimmune thyroiditis, which are not rare events after HSCT.42,43 However, unusual de novo immune dysregulation, such as myasthenia gravis, myositis, and sclerosing cholangitis, also occurred after HSCT. The critical role of thymic medulla regeneration early after HSCT to restore tolerance and prevent autoimmunity was demonstrated in experimental mouse models.44 One can speculate that pretransplant damage of the thymic medulla interferes with efficient reconstitution and may predispose to autoimmunity. Alternatively, it is possible that delayed or incomplete reconstitution of the Treg compartment may also contribute to the increased rate of posttransplant autoimmunity observed in this cohort. These hypotheses will need to be evaluated in future studies.
The goal of HSCT in patients with IEI is to achieve a rapid and durable restoration of immune function while avoiding GvHD and limiting toxicities. In this cohort, our analysis of immune reconstitution focused on CD4+ and naïve CD4+ T cell counts. In particular, the number of CD4+ CD45RA+ cells post-HSCT has been shown to represent a valid surrogate marker of thymic output45 and a biomarker predictive of long-term immune reconstitution.21 Our data showed that T-cell reconstitution was slower and incomplete in patients with pretransplant organ damage and in those ≥3.5 years of age. As the thymus is the central organ where T-cell reconstitution occurs, it is not surprising that thymic damage through pre-HSCT autoimmunity, inflammation and infections may hamper immunological reconstitution, especially when patients are diagnosed late and transplanted beyond infancy. In this series, with the exception of a single patient who received unconditioned HSCT, all other patients received a variety of CRs (RIC, RTC, and MAC), but no significant effects of these different regimens on either T-cell or B-cell reconstitution were observed (supplemental Figure 2; supplemental Table 7).
Conclusions
Poor pre-HSCT clinical status predicted an unfavorable HSCT outcome and slower naïve T-cell recovery. These findings advocate for early detection and early definitive treatment of patients with hypomorphic RAG1/RAG2 deficiencies. The completeness of ascertainment via NBS to identify these patients is currently unknown, but NBS may facilitate earlier diagnosis and improve outcome. OS of patients transplanted with ex vivo manipulation of the graft and from MMFD was poor, mainly because of infections. In addition, unusual de novo autoimmunity observed in this series might be related to thymic damage pre/during HSCT and persistent defects in central and/or peripheral tolerance early after HSCT.
Acknowledgments
We thank Sheree Hazelaar from the EBMT-IEWP office for excellent data management. We thank all patients’ families and the staff of the pediatric transplant wards for their dedication in patient care.
This work was in part supported by the Division of Allergy, Immunology, and Transplantation, National Institute of Allergy and Infectious Diseases (grant U54AI082973 to the Primary Immune Deficiency Treatment Consortium); the Office of Rare Diseases Research, National Center for Advancing Translational Sciences, National Institutes of Health through cooperative agreement. L.D.N. is supported by the Division of Intramural Research, National Institutes of Health, National Institute of Allergy and Infectious Diseases through grant ZIA AI001222. J.M.P. acknowledges support from the Lisa and Douglas Goldman Fund and the Jeffrey Modell Foundation. M.J.C. is supported by the California Institute of Regenerative Medicine (CIRM, CLIN2-10830). I.M. is a senior clinical investigator at FWO Vlaanderen and is supported by the Jeffrey Modell Foundation. Support by Rosemarie-Germscheid-Stiftung to C.S. is greatly appreciated.
Authorship
Contribution: C.S. and B.N. designed the study, interpreted compiled data, wrote the manuscript, and managed patients; J.G. and M.E. provided statistical analyses, interpreted data, and revised the manuscript; J.P. and A.L. contributed to the study design, interpreted data, and edited the manuscript; L.D.N. contributed to the study design, supervised V(D)J recombination assays, and reviewed the manuscript; T.K. performed V(D)J recombination assays; and the remaining authors contributed their patients’ data and reviewed the manuscript.
Conflict-of-interest disclosure: M.J.C. receives royalties from UpToDate and is on the scientific board with stock interest of Homology Medicines, Inc. J.H. receives royalties from UpToDate and is supported by a CSL Behring grant and clinical trial support from Regeneron. J.L. is supported by Horizon Therapeutics and Sobi; she is also employee and shareholder of bluebirdbio. I.M. is supported by a CSL Behring grant paid to KU Leuven. E.C.M. is supported by the NIHR UCLH Biomedical Research Centre and is a Scientific Founder and shareholder of Quell Therapeutics. J.P. discloses royalties from UpToDate and spousal employment at Invitae, a gene sequencing company.
Correspondence: Catharina Schuetz, Department of Paediatrics, Universitätsklinikum Carl Gustav Carus, Technische Universität Dresden, Fetscherstrasse 74, 01277 Dresden, Germany; e-mail: catharina.schuetz@ukdd.de; and Bénédicte Neven, Paediatric Immunology, Department of Haematology and Rheumatology, Necker-Enfants Malades, Paris, France; e-mail: benedicte.neven@aphp.fr.
References
Author notes
∗J.M.P and A.C.L. contributed equally to this study.
†L.D.N. and B.N. contributed equally to this study.
Data are available on request from the corresponding author, Catharina Schuetz (catharina.schuetz@ukdd.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal