Abstract
Treatment paradigms for B-cell non-Hodgkin lymphomas (B-NHL) have shifted dramatically in the last 2 decades following the introduction of highly active immunotherapies such as rituximab. Since then, the field has continued to witness tremendous progress with the introduction of newer, more potent immunotherapeutics, including chimeric antigen receptor T-cell therapy, which have received regulatory approval for and currently play a significant role in the treatment of these diseases. Bispecific antibodies (BsAb) are a novel class of off-the-shelf T-cell redirecting drugs and are among the most promising immunotherapeutics for lymphoma today. BsAb may target various cell-surface antigens and exist in different formats. Anti-CD20xCD3 BsAb have demonstrated remarkable single-agent activity in patients with heavily pretreated B-NHL with a manageable toxicity profile dominated by T-cell overactivation syndromes. Much work remains to be done to define the optimal setting in which to deploy these drugs for B-NHL treatment, their ideal combination partners, strategies to minimize toxicity, and, perhaps most importantly, pharmacodynamic biomarkers of response and resistance. In this review, we provide an update on BsAb development in B-NHL, from discovery to clinical applications, highlighting the achievements, limitations, and future directions of the field.
Introduction
B-cell non-Hodgkin lymphomas (B-NHL) are a heterogeneous group of neoplasms that are curable or highly treatable with conventional cytotoxic polychemotherapy. Treatment paradigms for these diseases have been profoundly reshaped over the years by the introduction of highly active immunotherapies that work in concert with the host immune system. The anti-CD20 monoclonal antibody rituximab, which significantly improved the chance of cure for patients with aggressive lymphoma1 and markedly increased the overall survival (OS) of those diagnosed with indolent lymphoma,2 works primarily through Fc-gamma receptor (FcγR)–mediated mobilization of cytotoxic and phagocytic host immune cells.3 More recently, autologous chimeric antigen receptor (CAR) T-cell therapy using genetically engineered T cells redirected against lymphoma-associated antigens, such as CD19, demonstrated significant clinical efficacy, emphasizing how non–major histocompatibility complex (MHC)–restricted T-cell receptor–mediated T-cell activation can induce potent antitumor activity. Prolonged responses in patients whose lymphoma was refractory to standard chemotherapy led to positioning autologous CAR T-cell therapy as an accepted standard of care in several clinical settings.4-10 However, broad adoption of this treatment strategy is limited by a combination of manufacturing delays and treatment-related toxicities. More recently, off-the-shelf bispecific antibodies (BsAb), which activate peripheral and intratumoral endogenous immune cells by cotargeting tumor antigens and T- or natural killer cells in an FcγR- and MHC-independent manner, have begun to emerge. Although the scope of their clinical development has been broad in hematological and solid tumors, these products have proven especially active in patients with B-NHL and may represent the next therapeutic milestone in these diseases.
Preclinical development
Structural properties
Bispecific products can be divided into those that possess a fragment crystallizable (Fc), and thus an immunoglobulin (Ig)–like structure, and those that do not. Various manufacturing technologies have been used for BsAb synthesis, each resulting in constructs with unique structural and pharmacologic properties. A systematic review of all BsAb formats is offered elsewhere.11 Here, we will concentrate on the characteristics of T-cell–engaging BsAb currently in clinical development for the treatment of B-NHL.
BsAb can be distinguished by the way in which moieties of different specificity are assembled. Because BsAb result from different combinations of heavy and light chain variable domains, random assembly of the heavy chains and/or mismatched coupling of heavy and light chains can compromise the purity of the final product and, therefore, its bispecificity. One way to overcome this problem is to generate individual antigen-binding fragments (scFv) and fuse them either chemically or through physical linkage, as is the case for bispecific T-cell engagers or dual affinity redirecting antibodies.11 Most BsAb in development for B-NHL, however, have a full-length, IgG-like structure, which shares the pharmacologic properties of monoclonal antibodies (Table 1). The first and best-characterized approach to manufacturing IgG-like BsAb has been the “knobs-into-holes” technology,12 where complementary mutations are introduced in the CH3 domain of each antibody moiety, thus allowing for consistent pairing of heavy chains. In another platform, “knobs-into-holes” or similar technology is used to allow heavy chains to heterodimerize, and light chain mispairing is overcome by crossover of the antibody’s entire Fab domain, variable domain, or constant domain within 1 Fab-arm of the BsAb.13,14 With this technology, bivalent (1:1), trivalent (2:1), or tetravalent (2:2) antibodies can be generated that retain the antigen-binding capacity of the parental antibody while displaying variable avidity for the target epitope and distinct cytotoxic potential.15 A variety of other technologies have been used to manufacture Ig-like BsAb, including controlled Fab-arm exchange, where a library of BsAb is first generated and then the best candidate is chosen based on its ability to bind both epitopes and induce cytotoxicity,16 as well as the creation of an IgG4-based heterodimer with different heavy chains but a common light chain.17
Comparative characteristics of CD20XCD3 BsAb currently in development
| Product name . | Schematic depiction . | Format . | Technology . | CD20:CD3 ratio . | CD3 clone . | CD20 clone . | Fc silencing mutations∗ . |
|---|---|---|---|---|---|---|---|
| Mosunetuzumab18 |  | IgG1 | Knobs-into-holes (different Fabs) | 1:1 | UCHT1v9 (CD3δε) | 2H7 (type 1 epitope, identical to rituximab) | N297G (no FcγR binding) |
| Glofitamab15 |  | IgG1 | Head-to-tail fusion | 2:1 | SP34-der.(CD3ε) | By-L1 (type 2 epitope, identical to obinutuzumab) | IgG1-P329G-LALA (no FcγR binding) |
| Epcoritamab16 |  | IgG1 | Controlled Fab-arm exchange | 1:1 | huCACAO (SP34-der.)(CD3ε) | 7D8 (type 1 epitope, shared by ofatumomab) | L234F,L235E,D265A (no FcγR,C1q binding) |
| Odronexamab17 |  | IgG4 | Heavy chains with different affinity | 1:1 | REG1250 (CD3δε) | 3B9-10 (type 1 epitope, shared by ofatumomab) | Modified IgG4 (no FcγRIII binding) |
| Plamotamab90 |  | IgG1 | Fab-Fc x scFv-Fc | 1:1 | α-CD3_H1.30 (SP34-der.)(CD3ε) | C2B8_H1_L1 (type 1 epitope, shared by rituximab) | G236R, L328R (no FcγR binding) |
| IgM 232319 |  | IgM | IgM + modified J chain | 10:1 | Not reported | Not reported | No |
| Product name . | Schematic depiction . | Format . | Technology . | CD20:CD3 ratio . | CD3 clone . | CD20 clone . | Fc silencing mutations∗ . |
|---|---|---|---|---|---|---|---|
| Mosunetuzumab18 |  | IgG1 | Knobs-into-holes (different Fabs) | 1:1 | UCHT1v9 (CD3δε) | 2H7 (type 1 epitope, identical to rituximab) | N297G (no FcγR binding) |
| Glofitamab15 |  | IgG1 | Head-to-tail fusion | 2:1 | SP34-der.(CD3ε) | By-L1 (type 2 epitope, identical to obinutuzumab) | IgG1-P329G-LALA (no FcγR binding) |
| Epcoritamab16 |  | IgG1 | Controlled Fab-arm exchange | 1:1 | huCACAO (SP34-der.)(CD3ε) | 7D8 (type 1 epitope, shared by ofatumomab) | L234F,L235E,D265A (no FcγR,C1q binding) |
| Odronexamab17 |  | IgG4 | Heavy chains with different affinity | 1:1 | REG1250 (CD3δε) | 3B9-10 (type 1 epitope, shared by ofatumomab) | Modified IgG4 (no FcγRIII binding) |
| Plamotamab90 |  | IgG1 | Fab-Fc x scFv-Fc | 1:1 | α-CD3_H1.30 (SP34-der.)(CD3ε) | C2B8_H1_L1 (type 1 epitope, shared by rituximab) | G236R, L328R (no FcγR binding) |
| IgM 232319 |  | IgM | IgM + modified J chain | 10:1 | Not reported | Not reported | No |
These Fc-silencing mutations do not abolish the binding of BsAb to neonatal FcR.
Other elements of differentiation among BsAb include number, distribution, and specificity of the Fab arms. CD20xCD3 BsAb may possess 112,16,18 or more CD20-binding Fabs13,14,19 and, as a consequence, different target-binding avidity and ability to elicit effector functions. For instance, in vitro, a CD20xCD3 BsAb with 2 CD20-binding sites (2:1 format) induced 40-fold greater tumor lysis than its 1:1 BsAb counterpart.15 Interestingly, a BsAb with CD20- and CD3-binding sites arranged in a head-to-tail fashion on the same Fab had greater potency than the variant with 1 binding site on each Fab.15 Overall, these 2 modifications resulted in increased binding avidity and stabilization of the tumor-T-cell synapse, indicating that BsAb-mediated tumor in vitro killing is in part influenced by the spatial configuration of the 2 binding moieties. Furthermore, distinct BsAb recognize different epitopes on the CD20 antigen (Table 1), which may have important implications for combinatorial strategies (see below). Finally, multispecific antibodies with 2:1 or 2:2 formats recognizing 2 distinct antigens on tumor cells (advantageous for tumors with antigen low-density or potential loss) or targeting CD3 and a T cell costimulatory antigen are being developed in different disease contexts.
A structural feature shared among most Ig-like BsAb is the presence of mutations in their Fc domain. These are designed to prevent antibody-dependent FcγR-mediated crossbinding of CD3 and T cells, which could result in antigen-independent T-cell activation and cytotoxicity, as well as fratricidal antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. In contrast, the neonatal FcR binding is usually preserved to prolong the drug half-life in vivo.12,16,18
Pharmacodynamics (PD)
A crucial step in developing effective BsAb is careful target selection. CD19 and CD2020 are relatively stable cell-surface antigens widely expressed on B cells and have been used as targets for most BsAb currently in development for B-NHL. In 1 experiment, BsAb cotargeting CD3 and other B-cell antigens (eg, CD22, CD37, CD70, CD79b, CD138, or HLA-DR) induced lower cytotoxicity compared with those cotargeting CD3 and CD20, even when the expression levels of these B-cell antigens were comparable.16 Other factors that can influence tumor cell killing are antigen-binding affinity, molecular size, flexibility, mobility, localization of the epitope on the cell surface, BsAb format, ease of immunological synapse formation, balance between costimulatory and coinhibitory molecules influencing T-cell activation, and the residual or concomitant presence of other competing therapeutic antibodies that could result in steric hindrance.
All BsAb work by simultaneously binding target tumor cells and immune effector cells (eg, T-cells, macrophages, or natural killer cells), which become activated and cause tumor cell killing. In the case of T-cell engagers, cytotoxicity occurs in a MHC-independent fashion, thus bypassing the restrictions imposed by the MHC-T-cell receptor interaction.11 This feature is critically important as many B-NHL, in particular diffuse large B-cell lymphoma (DLBCL), frequently exhibit genetic aberrations that abolish expression of MHC class I molecules.21,22 Affinity for T-cell binding (often via CD3) is equally important, as it affects the ability of the T cell to form a functional immune synapse and be optimally activated. Of note, BsAb against CD3, a marker expressed on all T-cell subpopulations, are likely to engage effector and noneffector T cells alike, and the net functional effects of indiscriminate T-cell activation are presently unknown. antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity are not expected to contribute to BsAb-mediated cell cytotoxicity due to the aforementioned mutations introduced in the Fc domain of most products.
CD19-directed BsAb
Blinatumomab is a CD19xCD3 bispecific T-cell engager comprised of 2 single-chain antibody fragments, respectively against CD19 and against CD3, connected by a linker.23 At femtomolar concentrations, blinatumomab simultaneously bound CD3 and CD19, increased T-cell motility, activation, and proliferation, and produced rapid, serial CD19+ malignant B-cell lysis in coculture experiments.24 Duvortuxizumab is an example of a CD19xCD3 dual affinity redirecting antibody in which the variable heavy chain domain of the first moiety is linked to the variable light chain domain of the second and vice versa. In a Burkitt’s lymphoma mouse model, duvortuxizumab induced CD4+ and CD8+ T-cell tumor infiltration, activation, effector memory (CD45RA− CCR7−) differentiation, and cytotoxicity.25
CD20-directed IgG BsAb
Mosunetuzumab, the first-in-class CD20xCD3 IgG-like BsAb, underwent extensive preclinical development. Experiments conducted in vitro and on mice engineered to express human CD20 and CD3ε revealed that the drug was active at very low concentrations and exerted cytotoxicity primarily through activated CD69+CD8+ T cells. Tumor killing peaked at 24 hours and decreased around day 3, when B cells had been mostly cleared, supporting the notion that mosunetuzumab-mediated T-cell activation occurs conditionally upon B-cell binding. Furthermore, mosunetuzumab retained its activity in combination with an effectorless variant of rituximab (which contributes to CD20 occupancy but not to cytotoxic activity), suggesting that coadministration of the 2 drugs may be feasible.18
The preclinical development of a second BsAb, glofitamab, stemmed from the observation that directly fusing a CD20 Fab and a CD3 Fab “head-to-tail”14 may elicit superior biological activity than placing a CD3 Fab and a CD20 Fab on each antibody arm. Indeed, incubation of tumor B cells with glofitamab resulted in tumor-T-cell synapse formation and marked tumor lysis as early as 4 hours following tumor encounter. In humanized nonobese diabetic/severe combined immunodeficiency gamma mice, weekly glofitamab resulted in peripheral and tissue B-cell clearance within 24 hours of the first full dose (0.5 mg/kg), and those remained undetectable throughout the experiment.15
The preclinical development of epcoritamab contributed additional insights into the BsAb mechanism of action. In vitro, epcoritamab induced dose-dependent activation of CD4+ and CD8+ T cells, release of perforin, and CD8+ T-cell–mediated reversible B-cell depletion when coincubated with various CD20+ B-NHL cell lines, regardless of CD20 expression level. In addition, both intravenous (IV) and subcutaneous (SC) epcoritamab suppressed the growth of CD20+ lymphoma xenografts in nonobese diabetic/severe combined immunodeficiency mice with a humanized immune system. An Fc-silenced rituximab variant coadministered in doses up to 10 mg/kg did not reduce epcoritamab’s cytotoxic potential.16
Clinical pharmacology
Unlike blinatumomab, which is a small molecule characterized by rapid clearance and a short half-life, thus requiring continuous infusion,26 full-length BsAb share pharmacokinetic (PK) characteristics with monoclonal antibodies and endogenous IgG and can therefore be dosed at longer intervals. In preclinical PK/PD studies, these agents exhibited at first nonlinear, time-varying PK due to initial rapid target-mediated clearance followed by a linear, more predictable clearance, allowing IV dosing every 1 to 4 weeks.27 In phase 1 trials, most BsAb administered IV showed a dose-dependent Cmax and a half-life of 6 to 14 days that was shorter than the typical 21-day half-life of most IgG, likely owing to the initial target-mediated drug clearance.17,28-30 Compared with the IV formulation, SC dosing was associated with slower absorption and a lower Cmax, but a similar area under the curve with both delayed and lower peak levels of inflammatory cytokines.30-32
Given the unique properties and mechanism of action of BsAb, novel integrated PK/PD models have been devised to identify optimally biologically effective (rather than maximum tolerated) doses by correlating drug exposure, changes in the B- and T-cell dynamics in different body compartments, and clinical response. These models demonstrated consistent PD effects with limited interindividual viariability using flat dosing, suggesting that weight- or body surface area–based BsAb dosing is likely unnecessary.33-36 In phase 1 trials, the dose-response relationship usually followed a sinusoidal pattern, with no meaningful clinical activity seen until a dose threshold, then a linear correlation between dose and response, and finally a plateau, beyond which larger doses did not produce incrementally higher response rates.28-30,36 The formation of antibodies directed against the BsAb has not been reported so far in patients.
Current clinical results in B-cell lymphoma
Blinatumomab was the first BsAb to enter the clinical arena. In a phase 1 trial in selected patients with relapsed or refractory (R/R) B-NHL, it produced encouraging response rates with durable benefit.26 Subsequent experiences in heavily pretreated individuals with DLBCL confirmed high efficacy, with an overall response rate (ORR) just above 40% and ∼20% complete responses (CR), a small number of which were durable.37-39 The cumbersome dosing schedule and significant neurological toxicities observed in these studies somewhat limited blinatumomab’s prospects for further clinical development. In contrast, the more favorable efficacy and safety profiles seen so far with CD20xCD3 IgG-like BsAb have hastened their development in patients with diverse R/R B-NHL (Table 2).
Conceptual overview of BsAb studies with available results
| Disease . | Setting . | Modifiers . | Trial ID . | Report format . | Phase . | Drug(s) . | Histology . | N. . | Median age, y (range) . | Median N. prior therapies (range) . | % Prior ASCT/prior CAR-T . | ORR (CR), % . | DOR, mo . | PFS, mo . | Follow-up (mo) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aNHL∗ | First line | R-CHOP candidate | NCT03677141 | Abs | I/II | MOSUN-CHOP | DLBCL | 40 | 65 (39-79) | 0 | NA | 82 (79) | NR | NR | NR |
| NCT03467373 | Abs | I | GLOFIT-R-CHOP | DLBCL | 26 | 68 (26-84) | 0 | NA | 100 (89) | NR | NR | NR | |||
| NCT04663347 | Abs | I/II | EPCOR-R-CHOP | DLBCL | 24 | 65 (30-82) | 0 | NA | 100 (73) | nr (1-6.5+) | NR | 1.3 (0.2-7.9) | |||
| Older/unfit | NCT03677154 | Abs | I/II | MOSUN (IV) | DLBCL | 29 | 82 (67-100) | 0 | NA | 63 (45) | nr (0.2-13+) | NR | 5.4 (0.3--16.2) | ||
| ≥second line | Transplant eligible | NCT04663347 | Abs | I/II | EPCOR-R-DHAX | DLBCL | 29 | 58 (28-75) | 1 (1-3) | 0/10 | 100 (86) | NR | NR | 5.8 (1.5-11.4) | |
| Transplant ineligible | NCT04663347 | Abs | I/II | EPCOR-GemOx | DLBCL | 26 | 71 (47-87) | 2 (1-13) | 12/12 | 92 (60) | NR | NR | 9 (1-15) | ||
| NCT02500407 | Paper | I/II | MOSUN (IV) | Multiple | 116 | 63 (19-91) | 3 (1-14) | 34/12 | 35 (19) | 7.6 (5.6-2.8) | 1.4 (1.4-2.9) | NR | |||
| NCT02500407 | Abs | I/II | MOSUN (SC) | Multiple | 50 | 68 (41-88)† | 3.5 (1-9)† | 17/42† | 29 (18) | NR | NR | 4.2 (0.1-7.8)† | |||
| NCT03075696 | Abs | I/II | GLOFIT | Multiple | 155 | 66 (21-90) | 3 (2-7) | 18/33 | 52 (39) | 18.4 (13.7-NE) | 4.9 (3.4-8.1) | 12.6 (0-22) | |||
| NCT03625037 | Paper | I/II | EPCOR | Multiple | 157 | 64 (20-83) | 3 (2-11) | 20/39 | 63 (39) | 12 (0+-15.5+) | 4.4 (3.0-7.9) | NR | |||
| NCT04082936 | Abs | I | IgM2323 | DLBCL | 18 | 64 (36-84)† | 3 (2-9)† | 8/20† | 31 (25) | nr (2-1.5+)† | NR | 7.8 (0.4-23.7)† | |||
| NCT02924402 | Abs | I | PLAMO | Multiple | 46 | 61.5 (31-82)† | 4 (1-10)† | 13/NR† | 51 (25)† | NR | NR | NR | |||
| NCT02290951 | Paper | I | ODRON | Multiple | 85 | 67 (57-73)† | 3 (2-5)† | 8/29† | 37 (24) | 4.4 (2.9-NE); nr (1.6-NE)‡ | 2 (0.9-5.3)‡ | 4.2 (1.5-11.5)† | |||
| NCT03671018 | Abs | I/II | MOSUN-pola | DLBCL | 60 | 68 (20-83) | 3 (1-8) | NR/40 | 65 (48) | nr (6.3 - NE) | 8.9 (3.5-NE) | 5.7 (0.7-27.5) | |||
| NCT03533283 | Abs | I/II | GLOFIT-pola | Multiple | 59 | 59 (29-82) | 2 (1-5) | NR/NR | 80 (51) | nr (0.5-23+) | NR | 3.7 (1.9-5.3) | |||
| Indolent B-NHL§ | ≥second line | After anti-CD20 and alkylating agents | NCT02500407 | Paper | I/II | MOSUN (IV) | Multiple | 68‖ | 60.5 (27-85) | 3 (1-11) | 18/6 | 66 (48) | 16.8 (11.7-NE) | 11.8 (8.4-NE) | NR |
| NCT02500407 | Paper | I/II | MOSUN (IV) | FL | 90‖ | 60 (29-90) | 3 (2-4) | 21/3 | 80 (60) | 22.8 (9.7-NE) | 17.9 (10.1-NE) | 18.3 (2-27.5) | |||
| NCT02500407 | Abs | I/II | MOSUN (SC) | FL | 12 | 68 (41-88)† | 3.5 (1-9)† | 17/42† | 82 (64) | NR | NR | 4.2 (0.1-7.8)† | |||
| NCT03075696 | Abs | I/II | GLOFIT | FL | 75 | 64 (22-86)† | 3 (1-13)† | 12/5† | 81 (69) | NR | NR | NR | |||
| NCT03075696 | Abs | I/II | GLOFIT-obin | FL | 19 | 61 (41-78) | 2 (1-5) | 16/0 | 100 (74) | NR | NR | 5.5 (5.4-6.3) | |||
| NCT03625037 | Paper | I/II | EPCOR | FL | 12 | 73 (63-76) | 4.5 (2.5-8) | 8/0 | 90 (50) | 6 (2.5-15.5) | NR | 13.6 (10.4-16.5) | |||
| NCT04082936 | Abs | I | IgM2323 | FL, MZL | 18 | 64 (36-84)† | 3 (2-9)† | 8/20† | 28 (22) | nr (2-21.5+)† | NR | 7.8 (0.4-23.7)† | |||
| NCT02924402 | Abs | I | PLAMO | Multiple | 17 | 61.5 (31-82)† | 4 (1-10)† | 13/NR† | 51 (25)† | NR | NR | NR | |||
| NCT02290951 | Paper | I | ODRON | FL, MZL | 46 | 67 (57-37)† | 3 (2-5)† | 8/29† | 76 (59)¶ | MZL: 18.1 (1.5-NE) | MZL: NR | 4.2 (1.5-11.5)† | |||
| NCT03467373 | Abs | I | GLOFIT-R-CHOP | FL, MZL (tFL) | 31 | 62 (34-78) | 2 (1-5) | NR/NR | 90 (81) | NR | NR | 9 (0-29) | |||
| NCT04246086 | Abs | I | MOSUN (IV)-len | FL | 29 | 59 (30-79) | 1 (1-6) | NR/NR | 90 (65) | nr (0.3-12.3+) | NR | 5.4 (3-12) | |||
| NCT04663347 | Abs | I/II | EPCOR-R-len | FL | 29 | 67 (42-80) | 1 (1-5) | 17/NR | 100 (92.3) | nr (0.3-6.5+) | NR | NR | |||
| MCL | ≥third line | post-BTKi | NCT03075696 | Abs | I/II | GLOFIT | MCL | 29 | 69 (41-84) | 3 (1-6) | NR/NR | 81 (67) | NR (1-28.5+) | NR | 1.4 (CI NR) |
| NCT02290951 | Paper | I | ODRON | MCL | 12 | 67 (57-37)† | 3 (2-5)† | 8/29† | 50 (33) | 10.9 (1.4-NE) | NR | 4.2 (1.5-11.5) |
| Disease . | Setting . | Modifiers . | Trial ID . | Report format . | Phase . | Drug(s) . | Histology . | N. . | Median age, y (range) . | Median N. prior therapies (range) . | % Prior ASCT/prior CAR-T . | ORR (CR), % . | DOR, mo . | PFS, mo . | Follow-up (mo) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aNHL∗ | First line | R-CHOP candidate | NCT03677141 | Abs | I/II | MOSUN-CHOP | DLBCL | 40 | 65 (39-79) | 0 | NA | 82 (79) | NR | NR | NR |
| NCT03467373 | Abs | I | GLOFIT-R-CHOP | DLBCL | 26 | 68 (26-84) | 0 | NA | 100 (89) | NR | NR | NR | |||
| NCT04663347 | Abs | I/II | EPCOR-R-CHOP | DLBCL | 24 | 65 (30-82) | 0 | NA | 100 (73) | nr (1-6.5+) | NR | 1.3 (0.2-7.9) | |||
| Older/unfit | NCT03677154 | Abs | I/II | MOSUN (IV) | DLBCL | 29 | 82 (67-100) | 0 | NA | 63 (45) | nr (0.2-13+) | NR | 5.4 (0.3--16.2) | ||
| ≥second line | Transplant eligible | NCT04663347 | Abs | I/II | EPCOR-R-DHAX | DLBCL | 29 | 58 (28-75) | 1 (1-3) | 0/10 | 100 (86) | NR | NR | 5.8 (1.5-11.4) | |
| Transplant ineligible | NCT04663347 | Abs | I/II | EPCOR-GemOx | DLBCL | 26 | 71 (47-87) | 2 (1-13) | 12/12 | 92 (60) | NR | NR | 9 (1-15) | ||
| NCT02500407 | Paper | I/II | MOSUN (IV) | Multiple | 116 | 63 (19-91) | 3 (1-14) | 34/12 | 35 (19) | 7.6 (5.6-2.8) | 1.4 (1.4-2.9) | NR | |||
| NCT02500407 | Abs | I/II | MOSUN (SC) | Multiple | 50 | 68 (41-88)† | 3.5 (1-9)† | 17/42† | 29 (18) | NR | NR | 4.2 (0.1-7.8)† | |||
| NCT03075696 | Abs | I/II | GLOFIT | Multiple | 155 | 66 (21-90) | 3 (2-7) | 18/33 | 52 (39) | 18.4 (13.7-NE) | 4.9 (3.4-8.1) | 12.6 (0-22) | |||
| NCT03625037 | Paper | I/II | EPCOR | Multiple | 157 | 64 (20-83) | 3 (2-11) | 20/39 | 63 (39) | 12 (0+-15.5+) | 4.4 (3.0-7.9) | NR | |||
| NCT04082936 | Abs | I | IgM2323 | DLBCL | 18 | 64 (36-84)† | 3 (2-9)† | 8/20† | 31 (25) | nr (2-1.5+)† | NR | 7.8 (0.4-23.7)† | |||
| NCT02924402 | Abs | I | PLAMO | Multiple | 46 | 61.5 (31-82)† | 4 (1-10)† | 13/NR† | 51 (25)† | NR | NR | NR | |||
| NCT02290951 | Paper | I | ODRON | Multiple | 85 | 67 (57-73)† | 3 (2-5)† | 8/29† | 37 (24) | 4.4 (2.9-NE); nr (1.6-NE)‡ | 2 (0.9-5.3)‡ | 4.2 (1.5-11.5)† | |||
| NCT03671018 | Abs | I/II | MOSUN-pola | DLBCL | 60 | 68 (20-83) | 3 (1-8) | NR/40 | 65 (48) | nr (6.3 - NE) | 8.9 (3.5-NE) | 5.7 (0.7-27.5) | |||
| NCT03533283 | Abs | I/II | GLOFIT-pola | Multiple | 59 | 59 (29-82) | 2 (1-5) | NR/NR | 80 (51) | nr (0.5-23+) | NR | 3.7 (1.9-5.3) | |||
| Indolent B-NHL§ | ≥second line | After anti-CD20 and alkylating agents | NCT02500407 | Paper | I/II | MOSUN (IV) | Multiple | 68‖ | 60.5 (27-85) | 3 (1-11) | 18/6 | 66 (48) | 16.8 (11.7-NE) | 11.8 (8.4-NE) | NR |
| NCT02500407 | Paper | I/II | MOSUN (IV) | FL | 90‖ | 60 (29-90) | 3 (2-4) | 21/3 | 80 (60) | 22.8 (9.7-NE) | 17.9 (10.1-NE) | 18.3 (2-27.5) | |||
| NCT02500407 | Abs | I/II | MOSUN (SC) | FL | 12 | 68 (41-88)† | 3.5 (1-9)† | 17/42† | 82 (64) | NR | NR | 4.2 (0.1-7.8)† | |||
| NCT03075696 | Abs | I/II | GLOFIT | FL | 75 | 64 (22-86)† | 3 (1-13)† | 12/5† | 81 (69) | NR | NR | NR | |||
| NCT03075696 | Abs | I/II | GLOFIT-obin | FL | 19 | 61 (41-78) | 2 (1-5) | 16/0 | 100 (74) | NR | NR | 5.5 (5.4-6.3) | |||
| NCT03625037 | Paper | I/II | EPCOR | FL | 12 | 73 (63-76) | 4.5 (2.5-8) | 8/0 | 90 (50) | 6 (2.5-15.5) | NR | 13.6 (10.4-16.5) | |||
| NCT04082936 | Abs | I | IgM2323 | FL, MZL | 18 | 64 (36-84)† | 3 (2-9)† | 8/20† | 28 (22) | nr (2-21.5+)† | NR | 7.8 (0.4-23.7)† | |||
| NCT02924402 | Abs | I | PLAMO | Multiple | 17 | 61.5 (31-82)† | 4 (1-10)† | 13/NR† | 51 (25)† | NR | NR | NR | |||
| NCT02290951 | Paper | I | ODRON | FL, MZL | 46 | 67 (57-37)† | 3 (2-5)† | 8/29† | 76 (59)¶ | MZL: 18.1 (1.5-NE) | MZL: NR | 4.2 (1.5-11.5)† | |||
| NCT03467373 | Abs | I | GLOFIT-R-CHOP | FL, MZL (tFL) | 31 | 62 (34-78) | 2 (1-5) | NR/NR | 90 (81) | NR | NR | 9 (0-29) | |||
| NCT04246086 | Abs | I | MOSUN (IV)-len | FL | 29 | 59 (30-79) | 1 (1-6) | NR/NR | 90 (65) | nr (0.3-12.3+) | NR | 5.4 (3-12) | |||
| NCT04663347 | Abs | I/II | EPCOR-R-len | FL | 29 | 67 (42-80) | 1 (1-5) | 17/NR | 100 (92.3) | nr (0.3-6.5+) | NR | NR | |||
| MCL | ≥third line | post-BTKi | NCT03075696 | Abs | I/II | GLOFIT | MCL | 29 | 69 (41-84) | 3 (1-6) | NR/NR | 81 (67) | NR (1-28.5+) | NR | 1.4 (CI NR) |
| NCT02290951 | Paper | I | ODRON | MCL | 12 | 67 (57-37)† | 3 (2-5)† | 8/29† | 50 (33) | 10.9 (1.4-NE) | NR | 4.2 (1.5-11.5) |
Reported abstract data refer to the time of their presentation; Only studies with ≥10 evaluable patients are listed; DLBCL includes DLBCL, not otherwise specified, high-grade B-cell lymphoma, and transformed indolent NHL.
ASCT, high-dose therapy and autologous stem cell support; B-NHL, B-cell non-Hodgkin lymphoma; BTKi, Bruton tyrosine kinase inhibitors; CAR-T, chimeric antigen receptor T-cell therapy; CR, complete response; DHAX, dexamethasone, cytarabine, oxaliplatin; DLBCL, diffuse large B-cell lymphoma; DOR, duration of response; EPCOR, epcoritamab; FL, follicular lymphoma; GemOx, gemcitabine, oxaliplatin; GLOFIT, glofitamab; len, lenalidomide; MCL, mantle cell lymphoma; MOSUN, mosunetuzumab; obin, obinutuzumab; MZL, marginal zone lymphoma; NA, not applicable; NE, not estimable; NR, not reported; nr, not reached; ODRON, odronextamab; ORR, overall response rate; PFS, progression-free survival; PLAMO, plamotamab; pola, polatuzumab, R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristin, prednisone; SC, subcutaneous.
Most studies excluded Richter syndrome and Burkitt lymphoma.
Refers to the entire study population.
Refers to patients with and without previous CAR T-cell therapy, respectively.
Most studies excluded chronic lymphocytic leukemia and lymphoplasmacytic lymphoma/Waldenström macroglobulinemia.
Partially overlapping populations.
Refers to patients with FL only.
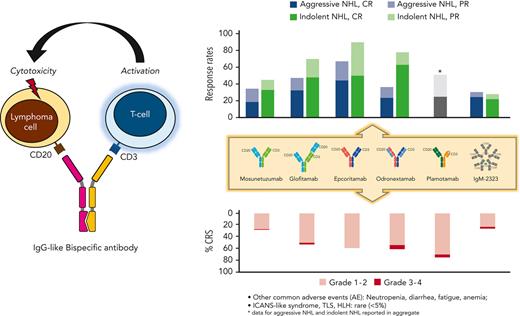
Single-agent clinical efficacy
Mosunetuzumab was evaluated in patients with R/R B-NHL in several phase 1 and 2 studies. In the first,28 mosunetuzumab was administered IV every 3 weeks for up to 8 cycles in patients who achieved a CR and up to 17 cycles in those who achieved a lesser response. Among 197 subjects, 43 were treated at a target dose of 13.5 mg, and 154 at 30 mg. About one-third of the patients had follicular lymphoma (FL), whereas the remaining had aggressive B-NHL (aNHL). The median number of prior therapies was 3, and 10% had previously received CAR T-cell therapy. Excluding the single-patient dose-escalation cohorts, the ORR, CR rate, and median duration of response (DOR) in patients with aNHL were 35%, 19%, and 7.6 months, respectively, with a median progression-free survival (PFS) of 1.4 months, whereas in those with indolent (i) NHL, these figures were 66%, 48%, 16.8 months, and 11.8 months, respectively. Responses were consistent across risk groups, including patients previously exposed to CAR T-cell therapy,28 and similar patients treated with IV or SC formulations.31 An analysis of 90 subjects with R/R FL treated in this study at the target dose of 30 mg was recently published.40 At an extended follow-up of 18.3 months, the best ORR was 80% and the CR rate was 60%. Responses were noted across demographic and risk groups, including patients older than 65 years,41 those refractory to anti-CD20 antibodies and alkylating agents, and those with early progression following first-line therapy.42 The median DOR and PFS were 22.8 months and 17.9 months, respectively, and the estimated 18-month OS rate was 90%.43 These data led to the approval of mosunetuzumab for patients with R/R FL after ≥2 prior lines of therapy by the European Medicines Agency.44
A report of the first 2 portions (dose escalation and dose expansion) of a 3-part international phase 1 study of glofitamab included 171 adults with CD20-positive B-NHL previously exposed to a median of 3 prior lines of therapy.29 Patients received a single 1000 mg dose of pretreatment obinutuzumab followed by fixed or step-up dosing IV glofitamab every 2 or 3 weeks. The drug exhibited dose-dependent clinical activity starting at 0.6 mg, and at doses ≥10 mg the ORR among patients with aNHL was 61%, including 49% CR. Similarly encouraging results were observed in 44 patients with grade 1-3A FL, with an ORR of 70% and a 48% CR rate. Most responses were observed after the first 6 weeks of therapy, and, despite the short observation time (2.9 months), the median duration of CR was not reached, with no relapses seen in complete responders 8 months from study entry.29 In a recent analysis of 155 patients with aNHL treated with glofitamab at the recommended phase 2 target dose of 30 mg, the ORR and CR rates were 52% and 39%, respectively, with similar rates of CR observed in the 52 subjects previously exposed to CAR T-cell therapy (35%) and in the 102 who were not (42%).45 At a median follow-up of 12.6 months, the median DOR was 18.4 months, the PFS was 4.9 months, and the OS was 11.5 months.46 In a separate analysis of patients with R/R FL treated with step-up dosing glofitamab with (N = 19) or without (N = 21) concomitant obinutuzumab, similar deep tumor volume reductions were observed regardless of obinutuzumab administration.47 Finally, in a preliminary report on 21 patients with R/R mantle cell lymphoma, an 81% ORR with 67% CR were observed with glofitamab, regardless of prior Bruton tyrosine kinase inhibitor therapy.48
Epcoritamab was tested in a phase 1/2 trial in 73 subjects with R/R B-NHL at doses ranging from 0.0128 to 60 mg.30 Treatment was given SC initially weekly, then every 2 weeks, then every 28 days. Among 22 patients with aNHL treated at doses between 12 mg (the minimum clinically active dose) and 60 mg, the ORR was 68% and the CR rate was 45%, and the median time to first and to CR were 1.4 and 2.7 months, respectively. At a median follow-up of 9.2 months, 75% of responding patients had remained relapse-free for at least 6 months. In the same phase 1/2 study, 9 of the 10 patients with R/R FL achieved a response, including 5 CR, and activity was seen in a small cohort of patients with mantle cell lymphoma.30 More recently, data from the phase 2 study cohort in subjects with R/R aNHL were reported. Among 157 patients, 61 of whom with prior CAR T-cell therapy, the ORR and CR rates were 63% and 39% respectively, with similar response rates observed in CAR-T–naïve (ORR, 69%; CR, 42%) and CAR-T–exposed (ORR, 54%; CR, 34%) individuals. At the 12-month mark, 80% of CRs were maintained, and 67% of patients were alive.49 A phase 3 trial of epcoritamab vs physician’s choice in patients with R/R DLBCL ineligible for curative therapy is underway.
Odronextamab was tested in a phase 1 trial in 145 patients with R/R B-NHL at doses from 0.1 to 320 mg weekly for 9 weeks, then every other week until progression. The median number of prior therapies was 3, and 41% of the 85 patients with DLBCL had previously received CAR T-cell therapy. Clinical activity was seen at doses ≥80 mg in patients with DLBCL and ≥5 mg in those with FL. In patients with DLBCL, responses were similar for those who had received CAR T-cell therapy and those who had not (ORR 33% and 39%, respectively; CR rate 24% in both groups). Among patients with FL, 78% had an objective response and 63% had a CR.36
Preliminary clinical data are also available for the pentameric CD20xCD3 BsAb IgM-2323 (N = 40), with objective responses seen in a third of patients and CR in 20%,50 and for plamotamab (N = 60), which yielded an ORR of 50% with 25% CR.51
Single-agent BsAbs are also being investigated in patients with previously untreated B-NHL, who would exhibit greater immune fitness and more consistent CD20 expression without having been exposed to chemotherapy or rituximab.52 In patients with newly diagnosed DLBCL unfit for immunochemotherapy, mosunetuzumab produced a best ORR of 68% with 42% CR.53 Similar studies are underway in patients with iNHL, and their results are eagerly awaited (supplemental Table, available on the Blood website).
Early safety observations and toxicity management
The safety profile of BsAb has been rather consistent across trials, and most adverse events (AEs) have been manageable, with rare treatment interruptions or discontinuations and only 5 fatal events causally linked to drug-related AEs in 2 studies.28,36 Toxicities related to T-cell overactivation dominated the safety profile of BsAb. Among these, cytokine release syndrome (CRS) was the most frequent, occurring in 15% to 80% of patients depending on the agent, route of administration, and dosing schedule (Table 3). Clinically, this syndrome presented with any combination of chills, fevers, skin rash, hypotension, hypoxia, and confusion, beginning 0.5 to 2 days after BsAb administration and generally resolving within 1.5 to 3 days. It occurred most frequently and with the greatest severity during the first cycle of therapy, and rarely persisted beyond the second cycle, reflecting conditional, target-dependent T-cell activation. Grading of CRS was adjudicated according to consensus criteria originally developed for CAR T-cell therapy.54,55 Most patients had grade 1 to 2 CRS, which resolved spontaneously or with minimal intervention, including IV hydration, acetaminophen, and corticosteroids. Few individuals experienced a more severe syndrome requiring treatment with tocilizumab, an anti-IL6 antibody, and, occasionally, intensive care unit admission for close monitoring and vasopressors. No fatalities related to CRS have been reported to date across CD20xCD3 BsAb trials.
Mitigation strategies used in BsAb clinical trials and resulting CRS rates
| Trial ID . | N. . | Histology, setting . | Drug(s) . | Route . | Full dose, mg . | Mitigation strategies during C1 . | % CRS‡ . | % serious CRS . | % toci use . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUD . | Hosp∗ . | Other† . | G1 . | G2 . | G3 . | G4 . | G5 . | ||||||||
| NCT03677154 | 29 | DLBCL, ND | MOSUN | IV | 13.5 (8), 30 (21) | Yes | Mandatory | 17 | 3 | 0 | 0 | 0 | NR | 0 | |
| NCT02500407 | 197 | Mixed, R/R | MOSUN | IV | 2.8-40.5 | Yes§ | Optional only in exp. cohorts | 21 | 6 | 1 | 0 | 0 | 7 | 1.5 | |
| 90 | FL, R/R | MOSUN | IV | 30 | Yes | Optional | 26 | 17 | 1 | 1‖ | 0 | NR | 7.8 | ||
| 39 | Mixed, R/R | MOSUN | SC | 45 | Yes | Optional | 11 | 4 | 0 | 0 | 0 | 178 | 10.3 | ||
| 27 | Mixed, R/R | MOSUN, rapid SUD | SC | 45 | Yes | Optional | 33 | 8 | 0 | 0 | 0 | 4 | 0 | ||
| NCT03075696 | 258 | Mixed, R/R | GLOFIT | IV | 0.6-30 | Yes§ | Mandatory | Obin¶ | 31 | 23 | 4 | 2 | 0 | 36 | 20% at RP2D |
| 155 | DLBCL, R/R | GLOFIT | IV | 30 | Yes | Mandatory | Obin¶ | 47 | 12 | 3 | 1 | 0 | NR | 32 | |
| 24 | FL, R/R | GLOFIT | IV | 16 (3), 30 (21) | Yes | Mandatory | Obin¶ | 63 | 13 | 4 | 0 | 0 | 50 | 8.3 | |
| 29 | FL, R/R | GLOFIT, ext. SUD | IV | 30 | Yes | Mandatory | Obin¶ | 35 | 21 | 0 | 0 | 0 | 31 | 21 | |
| 19 | FL, R/R | GLOFIT-obin | IV | 30 | Yes | Mandatory | Obin¶ | 53 | 26 | 0 | 0 | 0 | 26 | 26 | |
| 29 | MCL, R/R | GLOFIT | IV | 0.6-30 | Yes§ | Mandatory | Obin¶ | 35 | 21 | 0 | 3 | 0 | 38 | 24 | |
| NCT03625037 | 68 | Mixed, R/R | EPCOR | SC | 0.0128-60 | Yes§ | Mandatory | Post-dose CS | 29 | 29 | 0 | 0 | 0 | NR | 0 |
| 157 | LBCL, R/R | EPCOR | SC | 48 | Yes | Mandatory | Post-dose CS | 32 | 15 | 2 | 0 | 0 | NR | 14 | |
| NCT04082936 | 40 | Mixed, R/R | IgM2323 | IV | 0.5-1000 | Yes§ | Mandatory | 18 | 5 | 3 | 0 | 0 | NR | NR | |
| NCT02924402 | 64 | Mixed, R/R | PLAMO | IV | 0.7-450ug/Kg, or 50 | Yes§ | NR | 19 | 33 | <1 | <1 | 0 | NR | NR | |
| NCT02290951 | 145 | Mixed, R/R | ODRON | IV | 0.5-320 | Yes | Mandatory | Split-dose | 36 | 18 | 6 | 1 | 0 | NR | NR |
| NCT03677141 | 40 | DLBCL, ND | MOSUN-CHOP | IV | 30 | Yes | NR | 40 | 13 | 0 | 0 | 0 | NR | 5 | |
| NCT03467373 | 31 | Mixed, R/R | GLOFIT-R-CHOP | IV | 0.07-30 | Yes§ | Mandatory | GLOFIT from C2 | 32 | 13 | 10 | 0 | 0 | 26 | 26 |
| 26 | DLBCL, ND | GLOFIT-R-CHOP | IV | 30 | Yes | Mandatory | GLOFIT from C2 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | |
| NCT04663347 | 24 | DLBCL, ND | EPCOR-R-CHOP | SC | 24 (4), 48 (20) | Yes | Mandatory | Post-dose CS | 17 | 17 | 4 | 0 | 0 | NR | NR |
| 29 | DLBCL, R/R | EPCOR-R-DHAX | SC | 48 | Yes | Mandatory | Post-dose CS | 38 | 28 | 10 | 0 | 0 | NR | 7 | |
| 26 | DLBCL, R/R | EPCOR-GemOx | SC | 48 | Yes | Mandatory | Post-dose CS | 27 | 38 | 4 | 0 | 0 | NR | 19 | |
| NCT03671018 | 63 | DLBCL (60), FL (3), R/R | MOSUN-pola | IV | 30 | Yes | Optional | 16 | 2 | 0 | 0 | 0 | 8 | 0 | |
| NCT03533283 | 59 | Mixed, R/R | GLOFIT-pola | IV | 10 (6), 30 (53) | Yes | NR | GLOFIT from C1D8 | 29 | 12 | 0 | 0 | 2 | 24 | 11.9 |
| NCT04246086 | 29 | FL, R/R | MOSUN-len | IV | 30 | Yes | Optional | 24 | 3 | 0 | 0 | 0 | 14 | 0 | |
| NCT04663347 | 29 | FL, R/R | EPCOR-R-len | SC | 2 (3), 48 (26) | Yes | Mandatory | Post-dose CS | 28 | 14 | 7 | 0 | 0 | NR | NR |
| Trial ID . | N. . | Histology, setting . | Drug(s) . | Route . | Full dose, mg . | Mitigation strategies during C1 . | % CRS‡ . | % serious CRS . | % toci use . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUD . | Hosp∗ . | Other† . | G1 . | G2 . | G3 . | G4 . | G5 . | ||||||||
| NCT03677154 | 29 | DLBCL, ND | MOSUN | IV | 13.5 (8), 30 (21) | Yes | Mandatory | 17 | 3 | 0 | 0 | 0 | NR | 0 | |
| NCT02500407 | 197 | Mixed, R/R | MOSUN | IV | 2.8-40.5 | Yes§ | Optional only in exp. cohorts | 21 | 6 | 1 | 0 | 0 | 7 | 1.5 | |
| 90 | FL, R/R | MOSUN | IV | 30 | Yes | Optional | 26 | 17 | 1 | 1‖ | 0 | NR | 7.8 | ||
| 39 | Mixed, R/R | MOSUN | SC | 45 | Yes | Optional | 11 | 4 | 0 | 0 | 0 | 178 | 10.3 | ||
| 27 | Mixed, R/R | MOSUN, rapid SUD | SC | 45 | Yes | Optional | 33 | 8 | 0 | 0 | 0 | 4 | 0 | ||
| NCT03075696 | 258 | Mixed, R/R | GLOFIT | IV | 0.6-30 | Yes§ | Mandatory | Obin¶ | 31 | 23 | 4 | 2 | 0 | 36 | 20% at RP2D |
| 155 | DLBCL, R/R | GLOFIT | IV | 30 | Yes | Mandatory | Obin¶ | 47 | 12 | 3 | 1 | 0 | NR | 32 | |
| 24 | FL, R/R | GLOFIT | IV | 16 (3), 30 (21) | Yes | Mandatory | Obin¶ | 63 | 13 | 4 | 0 | 0 | 50 | 8.3 | |
| 29 | FL, R/R | GLOFIT, ext. SUD | IV | 30 | Yes | Mandatory | Obin¶ | 35 | 21 | 0 | 0 | 0 | 31 | 21 | |
| 19 | FL, R/R | GLOFIT-obin | IV | 30 | Yes | Mandatory | Obin¶ | 53 | 26 | 0 | 0 | 0 | 26 | 26 | |
| 29 | MCL, R/R | GLOFIT | IV | 0.6-30 | Yes§ | Mandatory | Obin¶ | 35 | 21 | 0 | 3 | 0 | 38 | 24 | |
| NCT03625037 | 68 | Mixed, R/R | EPCOR | SC | 0.0128-60 | Yes§ | Mandatory | Post-dose CS | 29 | 29 | 0 | 0 | 0 | NR | 0 |
| 157 | LBCL, R/R | EPCOR | SC | 48 | Yes | Mandatory | Post-dose CS | 32 | 15 | 2 | 0 | 0 | NR | 14 | |
| NCT04082936 | 40 | Mixed, R/R | IgM2323 | IV | 0.5-1000 | Yes§ | Mandatory | 18 | 5 | 3 | 0 | 0 | NR | NR | |
| NCT02924402 | 64 | Mixed, R/R | PLAMO | IV | 0.7-450ug/Kg, or 50 | Yes§ | NR | 19 | 33 | <1 | <1 | 0 | NR | NR | |
| NCT02290951 | 145 | Mixed, R/R | ODRON | IV | 0.5-320 | Yes | Mandatory | Split-dose | 36 | 18 | 6 | 1 | 0 | NR | NR |
| NCT03677141 | 40 | DLBCL, ND | MOSUN-CHOP | IV | 30 | Yes | NR | 40 | 13 | 0 | 0 | 0 | NR | 5 | |
| NCT03467373 | 31 | Mixed, R/R | GLOFIT-R-CHOP | IV | 0.07-30 | Yes§ | Mandatory | GLOFIT from C2 | 32 | 13 | 10 | 0 | 0 | 26 | 26 |
| 26 | DLBCL, ND | GLOFIT-R-CHOP | IV | 30 | Yes | Mandatory | GLOFIT from C2 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | |
| NCT04663347 | 24 | DLBCL, ND | EPCOR-R-CHOP | SC | 24 (4), 48 (20) | Yes | Mandatory | Post-dose CS | 17 | 17 | 4 | 0 | 0 | NR | NR |
| 29 | DLBCL, R/R | EPCOR-R-DHAX | SC | 48 | Yes | Mandatory | Post-dose CS | 38 | 28 | 10 | 0 | 0 | NR | 7 | |
| 26 | DLBCL, R/R | EPCOR-GemOx | SC | 48 | Yes | Mandatory | Post-dose CS | 27 | 38 | 4 | 0 | 0 | NR | 19 | |
| NCT03671018 | 63 | DLBCL (60), FL (3), R/R | MOSUN-pola | IV | 30 | Yes | Optional | 16 | 2 | 0 | 0 | 0 | 8 | 0 | |
| NCT03533283 | 59 | Mixed, R/R | GLOFIT-pola | IV | 10 (6), 30 (53) | Yes | NR | GLOFIT from C1D8 | 29 | 12 | 0 | 0 | 2 | 24 | 11.9 |
| NCT04246086 | 29 | FL, R/R | MOSUN-len | IV | 30 | Yes | Optional | 24 | 3 | 0 | 0 | 0 | 14 | 0 | |
| NCT04663347 | 29 | FL, R/R | EPCOR-R-len | SC | 2 (3), 48 (26) | Yes | Mandatory | Post-dose CS | 28 | 14 | 7 | 0 | 0 | NR | NR |
The table separates single-agent studies (upper portion) from combination studies (lower portion). Data generally refer to the CRS episode with highest frequency and/or of the highest grade following BsAb dosing. These varied among trials. Virtually all patients received acetaminophen, antihistamine, and corticosteroids premedication. The distinction between CTCAE-defined infusion-related reaction and CRS was either not explicitly made or left at the treating physician's discretion, thus it is possible that the true incidence of CRS be different than reported in some studies.
C, cycle; CS, corticosteroids; (D)LBCL, (diffuse) large B-cell lymphoma; EPCOR, epcoritamab; esc, escalation; exp, expansion; FL, follicular lymphoma; GLOFIT, glofitamab; G, grade; len, lenalidomide; hosp, hospitalization; MCL, mantle cell lymphoma; MOSUN, mosunetuzumab; ND, newly diagnosed, NR, not reported; obin, obinutuzumab; ODRON, odronextamab; PLAMO, plamotamab; pola, polatuzumab, RP2D, recommended phase-2 dose; R/R relapsed/refractoy; SC, subcutaneous; SUD, step-up dosing; toci, tocilizumab.
Prescribed on the day of expected highest risk of CRS.
In all reported studies, corticosteroids were used as part of the premedication during cycle 1 and, in some cases cycle 2.
Incidence as reported by authors, separately reported single components are not listed in this table.
Performed in only part of the study population, for example, at certain dose levels or in expansion cohorts only.
Patient with leukemic phase FL.
Generally administered on cycle 1, day 7; defined according to the ASBMT consensus criteria.
Neurological toxicities potentially related to T-cell overactivation have been observed with the use of BsAb, including delirium, dysphasia, tremor, lethargy, difficulty concentrating, agitation, confusional state, aphasia, depressed level of consciousness, encephalopathy, seizures, or cerebral edema.55 Although the term immune effector cell–associated neurotoxicity syndrome–like has been used to describe these AEs, the pathogenesis and clinical findings of neurological toxicity associated with BsAb and CAR T-cell therapy (for which the term immune effector cell–associated neurotoxicity syndrome was coined) may not be overlapping. CAR T-cells are known to traffic to the CSF, causing increased protein and cytokine levels,56 and potentially targeting CD19-expressing mural cells in the brain,57 but IgG-like BsAb are not expected to cross the blood-brain barrier, and virtually no information is available on the presence of activated T cells or inflammatory cytokines in the CSF of these patients. Accordingly, neurological AEs in BsAb clinical trials have been rare, generally mild, and self-resolving within hours of their onset.28-30,36
Several mitigation strategies have been pursued to prevent or minimize the severity of AEs due to T-cell overactivation (Table 3). A widely implemented approach is referred to as step-up dosing, where patients are given a small, “priming” dose followed by an intermediate dose, before receiving the full dose of the BsAb. In preclinical models, this strategy was shown to reduce peak systemic cytokine release without significantly compromising tumor cell killing.58 Investigators have used both rapid (2-step)31 and extended one (4-step)47 dose-escalation schemes. Other preventive measures have included slower IV infusion,29,36 the use of prophylactic corticosteroids during the first few weeks of therapy,30 inpatient administration of the dose at the highest risk of provoking CRS,28-30,36 and, in glofitamab trials, single-dose obinutuzumab pretreatment based on preclinical experiments suggesting that depleting circulating B cells may dampen T-cell activation, cytokine release, and subsequent endothelial cell activation.15 Preclinical data also suggested that modulating the affinity for CD3 may attenuate the severity of CRS while preserving the efficacy of the BsAb,59 an observation that has not yet been clinically validated.
Aside from AEs due to T-cell overactivation, the toxicity profile of BsAb is characterized by neutropenia (15%-33%), hypophosphatemia (13%-29%), anemia (19%-38%) fatigue (18%-42%), and diarrhea (15%-26%), all predominantly of grade 1 to 2 and reversible.28-30,36,50,51 It should be noted that long-term BsAb safety data are not yet available, and potential AEs related to delayed B-cell recovery will need to be ascertained.
BsAb-containing combinations
Preclinical work had demonstrated that, although T-cell numbers are decreased in patients treated with immunochemotherapy, surviving T cells can still be activated and kill lymphoma cells when engaged by a BsAb.60 Accordingly, the lymphoma killing activity of CD20xCD3 BsAb was not significantly affected when T-cell cytotoxic agents like cyclophosphamide or dexamethasone were coadministered, individually or in combination, both in vitro and in mice expressing human CD20 and CD3.34,61 Moreover, BsAb appear combinable with monospecific anti-CD20 antibodies because they target partially overlapping epitopes, do not compete for FcγR, and induce potent target-cell killing at low occupancy rates. Several studies combining BsAb with cytotoxic chemotherapy were commenced, and some results have begun to emerge (Table 2 and supplemental Table).
Glofitamab and epcoritamab have been integrated into standard-of-care platinum-based chemoimmunotherapy platforms for the treatment of patients with R/R aNHL. Clinical results have been promising (eg, ORR and CR rate of 100% and 86%, respectively, for epcoritamab-R-DHAX,62 and 92% and 60%, respectively, for epcoritamab-gemcitabine, oxaliplatin63) and registration-directed phase 3 trials are being launched (supplemental Table).
Both mosunetuzumab and glofitamab have been combined with the anti-CD79b antibody-drug conjugate polatuzumab vedotin in patients with R/R DLBCL. The ORR and CR rates of these combinations were 65% and 48%, respectively, for mosunetuzumab and 80% and 51%, respectively, for glofitamab. Although these results appear similar to those observed in the single-agent trials, the short follow-up precludes an exhaustive evaluation of the depth and durability of these responses.64
Additional studies have combined BsAb with immunomodulatory agents with the aim of restoring the immune synapse65 and potentiating BsAb-dependent cytotoxicity. In patients with R/R FL, mosunetuzumab and epcoritamab have been combined with lenalidomide, with or without rituximab. Both combinations exhibited remarkable preliminary activity, with responses seen in nearly all patients and CR in the majority of them.66-68 These results are still immature, and their long-term performance, the role of the addition of rituximab, or the optimal treatment duration remain to be defined. Nonetheless, phase-3, registration-directed trials of these combinations have been launched. Other approaches include combining CD20xCD3 BsAb with other BsAb that deliver costimulatory signals to engaged T cells, such as those targeting CD137 or CD28 (supplemental Table).
Use of BsAb in the initial management of patients with B-cell lymphoma
In patients with newly diagnosed DLBCL, 2 phase 1/2 studies combining mosunetuzumab or glofitamab with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) with or without rituximab produced positive efficacy signals.69,70 Given the already high efficacy of standard R-CHOP in unselected patients with DLBCL, one approach to incorporating BsAb is to reserve their use to higher-risk individuals. In a study of combined epcoritamab and R-CHOP in 33 patients with DLBCL with an international prognostic index of 3 to 5, all subjects responded, and 77% had a CR.71 Another high-risk DLBCL category includes patients with <2.5 log reduction in circulating tumor (ct)DNA after 2 cycles of R-CHOP,72 and a study of glofitamab-R-CHOP in patients with “ctDNA high-risk” DLBCL is underway. Phase 3 trials comparing BsAb plus R-CHOP–like platforms to standard immunochemotherapy are expected to commence. For patients with treatment-naïve FL, trials of epcoritamab combined with bendamustine and rituximab or lenalidomide and rituximab are accruing, while studies of mosunetuzumab as a single agent or in combination with polatuzumab vedotin or lenalidomide are being initiated (supplemental Table).
It is worth noting that in combination trials, the addition of BsAb to chemotherapy has not so far significantly interfered with the timely delivery of chemotherapy or produced new adverse safety signals, and the toxicity profile of each combination largely recapitulated that of its components. Interestingly, combining potentially T-cell cytotoxic chemotherapy did not seem to diminish the rate or severity of CRS, suggesting that BsAb-engaged T cells retain significant inflammatory activity in the presence of chemotherapy (Table 3).
Biomarkers of response, resistance, and toxicity
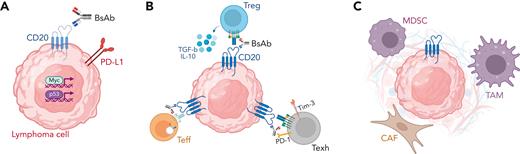
Remarkable activity and the potential for serious CRS represent the "yin-yang" of BsAb therapy. Consequently, the search for PD biomarkers of response, resistance, and toxicity has been intense. Potential mechanisms of BsAb resistance may be broadly separated into 3 categories, as depicted in Figure 1. First, selective pressure may drive the activation of programs that promote immune escape. Loss of the target antigen CD20 is known to occur after anti-CD20 monoclonal antibody therapy.73-75 Although higher baseline CD20 expression was not clearly associated with the achievement of CR after CD20xCD3 BsAb therapy,45,76 a loss of CD20 was observed in patients with progressive or recurrent disease and in some cases was associated with CD20 gene mutations.52,77 Furthermore, a detailed analysis of tumor biopsies from patients with DLBCL treated with glofitamab revealed a somewhat higher frequency of TP53 mutations or overactivated MYC signaling among patients with progression, suggesting that distinct oncogenic pathways might be associated with either de novo or acquired resistance to BsAb.78 Intriguingly, both TP53 mutations79-81 and MYC amplifications82-84 are known to promote resistance to immunotherapy, including CAR T-cell therapy.85
Mechanisms of resistance to BsAb within the lymphoma microenvironment. Potential mechanisms of resistance to BsAb therapy include (A) tumor cell–intrinsic mechanisms, such as antigen loss and activation of immune-evasive gene expression programs, (B) T-cell intrinsic mechanisms, including activation of regulatory T-cells, downregulation of the T-cell receptor, and development of T-cell exhaustion, and (C) T-cell extrinsic mechanisms, including recruitment of immunosuppressive myeloid and/or stromal cells. CAF, cancer-associated fibroblast; IL-10, interleukin-10; MDSC, myeloid-derived suppressor cell; PD-1, programmed death 1; PD-L1, programmed death ligand 1; TAM, tumor-associated macrophage; Teff, effector T cell; Texh, exhausted T cell; TGF-b, transforming growth factor beta; Tim-3, T-cell immunoglobulin mucin-3; Treg, regulatory T cell.
Mechanisms of resistance to BsAb within the lymphoma microenvironment. Potential mechanisms of resistance to BsAb therapy include (A) tumor cell–intrinsic mechanisms, such as antigen loss and activation of immune-evasive gene expression programs, (B) T-cell intrinsic mechanisms, including activation of regulatory T-cells, downregulation of the T-cell receptor, and development of T-cell exhaustion, and (C) T-cell extrinsic mechanisms, including recruitment of immunosuppressive myeloid and/or stromal cells. CAF, cancer-associated fibroblast; IL-10, interleukin-10; MDSC, myeloid-derived suppressor cell; PD-1, programmed death 1; PD-L1, programmed death ligand 1; TAM, tumor-associated macrophage; Teff, effector T cell; Texh, exhausted T cell; TGF-b, transforming growth factor beta; Tim-3, T-cell immunoglobulin mucin-3; Treg, regulatory T cell.
A second mechanism of resistance to BsAb is intrinsic or acquired T-cell dysfunction within the lymphoma microenvironment. The landscape of nonmalignant T cells within the lymphoma microenvironment is diverse and includes both CD4+ and CD8+ cells with cytotoxic as well as regulatory functions. Because BsAb-induced CD3-mediated T-cell activation is by definition nonselective, activation of regulatory or suppressive T cells within the lymphoma microenvironment might promote resistance.76 Correlative studies of phase 1 trials confirmed the preclinical observation that BsAb induce a rapid and transient decrease in peripheral blood CD3+ cells (thought to reflect T-cell redistribution),29,30,78 which was more pronounced in responders,78 and, simultaneously, a dose-dependent increase in activated (granzyme B+, Tim3+, or PD-1+) CD8+ and CD4+ T cells,28,29,53 which was sustained with repeated dosing.30,43,78 However, in ex vivo studies, BsAb therapy exerted greater tumor killing by peripheral blood than by intratumoral T cells,86 suggesting that tumor-resident T cells may be at least in part resistant to BsAb-dependent activation. This resistance may occur owing to the consequences of persistent TCR triggering, which is known to downregulate CD3 expression and may desensitize intratumoral T cells to further BsAb-dependent activity.87 Moreover, chronic TCR triggering promotes a dysfunctional T-cell phenotype known as T-cell “exhaustion,” which is associated with blunted antitumor activity.88 Finally, the lymphoma microenvironment contains several elements, such as tumor-associated macrophages, cancer-associated fibroblasts, and myeloid-derived suppressor cells, all of which can promote immunosuppression. Whether and how these cells directly limit BsAb activity remains to be determined.
Data on clinical and biological predictors of CRS have also begun to emerge. In one clinical study, pretreatment parameters such as age, stage, tumor burden, bone marrow involvement, and the presence of circulating lymphoma B cells were found to correlate with the risk of CRS.89 Other factors, including a low in vitro EC50, the dose of the BsAb, and its route of administration32 may influence this risk. Studies looking at changes in plasma levels of cytokines like interleukin-2, interleukin-6 interferon gamma, or tumor necrosis factor alfa as a function of treatment have been largely descriptive, and the correlation between cytokine levels and CRS is inconsistent.28,30,64,78,89
Conclusions
BsAb, primarily those targeting CD20xCD3, represent a breakthrough in the treatment of patients with B-NHL and will likely constitute an important addition to the available therapeutic armamentarium. Managing toxicities related to T-cell overactivation will likely require a learning curve. In this sense, physician and patient education, better identification of risk factors for CRS, and further development of prophylaxis and treatment guidelines will be key to the widespread adoption of these drugs. Following mosunetuzumab’s approval for R/R FL in Europe, BsAb are likely to receive additional approvals by health authorities for patients with R/R B-NHL.
As we learn more about the efficacy and safety of these drugs, several emerging questions will need to be answered (Table 4). Although CR rates observed with BsAb in unselected patients rival those seen with CAR T-cell therapy, a fair appraisal of these drugs will require additional information on the DORs and on whether cures are achievable, particularly in patients with aNHL. BsAb are being increasingly used in combination with other agents to improve the rate and DORs, and numerous such trials underway attest to the appeal of this new therapeutic modality. Similarly, efforts to move their use earlier in the disease course are accelerating. Understanding the determinants of response and resistance will be critical for patient selection, optimal positioning, and future rational combinations. Ultimately, well-designed clinical trials will be necessary to assess the role of BsAb in the management of patients with lymphoma and move their development forward.
Synopsis of areas of uncertainty in BsAb research and relative specific challenges
| Areas of uncertainty . | Challenges . |
|---|---|
| Management of T-cell overactivation syndromes | Identifying risk factors for CRS |
| Optimal step-up dosing, drug formulation, prophylaxis | |
| Outpatient administration | |
| Patient and provider education | |
| DOR | Optimal duration of BsAb therapy |
| Predictors of durable response | |
| Moving BsAb to earlier lines of therapy | Competitive landscape |
| Selecting the most appropriate patient populations (eg, high-risk disease) | |
| Optimal combinations | Moving beyond cytotoxic agents as partners |
| Rational (rather than expedient) combinations | |
| Understanding mechanisms of resistance | Identifying actionable tumor-intrinsic resistance mechanisms |
| Detailed characterization of T-cell function (and dysfunction) during BsAb therapy | |
| Dissecting the role of other players in the lymphoma immune microenvironment |
| Areas of uncertainty . | Challenges . |
|---|---|
| Management of T-cell overactivation syndromes | Identifying risk factors for CRS |
| Optimal step-up dosing, drug formulation, prophylaxis | |
| Outpatient administration | |
| Patient and provider education | |
| DOR | Optimal duration of BsAb therapy |
| Predictors of durable response | |
| Moving BsAb to earlier lines of therapy | Competitive landscape |
| Selecting the most appropriate patient populations (eg, high-risk disease) | |
| Optimal combinations | Moving beyond cytotoxic agents as partners |
| Rational (rather than expedient) combinations | |
| Understanding mechanisms of resistance | Identifying actionable tumor-intrinsic resistance mechanisms |
| Detailed characterization of T-cell function (and dysfunction) during BsAb therapy | |
| Dissecting the role of other players in the lymphoma immune microenvironment |
Acknowledgment
We are grateful to Terry Helms for creating the illustrations of the bispecific antibodies contained in this article.
This work was supported by a grant from the National Institutes of Health, National Cancer Institute to the Memorial Sloan Kettering Cancer Center (P30 CA008748).
Authorship
Contribution: All authors designed the research, summarized the data, wrote, and approved the paper.
Conflict-of-interest disclosure: L.F serves as a consultant for Genmab, AbbVie, and Hoffmann-La Roche/Genentech; has received research funding from Genmab, AbbVie, and Hoffmann-La Roche/Genetech; and has membership on advisory committees of ADC Therapeutics. S.A.V. has membership on advisory committees of Immunai Inc and receives consulting fees from Koch Disruptive Industries. G.A.S. has membership on advisory committees receives consulting fees from AbbVie, Bayer, Beigene, BMS/Celgene, Epizyme, Hoffmann-La Roche/Genetech, Genmab, Incyte, Janssen, Kite/Gilead, Loxo, Miltenyi, Molecular Partners, Morphosys, Nordic Nanovector, Novartis, Rapt, Regeneron, and Takeda; and is a shareholder in Owkin.
Correspondence: Gilles A. Salles, Lymphoma Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 530 E 74th St, New York, NY 10021; e-mail: sallesg@mskcc.org.
References
Author notes
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal