In this issue of Blood, Makishima et al1 use next-generation sequencing data from a large multinational cohort of patients with myeloid neoplasm (MN) (n = 9082; Japanese, 49%) to define the frequency and spectrum of pathogenic DDX41 germ line and somatic variants, estimate the age-related penetrance of DDX41 variants on the development of MNs, and elucidate key genetic and clinical features germane to DDX41-mutated MNs.
Of the many germ line variants that have causal predispositions to MNs, germ line mutations in DDX41, which encodes a DEAD-box type RNA helicase involved in various aspects of RNA metabolism and myeloid differentiation,2 are present in ∼2% to 5% of patients with acute myeloid leukemia (AML) and myelodysplastic neoplasm (MDS) and explain ∼80% of known germ line predisposition to MNs in adults.1,3 Recent data3-5 (previously commented on by Rio-Machin and Fitzgibbon6) across multiple cohorts have further recapitulated findings that predisposition to MNs associated with germ line DDX41 variants is characterized by late onset of MNs, propensity towards cytopenia, favorable prognosis relative to DDX41 wild-type (WT) MNs, normal karyotype, hypocellular bone marrow, and male sex skewing. In an improvement over the 2016 World Health Organization classification of MNs, the 2022 revisions have revised their classification of MNs with germ line predispositions to specifically include pathogenic/likely pathogenic (P/LP) germ line variants of DDX41 and have recognized hypoplastic MDS with DDX41 predispositions as a distinct disease type.7
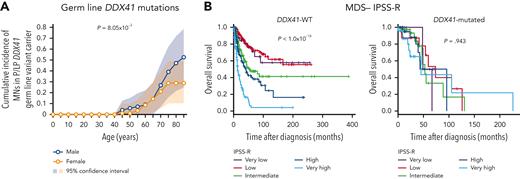
Although germ line DDX41 mutations clearly constitute a larger etiologic fraction of MN cases than other variants, the associated risk of developing MNs from carrying a germ line DDX41 variant has yet to be determined. To address this multifaceted topic, Makishima et al enrolled 9082 patients with MN and identified P/LP germ line variants in 293 patients. Of these 293 cases, 159 acquired secondary somatic DDX41 mutations, 64% of which were p.R525H, similar to previous findings.3,8 Among the 293 germ line variants, 197 were truncating, whereas most somatic variants (223/229) were non-truncating. Somatic truncating variants were all heterozygous and mutually exclusive with truncating germ line mutations, implicating that homozygosity in truncating alleles promotes cell lethality. To estimate the penetrance of these variants on the development of MN, Makishima et al calculated the cumulative incidence of MN in a cohort of 525 first-degree relatives of DDX41-mutated MN cases. The penetrance of P/LP variants was trivial until age 40 years but swiftly achieved ∼49% by age 90 years. Concordantly, a male preponderance was associated with germ line DDX41 mutations across all MN subtypes, and the penetrance associated with DDX41 risk alleles was significantly higher in males (52.5%) than females (28.7%) by age 85 years (see figure panel A). This estimation is perplexing given that DDX41 risk allele frequencies were found to be similar between sexes in the general population, further implicating sex-specific selection processes on disease penetrance.
(A) Age-related risk of developing MN in male and female carriers of DDX41 risk alleles. Cumulative incidence was estimated by kin-cohort analysis using 525 first-degree relatives. (B) Kaplan-Meier survival plots of MDS patients with DDX41-WT or DDX41-mutations stratified by IPSS-R. See Figures 3F and 6C in the article by Makishima et al that begins on page 534.
(A) Age-related risk of developing MN in male and female carriers of DDX41 risk alleles. Cumulative incidence was estimated by kin-cohort analysis using 525 first-degree relatives. (B) Kaplan-Meier survival plots of MDS patients with DDX41-WT or DDX41-mutations stratified by IPSS-R. See Figures 3F and 6C in the article by Makishima et al that begins on page 534.
When assessing the frequency of DDX41-mutated variants across the spectrum of MNs, DDX41 variants had the highest representation in high-risk MDS and secondary AML. This enrichment was correlated with greater frequencies of truncating variants in these cases, as carriers with truncated DDX41 variants were found to progress from MDS to secondary AML 2.5 times faster than those with DDX41-WT. This enhanced clonal evolution was not observed in carriers with non-truncating variants. Despite truncation status of DDX41 variants being a strong predictor of malignant transformation, the presence of DDX41-truncating alleles was not a predictor of overall survival (OS) in DDX41-mutated cases.
As an additional acknowledgment of DDX41-mutated MN as a distinct entity, Makishima et al identified that an enrichment of AML-associated type-1 mutations, a class of mutations often acquired during the progression from high-risk MDS to secondary AML,9 was not recapitulated in respective comparisons with DDX41 mutants. In patients with germ line P/LP DDX41 variants, the frequencies of secondary somatic DDX41 variants were often larger compared with other driver mutations, consistent with observations in which carriers of a heterozygous P/LP germ line DDX41 variant later acquired a secondary somatic missense mutation in the other allele prior to malignant transformation.3,10 Furthermore, DDX41-mutated cases have better OS, which, unlike in DDX41-WT cases, is not impacted by risk classification assigned by the revised/molecular international prognostic scoring system (IPSS-R/M) (see figure panel B) or TP53 mutation status. Intriguingly, retrospective analyses indicate that DDX41-mutated cases had better prognoses than unmutated cases, particularly when treated with hypomethylating agents.
Key takeaways that will guide risk management of affected patients include the following: (1) Truncation status of DDX41 variants can inform whether the variants are germ line in nature, as somatic DDX41 mutations are almost exclusively non-truncating. (2) Truncating DDX41 variants are associated with faster progression to leukemia, but truncation status itself is not a predictor of OS in DDX41-mutated cases. (3) The life-long risk of developing MN from a P/LP germ line DDX41 variant is ∼50%, and the penetrance associated with DDX41 risk alleles in males is almost twice that of the penetrance in females. (4) Screening for DDX41 mutational status in patients with MN is of paramount importance as prognosis of DDX41-mutant MDS is discordant with classical IPSS-R/M stratification and TP53 mutational status. (5) DDX41-mutated MNs have better responsiveness to hypomethylating agents.
Beyond clinical implications, these findings also raise important biological questions. For starters, why do these cases have lower frequencies of other mutations commonly observed in DDX41-WT MNs, and why do carriers of a monoallelic germ line P/LP DDX41 variant have higher propensities of acquiring a secondary somatic DDX41 mutation that can result in MN onset? One possibility is that mutations commonly observed in DDX41-WT cases are not compatible with germ line P/LP DDX41 variants in terms of hematopoietic stem cell fitness. This could potentially explain the observed higher frequency of secondary missense mutations (p.R525H) in DDX41 based on the absence of homozygous DDX41 truncating alleles, as somatic missense mutations such as p.R525H are likely hypomorphic.10 Further investigations into the underrepresentation of type-1 mutations in biallelic DDX41-mutated MNs may reveal opportunities for therapeutic interventions and provide further insights on alternative mechanisms of leukemic evolution.
Findings from Makishima et al elegantly reaffirm that correctly recognizing DDX41 mutational status will have widespread implications on the diagnosis and management of DDX41-related MNs and genetic counseling of affected family members to inform risk of MN development with associated DDX41 variants.
Conflict-of-interest disclosure: J.E.P. has served on advisory boards and received honoraria or clinical trial funding from Celgene/BMS and AbbVie. P.T. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal