Abstract
Agents targeting the unique biology of mycosis fungoides and Sézary syndrome are quickly being incorporated into clinical management. With these new therapies, we are now capable of inducing more durable responses and even complete remissions in advanced disease, outcomes which were exceedingly rare with prior therapies. Yet, even this new generation of therapies typically produce objective responses in only a minority of patients. As our therapeutic options increase, we are now challenged with selecting treatments from a growing list of options. To gain the full benefit of these novel agents, we must develop strategies to match treatments for the patients most likely to benefit from them. Here, we consider both the current approaches to treatment selection based on clinical features and the future of molecular biomarker-guided therapy for patients with this heterogeneous disease.
Introduction
Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common subtypes of cutaneous T-cell lymphoma (CTCL). Although the skin is the primary site of involvement, malignant T cells may expand in lymph node, visceral, and blood compartments. Most patients with MF or SS have a chronically relapsing disease course requiring multiple lines of therapy because individual treatments seldom result in lasting remission. Therefore, the goals of therapy include not just overall disease control but also symptom palliation and mitigation of treatment-related toxicities.
Traditionally, clinical stage has been a primary consideration in treatment selection. In early-stage MF, improved quality of life can be achieved with skin-directed therapies or milder systemic agents with less therapy-related morbidity. In contrast, patients with advanced-stage MF or SS are faced with a comparatively worse prognosis, necessitating consideration for more intensive systemic therapies that risk increased toxicity. For patients with relapsed or refractory advanced disease, allogeneic stem cell transplantation (allo-SCT) is potentially curative, though the significant risk of transplant-related morbidity and mortality warrants individualized consideration of its timing of and selection for use.
Historically, most systemic therapies utilized in CTCL were originally developed for other malignancies and subsequently adopted based on limited data. More recently, however, a wave of novel therapeutic approaches has emerged, built upon rationally selected targets for MF and SS. Although these novel agents represent a major advancement, they pose new challenges in matching patients to the therapies from which they are most likely to benefit. Here, we discuss the landscape of both established and novel therapeutics for patients with advanced-stage MF and SS requiring systemic therapy, focusing on the factors that currently guide treatment prioritization, as well as emerging strategies directed at further honing therapeutic precision amidst disease heterogeneity.
Traditional treatment options
Although treatment patterns are highly heterogeneous, in patients with advanced-stage MF and SS, systemic therapies are often considered. Numerous treatments of varying modalities and intensities have been explored, beginning with cytotoxic chemotherapy, extracorporeal photopheresis (ECP), interferon, methotrexate, bexarotene, and vorinostat. More recently, trials establishing the efficacy of romidepsin and pralatrexate heralded a shift toward exploring increasingly targeted, single-agent infusional drugs in CTCL, representing a bridge between historical and novel eras.1,2
As biologic and novel therapies have emerged over the past decade, accumulating evidence suggests that traditional cytotoxic chemotherapy is associated with comparatively greater risk and limited benefit in the routine treatment of MF and SS.3,4 A large international study of CTCL treatment patterns found the use of single-agent or combination chemotherapy to be independently associated with increased mortality.5 Thus, for patients requiring systemic therapy, we prioritize the use of nonchemotherapeutic drugs, reserving chemotherapy for later lines of treatment or specific situations detailed below. The true efficacies of traditional therapies are often difficult to discern given that data supporting their use were derived primarily from phase 2 studies. Subsequent phase 3 trials comparing historical systemic therapies to novel agents have helped contextualize their efficacy and safety profiles.
Novel systemic therapies
Brentuximab vedotin (BV), an anti-CD30 monoclonal antibody conjugated to the microtubule toxin monomethyl auristatin E, was approved for the treatment of CTCL on the basis of the randomized phase 3 ALCANZA trial.6 The study included 131 patients with CD30-positive (defined as ≥10% expression) MF having received at least 1 prior systemic therapy and compared BV with physician’s choice of methotrexate or bexarotene. Patients with SS were excluded. In the MF cohort, the primary end point of overall response rate sustained for at least 4 months (ORR4) favored BV as compared with the control arm (50% vs 10%). Updated trial analysis showed that BV also resulted in longer time to next treatment (median, 13.4 vs 5.6 months) and progression-free survival (PFS) (median, 16.1 vs 3.5 months).7 Peripheral neuropathy was more common in the BV arm, affecting 66% of patients overall (9% grade 3), and led to permanent discontinuation in 14% of cases.
Mogamulizumab, a defucosylated humanized IgG1κ monoclonal antibody targeting chemokine receptor 4 (CCR4), was approved for the treatment of CTCL on the basis of the randomized phase 3 MAVORIC trial.8 The study included 372 patients with stage IB-IVB MF/SS having received at least 1 prior systemic therapy and compared mogamulizumab with vorinostat. Patients with large cell transformation (LCT) were excluded. The primary end point of PFS favored mogamulizumab (median, 7.7 vs 3.1 months), as did overall response rate (ORR, 28% vs 5%). The most common adverse events were infusion-related reactions, diarrhea, fatigue, and drug-associated rash. The latter occurred in 24% of patients and led to treatment discontinuation in 7% of cases.
The ALCANZA and MAVORIC trials led to the Food and Drug Administration–approval of both BV and mogamulizumab in patients with CTCL having received at least 1 prior systemic therapy. The approval of these drugs as second-line or subsequent treatments raises important questions regarding frontline therapy in advanced MF and SS. Should BV and mogamulizumab be uniformly relegated for use behind the same traditional therapies against which they have demonstrated superiority? Are there scenarios in which they might be appropriate for use upfront? Putting the available data into practical context, we believe that the frontline use of either BV or mogamulizumab may be preferred in selected patients. Specifically, these agents should be considered as initial treatment in patients with advanced disease in need of rapid clinical response and whose clinical phenotype would be expected to respond especially favorably, discussed in further detail below.
Clinical factors guiding prioritization of systemic therapy in advanced MF and SS
Although many patients with advanced MF or SS initially respond to a given therapy, durations of response are generally limited. Thus, treatment selection is a repeatedly revisited task which requires an individualized approach, accounting for disease-related factors, notable drug toxicities, and whether future allo-SCT is planned.
Compartmental burden of disease
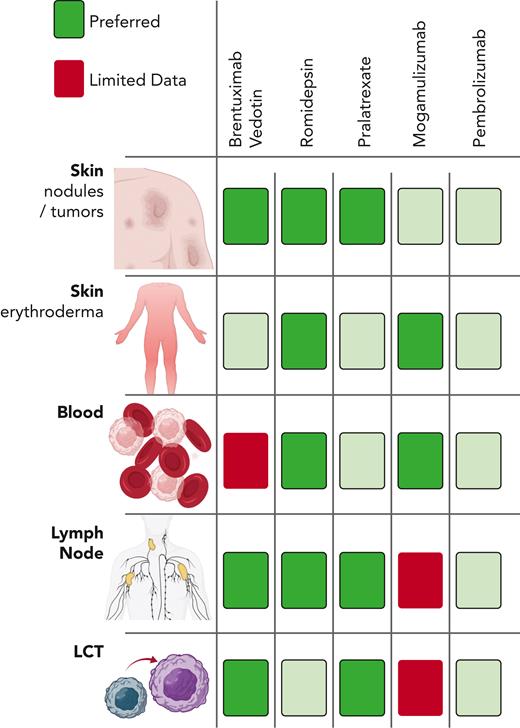
Because systemic treatments often exhibit differential efficacy across skin, blood, and lymph nodes, 1 useful framework to guide therapeutic prioritization is through an assessment of compartmental disease burden. More recent trials investigating single-agent infusional drugs have placed increased emphasis on describing their compartment-specific activity, allowing for a more nuanced approach to initial therapy selection (Figure 1).
Disease compartment driven drug selection. Relative activity of single-agent infusional therapies in various disease compartments in cutaneous T-cell lymphomas.
Disease compartment driven drug selection. Relative activity of single-agent infusional therapies in various disease compartments in cutaneous T-cell lymphomas.
Patients with significant blood burden (eg, B2) may particularly benefit from romidepsin or mogamulizumab. In this context, romidepsin has shown blood compartment response rates of up to 54%.9 In the MAVORIC study, mogamulizumab was associated with a blood ORR of 68%, a median time to response of 1.1 months, and median response duration of 26 months.8 Furthermore, a posthoc analysis found that the subset of patients eventually achieving long-term (≥12 months) responses to mogamulizumab were more likely to have SS or blood involvement.10 We find that mogamulizumab is often better tolerated than romidepsin, especially in patients with advanced age or decreased performance status, and thus consider it a frontline option in this setting. Though limited by selective availability and longer time to response, ECP is also effective in patients with erythrodermic MF or SS, especially when used earlier in a patient’s treatment course and in those with lower blood burden.11,12 In contrast, data regarding the utility of BV in this setting are limited because patients with SS were excluded from the ALCANZA study.13,14
Patients with erythrodermic skin involvement frequently have accompanying blood involvement, and so their treatment follows similar principles as described above. In patients with extensive skin tumors, however, the approach differs. Both romidepsin and pralatrexate have shown significant activity in patients with tumor-stage disease, with ORRs of 45% and 67%, respectively.2,15 However, based on the strength of data from the ALCANZA study, in which patients with tumor-stage MF treated with BV achieved striking response rates as compared with the control arm (ORR4, 63% vs 5%), we consider BV a frontline treatment option for these patients.6 In contrast, mogamulizumab is less effective in this context because only 16% of patients with tumor-stage MF in the MAVORIC study achieved a response.8
In patients with lymph node involvement, both romidepsin and pralatrexate are active, based on analyses of both specific CTCL cohorts and the landmark trials in nodal peripheral T-cell lymphomas (PTCLs).9,16 Similarly, BV is effective in treating nodal disease based on data from trials of both CTCL and nodal PTCLs.13,17,18 In the ALCANZA study, patients with extracutaneous involvement achieved an ORR4 of 46% vs 9% in the control arm.6 Thus, we view BV, romidepsin, and pralatrexate all as useful treatment options in these patients. In contrast, mogamulizumab is less effective, with only 17% of patients enrolled in the MAVORIC trial achieving a nodal response.8
In patients with extensive nodal or visceral involvement, the historically high ORRs associated with single-agent cytotoxic chemotherapies such as gemcitabine or liposomal doxorubicin provide an avenue for rapid disease control.19-21 Multiagent chemotherapy still holds an important role in managing patients with urgent need of clinical response and in obtaining maximal clinical response of extracutaneous compartments before allo-SCT. Given the limited response duration and risk of cumulative toxicity associated with chemotherapy, polychemotherapy is best used with a plan to transition to an alternative noncytotoxic agent or to proceed to allo-SCT once maximal disease response is achieved.
LCT
LCT is a consistent independent adverse prognostic factor in MF and SS.22 Historically, patients with LCT who required systemic treatment commonly received multiagent cytotoxic chemotherapy.23 Subsequent prospective studies of single-agent chemotherapies including gemcitabine, pentostatin, and liposomal doxorubicin specifically included patients with LCT, as did the pivotal PROPEL study which investigated pralatrexate in relapsed/refractory T-cell lymphomas.16,20,21,24 Taken together, these therapies result in ORRs of 25% to 60% and median response durations of <6 months.
LCT is now emerging as a predictive factor in the selection of novel agents. Although CCR4 is commonly expressed in MF or SS with LCT,25 mogamulizumab has not shown encouraging activity in this patient population,26 and patients with LCT were excluded from the MAVORIC study.8 In contrast, patients with LCT were included in the ALCANZA study, and BV was more effective in patients with LCT than in those without LCT (ORR4, 65% vs 39%), with a median PFS of 15.5 months.27 Together with emerging data confirming its effectiveness in typical clinical practice, BV should be considered a preferred option for patients with LCT.17
Planned allo-SCT
Despite the introduction of highly effective novel treatments, allo-SCT remains the only curative treatment in advanced MF and SS. However, because of high morbidity and mortality, only a small minority of patients undergo transplantation. Criteria for transplantation remain controversial and vary across experienced CTCL centers, with SCT usually reserved for patients with high-risk or multiple relapsed/refractory advanced-stage disease. Traditional transplantation regimens are associated with a 5-year PFS of 17% to 26% and overall survival of 32% to 38%.28,29 The introduction of novel reduced intensity conditioning regimens that include total skin electron beam therapy have improved outcomes, with a 5-year overall survival of >50% because of decreased peritransplant mortality.30,31 These treatment protocols may extend the possibility of allo-SCT to older and higher risk patients earlier in their disease course. A large international study of prognostic factors in advanced-stage MF and SS (retro-CLIPI) identified 3 risk groups with distinct outcomes based on clinical characteristics (age >60, stage IV, LCT, and elevated lactate dehydrogenase). Patients in the high-risk group, with 5-year overall survival of 28%, may generally be appropriate for allo-SCT, if eligible.22 Improved prognostic models based on molecular features are needed to further stratify decision-making in intermediate- and low-risk patients. Ultimately, the decision to undergo transplantation is individualized and requires weighing multiple factors including the prospect of durable remission, the risk of graft-versus-host disease, the possibility of post-transplant disease relapse, and remaining treatment options if transplantation is not pursued.
Although BV can effectively serve as bridging therapy without enhancing pretransplant toxicity,32 mogamulizumab should be used cautiously. In patients with adult T-cell leukemia/lymphoma, the use of mogamulizumab before transplant was associated with increased risk of severe acute graft-versus-host disease and nonrelapse mortality because of depletion of CCR4-expressing regulatory T cells.33 This association has not been established in patients with CTCL, with 1 study showing no clear evidence of increased risk of acute graft-versus-host disease in patients who had received mogamulizumab ≥6 months before transplantation.34
Maximizing therapeutic benefit of novel systemic agents
Decisions regarding treatment duration must balance conflicting goals of maximizing efficacy and minimizing toxicity. Because responses to traditional treatments are usually partial and short-lived, modern therapies are typically continued until progression. Although only occurring in a minority of patients, treatment with the latter may result in deeper, even complete responses.6,10,35,36 Thus, some patients may continue infusional treatments for years, accumulating toxicity and cost, and interfering with quality of life. Although prospective trials are ongoing, limited evidence supports strategies including dose-reduction or extension of dosing interval between infusions, in patients receiving continuous therapies.37-39
BV is associated with cumulative neurotoxicity which can be mitigated by limiting treatment to 16 cycles. Even so, most patients may still experience some degree of neuropathy, which can be permanent.40 Thus, for patients with a complete or good partial response, the emergence of neuropathy should prompt consideration for reduction in dose or a treatment holiday to ensure resolution. Upon disease relapse, such patients typically can be safely and effectively treated again with BV, thereby extending its utility while simultaneously reducing the risk of disabling neuropathy.41 Prospective evaluation of reduced-dose BV is ongoing, including 1 study showing a 42% ORR in patients with MF treated with BV at 0.9 mg/kg (50% of the dose used in ALCANZA), which may allow for longer treatment courses, maintained clinical benefit, and reduced neurotoxicity.39,42
Mogamulizumab can be continued long-term, but a symptomatic, drug-associated rash may be observed in a significant fraction of patients.8,43-45 In addition, ongoing treatment is costly and inconvenient, requiring infusions every 2 weeks. Like BV, we have found that patients discontinuing mogamulizumab in the setting of a good response can be successfully retreated after relapse.44 For patients with a complete response to mogamulizumab, we believe that a fixed duration of treatment may be considered to reduce the risk of mogamulizumab-associated rash and costs associated with indefinite maintenance.
Combination therapies
Additional efforts have been sought to combine treatments with nonoverlapping toxicity profiles to increase efficacy. ECP-based combinations with bexarotene or interferon have been extensively investigated, which augments response rates compared with ECP alone.46 Romidepsin-based combinations with either pralatrexate or azacitidine have shown encouraging activity in patients with PTCL,47,48 and in those with CTCL Emerging trials include combinations with pembrolizumab (NCT03278782) or lenalidomide/carfilzomib.49 Combinations based on novel therapies are also being explored, including mogamulizumab or BV combined with total skin electron beam therapy (NCT04256018, NCT05357794), ECP (NCT04930653, NCT04676087), romidepsin (NCT02616965), lenalidomide (NCT03302728, NCT03409432), nivolumab (NCT02581631, NCT01703949), or with each other (NCT05414500). Combination strategies may yield more reliable responses across multiple disease compartments but require further study to show an advantage over using these therapies sequentially.50
Improving biomarker-guided treatment approaches with emerging therapies
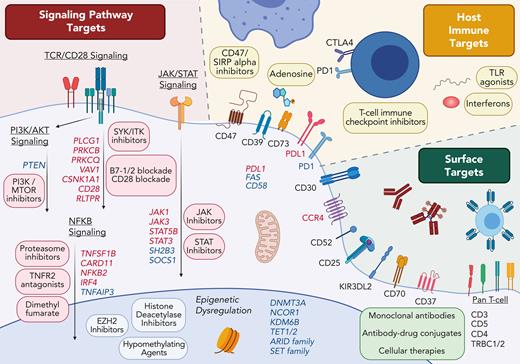
Decoding the molecular drivers of CTCL has catalyzed research into personalized treatment for this markedly heterogeneous disease, fueling both the discovery of new targeted treatments and the need for novel biomarkers predictive of therapeutic benefit. Emerging therapies can be broadly categorized into 3 groups, each associated with its own class of biomarker: (1) Targeting surface molecules, (2) Targeting disrupted cellular pathways, and (3) Targeting the host immune system (Figure 2).
Therapeutic targets in cutaneous T-cell lymphoma. Genes recurrently affected by gain-of-function genomic variants (red) or loss-of-function variants (blue) are shown with each altered pathway.
Therapeutic targets in cutaneous T-cell lymphoma. Genes recurrently affected by gain-of-function genomic variants (red) or loss-of-function variants (blue) are shown with each altered pathway.
Surface targets in CTCL
In contrast to B-cell malignancies, in which lineage molecules can be relatively safely targeted, targeting entire T-cell lineage markers is often hindered by unacceptable toxicity.51-53 Efforts in identifying more specific T-cell lymphoma surface markers including CCR4, CD30, CD25,54 CD70,55 CD37,56 TRBC1/2,57 and KIR3DL2 have produced a stream of antibody and cellular-based therapies with lesser toxicity. For example, in a phase 1 or 2 trial of patients with SS, the anti-KIR3DL2 antibody, lacutamab, was active (ORR, 43%) with few adverse events.58
BV represents a cautionary example in the application of predictive biomarkers for a biomarker-specified treatment in CTCL. In the ALCANZA study, positive CD30 expression was defined as having at least 1 biopsy with ≥10% CD30-positive malignant cells, but this cutoff was not based on data for superior outcomes in patients with higher CD30 expression. It is becoming clear that in CTCL, as with other lymphoid malignancies, CD30 expression is not a reliable predictive biomarker of response to BV.14,35,59,60 Remarkably, responses can be observed in CTCL even in the absence of immunohistochemical detection of CD30.14,61,62 Several hypotheses have been posited for this discordance, with one of the best validated explanations identifying a high intrapatient variability of CD30 expression.27 A biopsy of a single site may poorly represent CD30 expression in other areas, inappropriately selecting out patients who may nevertheless significantly benefit from BV.
Although the high interlesional variability of CTCL has been best demonstrated in the setting of CD30 expression based on the high molecular intratumoral heterogeneity of CTCL,63-66 it is likely that other putative biomarkers for novel therapies will similarly suffer sampling biases, limiting their utility. Ideally, future targeted therapies will require sampling of multiple lesions. Acknowledging the limitations of target detection, we believe that target expression should not necessarily be required as part of eligibility criteria for future trials but rather explored via planned analysis to determine whether expression correlates with clinical responses.
The revolution of cellular-based therapies such as chimeric antigen receptor T-cell therapy is now entering clinical exploration in CTCL. The adoption of cellular therapies into the treatment of T-cell lymphoma has been hampered by the challenges imposed by T-cell biology. T-cell based therapies, such as chimeric antigen receptor T-cell therapy, must circumvent fratricide whereby the therapeutic T cells are impeded by self-targeting. Additionally, it is unclear if any CTCL surface target exists that can yield durable responses comparable to those seen by CD19-directed T cells in B-cell malignancies, without additionally causing lasting T-cell aplasia. Although several cellular therapies are being studied in CTCL, efficacy data are limited. Notably, CD70-directed allogeneic chimeric antigen receptor T-cell therapy represents one example showing early promise in patients with CTCL.67
Targeting disrupted pathways in CTCL
It was hoped that increased understanding of the molecular pathogenesis of CTCL would lead to a new era of precision medicine. However, the marked genomic heterogeneity of CTCL has complicated these efforts.63,68-77 Even the most frequently mutated gene in CTCL, TP53, is mutated in only 15% of patients. Although recurrent sequence variants are rare, structural variants and copy number alterations are more stereotyped in CTCL.70,77,78 In addition, structural variants appear to vary by disease stage, with more frequent aberrations (including deletions of 17p and 10q and amplification of 17q) in patients with SS than in those with MF.79 Thus, efforts to personalize treatment in CTCL may require improved availability of assays to detect key copy number variants, rather than the current generation of targeted cancer sequencing panels focusing on sequence variants. Despite the genomic heterogeneity of CTCL, a clear pattern has emerged with recurrent clustering of genomic alterations within key pathways. These include activation of T-cell receptor signaling, T-cell receptor costimulation, JAK-STAT signaling, and the NF-κB pathway. As in other lymphomas, there is also frequent disruption of cell cycle and epigenetic regulators. Although the detection of these pathway alterations may be used to select targeted therapies, they are so frequently affected by various mechanisms that their predictive utility may be limited. For example, 1 case series found deletions or sequence variants in the ARID or SMARC gene families in 92% of patients with SS,72 whereas response rates in patients with SS to the histone deacetylase inhibitors vorinostat or romidepsin range from 2% to 34%.8,9
JAK inhibition represents the most practical initial candidate for a personalized treatment because of the wide availability of Food and Drug Administration––approved inhibitors. Single nucleotide variants in JAK1, JAK3, STAT3, or STAT5B cumulatively occur in 10% to 15% of patients with CTCL.72,80 A trial of ruxolitinib (a dual JAK1/JAK2 inhibitor) in T-cell lymphoma provides support for the concept that lymphomas with mutations promoting JAK/STAT signaling may respond better to JAK inhibitors.81 Likely because of the rarity of JAK and STAT mutations, no patients with CTCL were enrolled in cohort consisting the JAK/STAT mutation, highlighting the challenges of studying precision targeted strategies in prospective trials of rare diseases.
Targeting the host immune system in CTCL
Immune therapies play an increasingly important role in CTCL. In particular, programmed cell death protein 1 (PD1) targeting therapies are among the few treatments shown to induce lasting responses in patients.82 Pembrolizumab, a monoclonal antibody targeting PD1, was explored in a phase 2 study of 24 patients with relapsed or refractory stage IB-IV MF/SS,83 showing an ORR of 38%. Responses were observed across all disease stages; at a median follow-up of 58 weeks, median response duration was not reached, and 1-year PFS was 65%.
The future of PD1/ programmed cell death ligand 1 (PDL1) inhibition in CTCL depends on improved selection of those patients who are most likely to respond. Thus far, biomarkers for immune checkpoint response established in other malignancies do not appear to validate in CTCL. PDL1 expression has not correlated with response in the small studies to-date.82,83 However, structural variants of PDL1 occurring in a subset of patients with LCT may predict clinical benefit to immune checkpoint inhibitors.84 It is possible that some patients with LCT may show a more immunogenic phenotype, perhaps because of the higher tumor mutational burden seen in this setting.75,85 Other genomic events that promote immune evasion, such as CD58 and FAS mutations, are rarely found in CTCL but warrant study as potential immunotherapy biomarkers.
Immune profiling may reveal predictive biomarkers for other classes of immune mediated therapies being explored in CTCL including Toll-like receptor agonists, interferons, interleukins, and other immune checkpoint inhibitors. CD47 inhibitors have shown promise in CTCL with a response rate of 26%.86 Phenotyping of the macrophage component of the skin microenvironment is being explored as a biomarker for CD47 therapy. Despite single-agent activity, the future of CD47 blockade in CTCL is likely to be a part of combination therapies. The combination of CD47 inhibition with tumor-targeting monoclonal antibodies appears particularly promising.87,88
The most accurate predictive biomarkers for immunotherapies in CTCL would likely include not only tumor-intrinsic features, but also features of the tumor microenvironment and the host (eg, the microbiome).89,90 Components of the tumor microenvironment, such as tumor-associated macrophages, myeloid-derived suppressor cells, regulatory T cells, and cancer-associated fibroblasts, have been shown to contribute to disease pathogenesis by maintaining a strongly immune suppressive environment.91 Tumor microenvironment–based biomarkers of immunotherapy in CTCL may require not only quantification of these components but also more complex analysis of their spatial organization.92 Dysregulation of immune suppressive metabolic pathways should also be explored. In particular, overexpression of CD39 and CD73 in SS points to dysregulation of the adenosine pathway as a target for future immune based therapies.93,94
An additional challenge in adopting immunotherapy in CTCL treatment, and particularly in the targeting of T-cell immune checkpoints such as PD1, is the threat of “hyperprogression” or an acceleration of disease growth. Blockade of PD1 signaling may release malignant T cells from negative regulators of T-cell activation resulting in rapid proliferation. Although PD1 is commonly expressed in advanced-stage CTCL,95,96 the PDCD1 gene is paradoxically often deleted in advanced disease.79 Multiple studies have shown the potential for deletion of PD1 to promote lymphoma growth,79,97 and in vitro blockade of PD1 signaling to stimulate CTCL proliferation.98 However, as opposed to studies of other T-cell lymphomas,99,100 there have not yet been well-documented cases of hyperprogression in CTCL after PD1 blockade, even in lymphomas with PD1 deletion.83 Disease flaring was observed in patients treated with pembrolizumab with high PD1 expression but typically remitted without intervention and could evolve into clinical responses.83 Nevertheless, until there is more experience with immune checkpoint inhibitors, we cannot exclude their potential for hyperprogression in a subset of patients.
Conclusion
The escalating pace of drug discovery in CTCL continues to expand our therapeutic options. As we study these new treatments, we may need to adjust our measurement of clinical success. The combination of highly targeted therapies and a highly heterogeneous disease means that efficacy may not always be reflected in improved response rates in unselected patients. Rather, successful treatments may be manifested by extraordinary, durable responses in a subset of patients with susceptible disease. Though currently predominantly being guided by clinical factors, molecular biomarkers must become a part of our treatment paradigms if we are to take the leap from incremental gains to long-term improvements in outcomes for patients with advanced MF and SS.
Acknowledgments
Figures were created with Biorender.com.
Authorship
Contribution: M.S.K., E.M., and Y.H.K. designed and wrote the paper.
Conflict-of-interest disclosure: M.S.K. has received research funding from CRISPR Therapeutics and Nutcracker Therapeutics, consulting fees from Myeloid Therapeutics, and advisory board participation from Daiichi Sankyo. Y.H.K. has received research funding from Kyowa Kirin, Innate Pharma, Corvus Pharmaceuticals, Galderma Pharmaceuticals, CRISPR Therapeutics, Trillium Therapeutics, and advisory board participation from Kyowa Kirin, Galderma Pharmaceuticals, and Secura Bio. E.M. declares no competing financial interests.
Correspondence: Michael S. Khodadoust, 1701 Page Mill Rd, Palo Alto, CA 94304; e-mail: mkhodado@stanford.edu; and Youn H. Kim, 780 Welch Rd, CJ220D, Stanford, CA 94305; e-mail: younkim@stanford.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal