Key Points
Children with SCD had normalization of cerebral hemodynamics after stem cell transplant similar to children in the control group.
Stem cell transplant may restore cerebral oxygen delivery reserve, providing greater stroke protection than chronic transfusion therapy.
Abstract
Children with sickle cell disease (SCD) demonstrate cerebral hemodynamic stress and are at high risk of strokes. We hypothesized that curative hematopoietic stem cell transplant (HSCT) normalizes cerebral hemodynamics in children with SCD compared with pre-transplant baseline. Whole-brain cerebral blood flow (CBF) and oxygen extraction fraction (OEF) were measured by magnetic resonance imaging 1 to 3 months before and 12 to 24 months after HSCT in 10 children with SCD. Three children had prior overt strokes, 5 children had prior silent strokes, and 1 child had abnormal transcranial Doppler ultrasound velocities. CBF and OEF of HSCT recipients were compared with non-SCD control participants and with SCD participants receiving chronic red blood cell transfusion therapy (CRTT) before and after a scheduled transfusion. Seven participants received matched sibling donor HSCT, and 3 participants received 8 out of 8 matched unrelated donor HSCT. All received reduced-intensity preparation and maintained engraftment, free of hemolytic anemia and SCD symptoms. Pre-transplant, CBF (93.5 mL/100 g/min) and OEF (36.8%) were elevated compared with non-SCD control participants, declining significantly 1 to 2 years after HSCT (CBF, 72.7 mL/100 g per minute; P = .004; OEF, 27.0%; P = .002), with post-HSCT CBF and OEF similar to non-SCD control participants. Furthermore, HSCT recipients demonstrated greater reduction in CBF (−19.4 mL/100 g/min) and OEF (−8.1%) after HSCT than children with SCD receiving CRTT after a scheduled transfusion (CBF, −0.9 mL/100 g/min; P = .024; OEF, −3.3%; P = .001). Curative HSCT normalizes whole-brain hemodynamics in children with SCD. This restoration of cerebral oxygen reserve may explain stroke protection after HSCT in this high-risk patient population.
Introduction
Children with sickle cell disease (SCD) have high risks of overt strokes and silent cerebral infarctions (SCI).1-3 Although chronic red blood cell transfusion therapy (CRTT) reduces first and recurrent infarcts, up to 20% of children receiving CRTT have second overt strokes, and up to 25% have new or enlarging SCIs.4,5 Contributors to stroke recurrence are incompletely known but include cerebral vasculopathy/moyamoya, acute exacerbations of chronic anemia, and intercurrent illnesses affecting cerebral oxygen delivery.6 After curative hematopoietic stem cell transplant (HSCT), the risk of recurrent stroke or SCI is reduced compared with patients continuing on CRTT.7-9
Noninvasive brain magnetic resonance imaging (MRI) techniques have elucidated cerebral hemodynamic changes in children with SCD. Cerebral blood flow (CBF) measures the volume of blood delivered to brain tissue per minute, whereas oxygen extraction fraction (OEF) is the fraction of oxygen removed from hemoglobin (Hb) in brain tissue. Cerebral compensation mechanisms increase CBF and OEF in response to anemia. In adults and children with SCD, CBF is elevated compared with non-anemic controls and with anemic subjects.10-13 Whole-brain OEF is elevated in children with SCD and correlates negatively with Hb concentration.10 In children with SCD, OEF is highest in the brain regions with the lowest CBF, which are also the regions at greatest risk for SCIs.10,14 Furthermore, these elevations are highest in children receiving no disease-modifying therapy, with lesser elevations in those treated with hydroxyurea or CRTT.15 Within individuals with SCD, OEF, and to a lesser extent CBF, improve rapidly after a red blood cell (RBC) transfusion but remain elevated compared with controls.16 Curative HSCT has improved CBF and OEF in small series of children and adults with SCD,17,18 but post-HSCT CBF and OEF normalization has not been demonstrated compared with control participants.
In this prospective cohort study, we quantified the effects of HSCT on cerebral hemodynamics measured with MRI. We tested the primary hypothesis that within-subject whole-brain CBF and OEF normalize after HSCT, with post-HSCT CBF and OEF similar to non-SCD control participants. Furthermore, we tested the secondary hypothesis that children with SCD have greater reduction in CBF and OEF after HSCT than CRTT recipients experience after an RBC transfusion.
Methods
Enrollment of participants and clinical data
Participants with SCD receiving HSCT or CRTT were recruited from a single center and informed consent was obtained from participants or legal guardians. The study was approved by the Human Research Protection Office at Washington University in St. Louis, St. Louis, MO. Children were referred for HSCT or prescribed CRTT by their treating hematologist based on SCD severity, due to overt or silent strokes, abnormal transcranial Doppler ultrasound (TCD) velocities indicating high stroke risk, frequent vaso-occlusive pain episodes (VOEs), acute chest syndrome (ACS), or end-organ damage. The inclusion criteria were age ≥5 years, HSCT or CRTT to treat SCD (any genotype), and the ability to undergo brain MRI without sedation. Exclusion criteria were neurological conditions unrelated to SCD, such as congenital brain malformations or history of traumatic brain injury, or HSCT for reasons other than SCD. Three individuals were originally recruited as CRTT participants and later underwent HSCT. They were included only as HSCT participants in the current analysis but were part of the CRTT cohort in a previous study.16 A non-SCD control group comprising siblings of SCD patients was included, as described previously.10
Brain MRI and magnetic resonance angiography (MRA) were performed at participants’ pretransplant baseline, 1 to 3 months before the transplant. For all 7 children receiving CRTT before HSCT, the baseline MRI/MRA was performed within 24 hours before their scheduled blood transfusion, and a second MRI repeating CBF and OEF sequences was performed within 24 hours after transfusion when possible. In the analyses below, pre-HSCT refers to studies done before transfusion, unless otherwise specified. Brain MRI/MRA was repeated 12 to 24 months after HSCT, including clinical sequences and CBF and OEF sequences. The CRTT cohort participants underwent MRI/MRA within 24 hours before and 24 hours after a scheduled transfusion. Hb/hematocrit (Hct) and Hb S quantitation were performed within 2 days of each MRI. For 1 MRI time point with missing lab values in a participant after HSCT, the mean of values obtained 1 to 2 months before and after the time point was used.
MRI protocol
All brain images were acquired on a 3T TRIO or PRISMA (Siemens, Erlangen, Germany). Standard clinical sequences, including 3-dimensional T1-weighted, magnetization-prepared rapid acquisition gradient echo, fluid-attenuated inversion recovery, and time-of-flight MRA were obtained without contrast. Study sequences consisted of a double-echo asymmetric spin echo sequence (echo time/repetition time = 64.0/4400 ms; field of view, 220 mm; resolution = 1.7 × 1.7 × 3.0 mm) measuring tissue deoxyhemoglobin, permitting OEF quantification as described previously.19-21 Based on consensus recommendations,22,23 CBF was measured with a multi-slice, 2-dimensional pseudo-continuous arterial spin labeling (pCASL) sequence (echo time/repetition time = 12/3780 ms; resolution = 3.0 × 3.0 × 5.0 mm; labeling duration = 1500 ms) with a 1.0- or 1.5-second post-labeling delay (PLD). Forty pairs of label and control images were acquired without background suppression. CBF was measured using a single-compartment model.22,23 At the study's inception, we measured CBF after 1.0 second because SCD is known to increase CBF velocity. Fifteen months after initiation, we changed the sequences to measure pCASL with 1.5-second PLD following a consensus recommendation.23 In our larger SCD hemodynamics study, 56 participants underwent pCASL with both 1.0- and 1.5-second PLD, allowing imputation of 1.5-second PLD CBF (CBF1.5s) from 1.0-second PLD CBF (CBF1s) (supplemental Material, available on the Blood website). For the current analysis, CBF1.5s determinations were used when available. When only CBF1s was obtained, CBF1.5s was imputed; this occurred with 1 HSCT recipient’s pre-HSCT images, another HSCT recipient’s post-HSCT images, 11 CRTT recipients’ pre- and posttransfusion images, and 7 control participants’ images.
Image processing
T1-weighted images were skull-stripped and segmented using the statistical parametric mapping software (SPM12, Wellcome Institute of Neurology, London, United Kingdom) to generate white matter and gray matter masks. A board-certified stroke neurologist manually delineated infarct lesions on all MRIs using the Medical Image Processing, Analysis, and Visualization software (mipav.cit.nih.gov/) to exclude these voxels allowing calculation of CBF and OEF within the normal-appearing brain tissue. To improve the accuracy of CBF, blood T1, which varies with Hct, was measured in each participant using an inversion-recovery echoplanar imaging sequence, with an adiabatic nonselective inversion pulse, in the superior sagittal sinus and estimated using a four-parameter model.24
Statistical analysis
Analyses were performed in SPSS version 27 (IBM, Armonk, NY) and SAS software version 9.4 (SAS Institute Inc, Cary, NC). All analyses used nonparametric tests owing to the small sample size. Continuous variables were compared across groups using the Kruskal-Wallis test with the post hoc Dwass-Steel-Critchlow-Flinger pairwise comparison to control for multiple statistical inquiries. Categorical variables were compared with Fisher exact test. Spearman’s ρ was used for bivariate correlations. Pre- and postintervention outcomes were compared using the Wilcoxon signed-rank test. To assess predictors of OEF and CBF, an exploratory multivariable linear regression model was constructed with post-HSCT reciprocally-transformed Hb (1/Hb) or Hct (1/Hct), age, and donor genotype (Hb AA vs AS). Because the analyses were exploratory and used nonparametric data, confidence intervals (CIs) and β are not reportable.
Results
Participant characteristics
Thirteen participants were enrolled before planned HSCT from 2015 to 2019. HSCT was deferred in 1 participant due to medical nonadherence and psychological concerns, 1 became ineligible for the hemodynamics study owing to new-onset epilepsy unrelated to stroke/SCI, and 1 died of SCD complications before beginning the preparative regimen. Twenty CRTT recipients and 20 siblings of patients with SCD who underwent MRI/MRA in our earlier studies were included for comparison.10,15 The 3 groups had similar age distribution and sex, and the HSCT and CRTT cohorts had similar cerebral infarct and vasculopathy prevalence, pre-treatment Hb, and pre-treatment Hb S (Table 1). Nine controls had AA genotype, 7 had AS, and 4 had unknown genotype. One HSCT recipient had unilateral cerebral vasculopathy/moyamoya (defined as >50% narrowing or occlusion of the internal carotid artery and/or middle cerebral artery on time-of-flight MRA, with or without moyamoya collaterals) and had undergone cerebral revascularization via pial synangiosis. Four CRTT participants had cerebral vasculopathy (2 had bilateral, 2 had unilateral), of whom 2 had undergone pial synangiosis.
Comparison between HSCT, CRTT, and control cohorts at baseline
| . | HSCT (n = 10) . | CRTT (n = 20) . | Control (n = 20) . |
|---|---|---|---|
| Median age, y (IQR) | 11.6 (9.5-16.7) | 13.1 (10.1-18.1) | 11.3 (7.8-14.2) |
| Sex, n (% female) | 4 (40) | 13 (65) | 7 (35) |
| Median Hb, g/dL (IQR) | 9.1 (7.5-10.1) | 9.1 (8.8-9.8) | NA |
| Median Hb S, % (IQR) | 45.75 (35.9-74.2) | 37.4 (29.7-47.5) | NA |
| Presence of infarction, n (%) | 8 (80) | 18 (90) | NA |
| Cerebral vasculopathy, n (%) | 1 (10) | 4 (20) | NA |
| Abnormal TCD, n (%) | 3 (30) | 6 (30) | NA |
| Median whole-brain CBF, mL/100 g per min (IQR)∗ | 93.5 (75.9-109.9) | 86.8 (73.3-103.4) | 69.8 (56.0-80.0) |
| Median whole-brain OEF, % (IQR)† | 36.8 (34.3-42.2) | 35.3 (32.7-38.6) | 30.9 (27.5-31.9) |
| . | HSCT (n = 10) . | CRTT (n = 20) . | Control (n = 20) . |
|---|---|---|---|
| Median age, y (IQR) | 11.6 (9.5-16.7) | 13.1 (10.1-18.1) | 11.3 (7.8-14.2) |
| Sex, n (% female) | 4 (40) | 13 (65) | 7 (35) |
| Median Hb, g/dL (IQR) | 9.1 (7.5-10.1) | 9.1 (8.8-9.8) | NA |
| Median Hb S, % (IQR) | 45.75 (35.9-74.2) | 37.4 (29.7-47.5) | NA |
| Presence of infarction, n (%) | 8 (80) | 18 (90) | NA |
| Cerebral vasculopathy, n (%) | 1 (10) | 4 (20) | NA |
| Abnormal TCD, n (%) | 3 (30) | 6 (30) | NA |
| Median whole-brain CBF, mL/100 g per min (IQR)∗ | 93.5 (75.9-109.9) | 86.8 (73.3-103.4) | 69.8 (56.0-80.0) |
| Median whole-brain OEF, % (IQR)† | 36.8 (34.3-42.2) | 35.3 (32.7-38.6) | 30.9 (27.5-31.9) |
All other comparisons are not significant.
IQR, interquartile range; NA, not applicable.
Kruskal-Wallis test P = .001; HSCT vs CRTT not significant (P = .811), HSCT vs control (P = .023), CRTT vs control (P = .003) (Dwass-Steel-Critchlow-Flinger post hoc pairwise P values adjusted for multiple comparisons).
Kruskal-Wallis test P < .001; HSCT vs CRTT not significant (P = .600), HSCT vs control (P = .010), CRTT vs control (P =.001) (Dwass-Steel-Critchlow-Flinger post hoc pairwise P values adjusted for multiple comparisons).
Ten participants completed pre- and post-HSCT imaging. Seven participants had Hb SS disease; 3 participants had Hb S-β+ thalassemia with <10% Hb A at baseline and severe disease manifestations (frequent vaso-occlusive pain episodes, ACS, SCIs, overt stroke, and/or abnormal TCD). Pre-HSCT disease manifestations and hematological parameters are delineated in Table 2. Before HSCT, 5 participants had SCIs, 3 had overt strokes, and 2 had brain MRIs free of cerebral infarctions. Seven children were treated with CRTT before HSCT: 3 with overt strokes, 2 with abnormal TCD plus SCIs, 1 with abnormal TCD without SCIs, and 1 with SCIs but normal TCD. One of the 2 children with abnormal TCD plus SCIs had progressive cerebral vasculopathy/moyamoya disease and progressive SCIs concurrent with first HSCT graft rejection. She resumed CRTT and underwent unilateral pial synangiosis 1 year before her second HSCT from the same HLA-identical sibling donor. Before treatment, both HSCT and CRTT cohorts had elevated CBF and OEF compared with controls (Table 1).
Characteristics of HSCT recipients
| Subject . | Genotype . | Transplant indications . | Sex . | Age at HSCT, y . | Pre-HSCT Hb, g/dL . | Pre-HSCT Hb S, % . | Donor, stem cell source, genotype . | Post-HSCT Hb, g/dL . | Post-HSCT Hb S, % . | Myeloid engraftment, % donor . |
|---|---|---|---|---|---|---|---|---|---|---|
| A∗ | Hb S-β+ | ACS, TCD (no SCI), CRTT | M | 6.5 | 8.4 | 41.6 | MSD, PBSCs, AS | 11.7 | 40.7 | 95 |
| B | Hb SS | ACS, SCI, CRTT | M | 7.7 | 9.5 | 49.9 | MSD, marrow, AS | 12.3 | 39.7 | 62 |
| C | Hb SS | VOE, SCI | F | 9.5 | 10.1 | 77.6 | MSD, marrow, AS | 12.5 | 26.8 | 100 |
| D | Hb SS | ACS, TCD, SCI, sickle hepatopathy, CRTT | M | 11.0 | 8.6 | 25.3 | MSD, marrow, AA | 12.4 | 0 | 70 |
| E | Hb SS | TCD, cerebral vasculopathy, SCI, HSCT rejection, CRTT | F | 11.5 | 10.0 | 35.9 | MSD, PBSCs, AS | 12.2 | 41.5 | 66 |
| F | Hb SS | VOE, HSCT rejection | F | 11.7 | 7.5 | 51.5 | MSD, PBSCs, AS | 11.3 | 35.6 | 65 |
| G∗ | Hb S-β+ | ACS, VOE, SCI, autoimmune hemolytic anemia | M | 12.4 | 7.1 | 82.0 | MSD, PBSCs, AS | 11.8 | 40.8 | 100 |
| H | Hb SS | Overt stroke, CRTT | F | 16.6 | 7.4 | 74.2 | MUD, marrow, AA | 13.6 | 0 | 97 |
| I | Hb S-β+ | ACS, VOE, SCI, overt stroke, CRTT | M | 19.0 | 12.9 | 34.0 | MUD, marrow, AA | 16.7 | 0 | 92 |
| J | Hb SS | Overt stroke, CRTT | M | 21.6 | 11.5 | 39.7 | MUD, marrow, AA | 15.7 | 0 | 100 |
| Subject . | Genotype . | Transplant indications . | Sex . | Age at HSCT, y . | Pre-HSCT Hb, g/dL . | Pre-HSCT Hb S, % . | Donor, stem cell source, genotype . | Post-HSCT Hb, g/dL . | Post-HSCT Hb S, % . | Myeloid engraftment, % donor . |
|---|---|---|---|---|---|---|---|---|---|---|
| A∗ | Hb S-β+ | ACS, TCD (no SCI), CRTT | M | 6.5 | 8.4 | 41.6 | MSD, PBSCs, AS | 11.7 | 40.7 | 95 |
| B | Hb SS | ACS, SCI, CRTT | M | 7.7 | 9.5 | 49.9 | MSD, marrow, AS | 12.3 | 39.7 | 62 |
| C | Hb SS | VOE, SCI | F | 9.5 | 10.1 | 77.6 | MSD, marrow, AS | 12.5 | 26.8 | 100 |
| D | Hb SS | ACS, TCD, SCI, sickle hepatopathy, CRTT | M | 11.0 | 8.6 | 25.3 | MSD, marrow, AA | 12.4 | 0 | 70 |
| E | Hb SS | TCD, cerebral vasculopathy, SCI, HSCT rejection, CRTT | F | 11.5 | 10.0 | 35.9 | MSD, PBSCs, AS | 12.2 | 41.5 | 66 |
| F | Hb SS | VOE, HSCT rejection | F | 11.7 | 7.5 | 51.5 | MSD, PBSCs, AS | 11.3 | 35.6 | 65 |
| G∗ | Hb S-β+ | ACS, VOE, SCI, autoimmune hemolytic anemia | M | 12.4 | 7.1 | 82.0 | MSD, PBSCs, AS | 11.8 | 40.8 | 100 |
| H | Hb SS | Overt stroke, CRTT | F | 16.6 | 7.4 | 74.2 | MUD, marrow, AA | 13.6 | 0 | 97 |
| I | Hb S-β+ | ACS, VOE, SCI, overt stroke, CRTT | M | 19.0 | 12.9 | 34.0 | MUD, marrow, AA | 16.7 | 0 | 92 |
| J | Hb SS | Overt stroke, CRTT | M | 21.6 | 11.5 | 39.7 | MUD, marrow, AA | 15.7 | 0 | 100 |
F, female; Hb SS, hemoglobin SS disease; Hb S-β+, hemoglobin S-β+ thalassemia; M, male; PBSCs: peripheral blood stem cells; VOE, vaso-occlusive pain event.
Full siblings.
Transplant preparative regimen and outcomes
Seven participants received HSCT from HLA-identical matched sibling donors (MSD: 3 bone marrow, 4 peripheral blood stem cells), 3 had 8 out of 8 matched unrelated bone marrow donors (MUD). Two subjects experienced rejection of the first MSD transplant before enrollment in this study, with recurrence of hemolytic anemia and Hb S fraction exceeding Hb A fraction. They were enrolled before reconditioning and second HSCT from their original donors. The conditioning regimen for first transplants consisted of alemtuzumab, fludarabine, and melphalan (Table 3).25,26 During the study time period, the regimen was modified to include thiotepa (for participants A, G, and H) and the expanded use of hydroxyurea (all participants except A, C, E, and F). The 2 children who had first transplant rejection were reconditioned with fludarabine 25 to 30 mg/m2 (days −6 through −2), thymoglobulin 2.5 mg/kg (days −5 through −2), and cyclophosphamide 60 mg/kg (days −3 and −2). Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus and methotrexate, plus abatacept for MUD recipients and 1 child aged >10 years old with an MSD. Tacrolimus was weaned starting on day +100 and was discontinued before post-HSCT MRI in 8 out of 10 participants; the remaining 2 discontinued tacrolimus 3 to 4 months after post-HSCT MRI. Myeloid engraftment status within 2 months of post-HSCT MRI ranged from 62% to 100%, and all recipients had normal Hb concentration and Hb S fraction consistent with donor 1 to 2 years after HSCT (Table 2). None of the participants had post-HSCT overt strokes, posterior reversible encephalopathy syndrome (PRES), severe acute or chronic GVHD, or new SCIs on post-HSCT imaging.
Preparative regimen for first HSCT
| Drug . | Dose . | Days given . | Comments . |
|---|---|---|---|
| Hydroxyurea | 30 mg/kg | −48 to −21 | Participants B, D, G, H, I, J |
| Alemtuzumab | 3, 10, 15, 20 mg | −22 to −19 | Fixed dosing for all recipients |
| Fludarabine | 30 mg/m2 | −8 to −4 | |
| Melphalan | 140 mg/m2 | −3 | |
| Thiotepa | 4 mg/kg × 2 doses | −3 | Participants A, G, H |
| Tacrolimus | −3 through +100, then weaned as tolerated | ||
| Abatacept | 10 mg/kg | −1, +5, +14, +28, +60, +100, +180, +270, +360 | Participants D, H, I, J |
| Methotrexate | 7.5 mg/m2 | +2, +3, +6 |
| Drug . | Dose . | Days given . | Comments . |
|---|---|---|---|
| Hydroxyurea | 30 mg/kg | −48 to −21 | Participants B, D, G, H, I, J |
| Alemtuzumab | 3, 10, 15, 20 mg | −22 to −19 | Fixed dosing for all recipients |
| Fludarabine | 30 mg/m2 | −8 to −4 | |
| Melphalan | 140 mg/m2 | −3 | |
| Thiotepa | 4 mg/kg × 2 doses | −3 | Participants A, G, H |
| Tacrolimus | −3 through +100, then weaned as tolerated | ||
| Abatacept | 10 mg/kg | −1, +5, +14, +28, +60, +100, +180, +270, +360 | Participants D, H, I, J |
| Methotrexate | 7.5 mg/m2 | +2, +3, +6 |
Cerebral hemodynamics normalize after HSCT
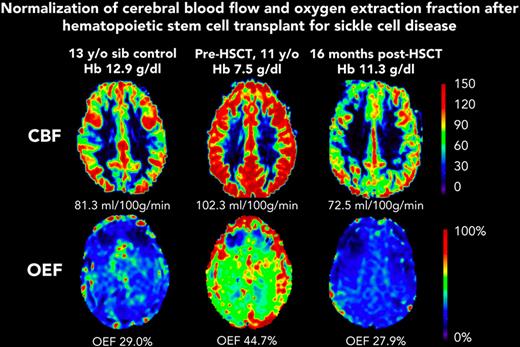
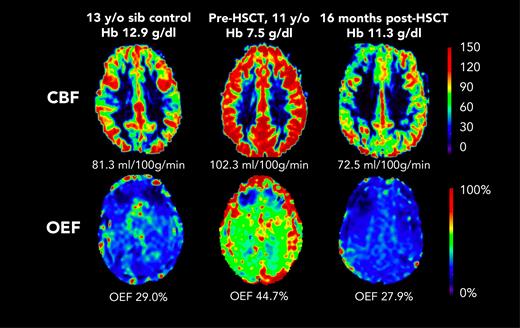
Before HSCT, participants had elevated CBF (median, 93.5 mL/100 g/min; IQR, 75.9-109.9) compared with the participants in the control group (median, 69.8 mL/100 g/min; IQR, 56.0-80.0; Kruskal-Wallis test P = .023) (Table 1). At the post-HSCT time point, CBF was significantly lower (median, 72.7 mL/100 g/min; IQR, 61.7-86.0; Wilcoxon signed-rank test P = .004). Likewise, OEF was elevated before HSCT (median, 36.8%; IQR, 34.3-42.2) compared with controls (median, 30.9%; IQR, 27.5-31.9; Kruskal-Wallis test P = .010). At the post-HSCT time point, OEF was significantly lower (median, 27.0%; IQR, 25.0-30.5%; Wilcoxon signed-rank test P = .002). After HSCT, recipients and controls had similar median CBF (HSCT: 72.7 mL/100 g/min; IQR, 61.7-86.0; controls: 69.8 mL/100 g/min; IQR, 56.0-80.0; P = .653) and median OEF (HSCT: 27.0%; IQR, 24.4-31.0; controls: 30.9%; IQR, 27.5-31.9; P = .168). Figure 1 shows representative maps of CBF and OEF in a child with SCD before and after HSCT compared with a sex–matched, similar-aged control participant, demonstrating that post-HSCT CBF and OEF are similar to control values. The supplemental Table shows each HSCT recipient’s pre- and post-HSCT Hb, CBF, and OEF.
Representative maps from participant F before HSCT (age 11 years) and after HSCT (age 12 years). CBF and OEF were elevated at baseline, and were similar to those of a sex–matched, 13-year-old control participant, 1 year after curative HSCT. sib, sibling.
Representative maps from participant F before HSCT (age 11 years) and after HSCT (age 12 years). CBF and OEF were elevated at baseline, and were similar to those of a sex–matched, 13-year-old control participant, 1 year after curative HSCT. sib, sibling.
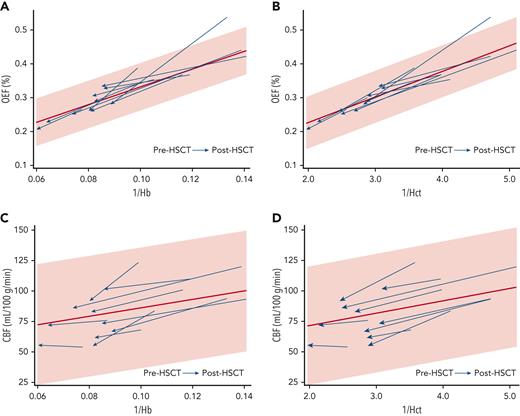
We investigated which clinical variables correlated with improved cerebral hemodynamics after HSCT. 1/Hb correlated with OEF elevation before HSCT (Spearman’s ρ, 0.818; P = .004) and remained correlated with OEF after HSCT (Spearman’s ρ, 0.842; P = .002) (Figure 2A). 1/Hct correlated slightly more strongly with OEF before HSCT (Spearman’s ρ, 0.869; P = .0011) and after HSCT (Spearman’s ρ, 0.952; P < .0001) (Figure 2B). Univariate analyses showed a negative correlation between post-HSCT OEF and post-HSCT age (Spearman’s ρ, −0.636; P = .048). The median post-HSCT OEF was lower in those with Hb AA donors (23.7%; IQR, 21.1-25.7) than in those with Hb AS donors (29.7%; IQR, 27.4-32.9, Mann-Whitney U test P = .011); however, the recipients with AA donors were also older and had higher post-HSCT Hb concentrations (Table 2). An exploratory multivariate model including post-HSCT 1/Hb, age, and donor genotype (AS vs AA) was not significant in predicting post-HSCT OEF (P = .384), and only post-HSCT 1/Hb remained as a significant predictor. Substituting 1/Hct in the model produced a similar nonsignificant result (P = .318). 1/Hb was not significantly correlated with CBF before HSCT (Spearman’s ρ, 0.479; P = .162) or after HSCT (Spearman’s ρ, 0.115; P = .751) (Figure 2C). Likewise, 1/Hct was not significantly correlated with CBF before HSCT (P = .162) or after HSCT (P = .726) (Figure 2D).
Hb and Hct are inversely correlated with OEF before and after HSCT. Each HSCT recipient’s data points are shown with an arrow oriented from before HSCT to after HSCT. The 95% CI (shaded) and regression line (red) are derived from the OEF and CBF measurements of the CRTT cohort. (A) Correlation between 1/Hb and OEF before HSCT (Spearman's ρ, 0.818; P = .004) and after HSCT (Spearman’s ρ, 0.842; P = .002). (B) Correlation between 1/Hct and OEF before HSCT (Spearman’s ρ, 0.869; P = .0011) and after HSCT (Spearman’s ρ, 0.952; P < .0001). (C) Correlation between 1/Hb and CBF before HSCT (Spearman’s ρ, 0.479; P = .162) and after HSCT (Spearman’s ρ, 0.115; P = .751). (D) Correlation between 1/Hct and CBF before HSCT (P = .162) or after HSCT (P = .726).
Hb and Hct are inversely correlated with OEF before and after HSCT. Each HSCT recipient’s data points are shown with an arrow oriented from before HSCT to after HSCT. The 95% CI (shaded) and regression line (red) are derived from the OEF and CBF measurements of the CRTT cohort. (A) Correlation between 1/Hb and OEF before HSCT (Spearman's ρ, 0.818; P = .004) and after HSCT (Spearman’s ρ, 0.842; P = .002). (B) Correlation between 1/Hct and OEF before HSCT (Spearman’s ρ, 0.869; P = .0011) and after HSCT (Spearman’s ρ, 0.952; P < .0001). (C) Correlation between 1/Hb and CBF before HSCT (Spearman’s ρ, 0.479; P = .162) and after HSCT (Spearman’s ρ, 0.115; P = .751). (D) Correlation between 1/Hct and CBF before HSCT (P = .162) or after HSCT (P = .726).
Comparison of post-HSCT hemodynamics with posttransfusion hemodynamics
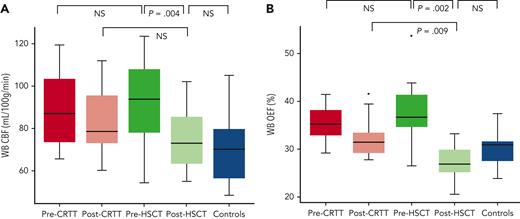
As expected, median of post-treatment Hb was higher in HSCT recipients (median, 12.4 g/dL; IQR, 11.8-14.1) than in CRTT recipients (median, 10.6 g/dL; IQR, 10.3-11.4; Mann-Whitney U test P < .001). HSCT recipients experienced a larger decline in OEF after HSCT (median change, −8.1%; IQR, −12.8 to −5.6) compared with CRTT recipients' change after transfusion (median change, −3.3%; IQR, −4.8 to −1.7; Wilcoxon rank-sum test P = .001). Median post-HSCT OEF (27.0%; IQR, 25.0-30.5) was lower than median post-transfusion OEF (31.5%; IQR, 29.2-33.8; Wilcoxon rank-sum test P = .009) (Figure 2). CBF declined more after HSCT (median change, −19.4 mL/100 g/min; IQR, −28.8 to −6.4) than after a CRTT transfusion (median change, −0.9 mL/100 g/min; IQR, −15.8 to 2.2; Wilcoxon rank-sum test P = .024). Although post-HSCT subjects had lower CBF (median, 72.7 mL/100 g/min; IQR, 61.7-86.0) than CRTT subjects after transfusion (median, 78.0 mL/100 g/min; IQR, 72.1-95.4), this comparison was not statistically significant (Wilcoxon rank-sum test P = .180) (Figure 3).
Comparison of CBF and OEF between CRTT and HSCT SCD participants and children in the control group. (A) CBF is elevated before HSCT, similar to CRTT participants, and declines to control values after HSCT. (B) OEF is elevated before HSCT, similar to CRTT participants, and declines to control values after HSCT. This change is greater than the OEF improvement seen after a scheduled transfusion among CRTT recipients. NS, not significant; WB, whole brain.
Comparison of CBF and OEF between CRTT and HSCT SCD participants and children in the control group. (A) CBF is elevated before HSCT, similar to CRTT participants, and declines to control values after HSCT. (B) OEF is elevated before HSCT, similar to CRTT participants, and declines to control values after HSCT. This change is greater than the OEF improvement seen after a scheduled transfusion among CRTT recipients. NS, not significant; WB, whole brain.
To evaluate whether within-subject CBF and OEF changes were greater after HSCT than after transfusion, 4 subjects who were receiving CRTT before HSCT underwent MRI before and after a scheduled pre-HSCT transfusion. In 3 of these participants, the CBF and OEF were lower after HSCT than after a scheduled pre-HSCT transfusion, whereas the fourth participant had slightly higher CBF and OEF after HSCT than before HSCT after transfusion (Wilcoxon rank-sum test P = .144).
Discussion
In this cohort of children with severe SCD, we demonstrate that CBF and OEF normalize after a successful HSCT. Normalization of OEF and CBF likely represents a relaxation of compensatory mechanisms due to restoration of oxygen delivery reserve, explaining the greater protection from overt and silent stroke recurrence after HSCT than in individuals who receive CRTT.
By comparing CBF and OEF in children before and after HSCT with children receiving CRTT and non-SCD control participants, our study confirms and expands on previous work. In a cohort of children with SCD who either underwent HSCT (n = 17) or commenced CRTT (n = 9), HSCT recipients had a significant decline in CBF, whereas CBF in CRTT recipients was unchanged.17 That study’s design differs from the current work because the post-CRTT CBF measurements were not obtained at a uniform time point relative to transfusion, so the authors were unable to determine the acute impact of a blood transfusion on CBF.17 A series of 4 adults with SCD demonstrated reductions in OEF and CBF after HSCT.18 Together, these studies support the role of cerebral hemodynamics in stroke prevention after HSCT. Importantly, the pediatric studies demonstrated no post-HSCT cerebral infarcts, whereas one reported adult HSCT recipient had a new SCI in the hemisphere affected by moyamoya vasculopathy.18 This suggests that children may be less likely to experience strokes or SCIs after HSCT compared with adults, supporting a role for earlier transplantation. Adults with SCD have less relative reduction in CBF after a single RBC transfusion than children, suggesting that cerebral compensatory changes in response to change in Hb are not as robust in adults compared with children.27 Despite the greater improvement in Hb after HSCT compared with transfusion, adults or those with moyamoya syndrome may have arteriopathy that limits cerebral oxygen delivery, in turn leaving patients vulnerable to post-HSCT strokes. HSCT in childhood for those with MSDs was recently supported by the American Society of Hematology 2021 Guidelines for Sickle Cell Disease: Stem Cell Transplantation, owing to better engraftment and survival outcomes in children compared with teens and adults.28,29 Although HSCT carries risks of acute strokes, intracranial hemorrhage, and PRES, these risks have been mitigated with improved supportive care and immune suppression regimens.25,30-32
Although strokes and SCIs are considered fixed lesions that will not improve after HSCT, more subtle forms of neurological and cognitive dysfunction may be reversible with HSCT, including substructural white matter injury measured by diffusion tensor imaging (DTI).33 Improvement in fractional anisotropy, which measures the movement of water molecules along axons, has been found in children and adults with SCD after haploidentical HSCT.34 Normalization of cerebral hemodynamics may underlie this improvement in white matter integrity.6 Notably, a subset of participants in this study had worsening fractional anisotropy after HSCT,34 which could result from acute neurological complications during HSCT, including PRES. Such complications may cause persistent substructural white matter damage that persists in post-HSCT DTI despite engraftment and eventual normalization of CBF and OEF. Our work and the DTI data suggest that some, but not all, aspects of SCD-induced cerebral injury are reversible with curative therapy.
Furthermore, normalization of cerebral hemodynamics and improvement in white matter integrity after HSCT may stabilize or even improve cognition and executive function (EF), which deteriorate over time in SCD.35 Thirteen children with SCD had stable cognition 2 years after MUD HSCT, despite a high pre-HSCT stroke/SCI burden, and 5 out of 13 having experienced PRES after corticosteroid treatment for GVHD.36 In a cohort of children receiving CRTT, EF (but not general cognition) was better after transfusion than before transfusion.37 This parallels the CBF and OEF changes with transfusion; if executive dysfunction in SCD results in part from cerebral metabolic stress, it is reasonable to hypothesize that children may experience stable or improved EF after HSCT, similar to the improvements in cerebral hemodynamics. As GVHD prophylaxis strategies are refined, neurological complications of HSCT will likely be reduced, in turn limiting the cognitive risks of HSCT. The relationship between cerebral hemodynamic stress, substructural white matter injury, and cognitive impairment should be explored further, especially as disease–modifying and curative therapies for SCD expand.
Our data suggest that hemodynamic normalization after HSCT is driven by normalizing Hb/Hct and thus blood oxygen content. CBF and OEF increase in SCD to compensate for chronic anemia and low arterial oxygen content (CaO2). Areas of physiologically-limited CBF, such as the deep white matter watershed zone, have nadir CBF, highest infarct density,14 and extreme OEF elevation10 in children with SCD. These findings suggest that if compensation mechanisms fail or CaO2 drops acutely, insufficient tissue oxygen delivery may ensue, causing a cerebral infarction. Our group16 and others27 have demonstrated that OEF and, to a lesser degree, CBF improve acutely following RBC transfusion, demonstrating that this compensation is dynamic and can be mitigated by increasing Hb/Hct. CRTT recipients remain anemic even immediately after transfusion because standard CRTT strategies target posttransfusion Hb of 10 to 11 g/dL.38 Hb may fluctuate from 1 to 3 g/dL between transfusions. Our group’s previous work showed that patients receiving CRTT have significantly higher OEF and CBF before transfusion compared with after transfusion, indicating that the beneficial effect of CRTT on cerebral hemodynamics is not maintained during the weeks between scheduled transfusions.16 Furthermore, patients with SCD receiving CRTT remain at risk for acute SCD complications including delayed hemolytic transfusion reactions or ACS.39,40 These could cause a sudden drop in CaO2 and acutely increase cerebral metabolic stress beyond compensation capacity, resulting in inadequate brain tissue oxygen delivery, which manifests as an overt stroke or SCI. In a cohort of children with SCD receiving CRTT for secondary stroke prophylaxis, 45% of participants had recurrent overt stroke or SCI progression, demonstrating that CRTT provides incomplete protection from recurrent neurological injury4 and extreme OEF elevation.10 These findings suggest that if compensation mechanisms fail or CaO2 drops acutely, insufficient tissue oxygen delivery may ensue, causing a cerebral infarction. Our group16 and others27 have demonstrated that OEF and to a lesser degree CBF improve acutely after packed RBC transfusion, demonstrating that this compensation is dynamic and can be mitigated by increasing Hb/Hct. CRTT recipients remain anemic even immediately after transfusion, because standard CRTT strategies target post-transfusion Hb of 10 to 11 g/dL.38 Hb fluctuations of 1 to 3 g/dL occur commonly between transfusions. Our group’s previous work showed that patients receiving CRTT have significantly higher OEF and CBF before transfusion than after transfusion, indicating that the beneficial effect of CRTT on cerebral hemodynamics is not maintained during the weeks between scheduled transfusions.16 Furthermore, patients with SCD receiving CRTT remain at risk for acute SCD complications including delayed hemolytic transfusion reactions or ACS.39,40 These could cause a sudden drop in CaO2 and acutely increase cerebral metabolic stress beyond compensation capacity, resulting in inadequate brain tissue oxygen delivery which manifests as an overt stroke or SCI. In a cohort of children with SCD receiving CRTT for secondary stroke prophylaxis, 45% of participants had a recurrent overt stroke or SCI progression, demonstrating that CRTT provides incomplete protection from recurrent neurological injury.4
In contrast to CRTT, successful HSCT resolves chronic hemolytic anemia and Hb fluctuations, stabilizing cerebral oxygen delivery and minimizing stroke risk. Hb and oxygen delivery normalization relieves the chronic metabolic stress of anemia and obviates the need for cerebral hemodynamic compensation, resulting in the normalization of CBF and OEF. This is consistent with the observation that children with SCD whose abnormal TCD blood flow velocities remained abnormal despite CRTT experienced an improvement in TCD after HSCT.8
The correlations between 1/Hb or 1/Hct and OEF among HSCT recipients fall within the CRTT cohort’s 95% CIs, supporting the hypothesis that the improvement in hemodynamics results primarily from the normalization of Hb/Hct. Surprisingly, the correlation between OEF and 1/Hct was stronger than the correlation between OEF and 1/Hb. Hb is a measured value of blood Hb concentration, whereas Hct, the calculated product of mean corpuscular volume and number of RBCs, describes RBC mass. RBC mass influences blood viscosity, and in SCD, viscosity increases disproportionately with increases in Hct in the low-shear environment of capillaries and venules.41 Potentially, changes in Hb and RBC mass may have different effects on compensation mechanisms for chronic anemia, accounting for the differences in relationship strength. Furthermore, post-HSCT changes, such as fewer dense RBCs, normal RBC morphology, and resolution of endothelial injury, may also contribute to hemodynamic normalization.42
This work is limited in that most participants received transplants from MSDs with Hb AS genotype. Although all HSCT recipients had severe disease manifestations, we cannot generalize our findings to less severe SCD such as Hb SC disease because cerebral hemodynamics have not been investigated in these patients. Due to the sample size, we cannot determine whether donor genotype independently predicts post-HSCT hemodynamics. Multivariate modeling did not find an association between post-HSCT OEF or CBF and donor genotype when age and Hb were controlled. Our group has shown similar OEF and CBF in adults with Hb AS and Hb AA,43 so we would not expect significant differences based on Hb AA vs AS donor status. Participants received similar reduced-intensity preparative regimens, so we cannot evaluate the impact of different regimens. No participant experienced neurological HSCT complications, but subclinical hemodynamic perturbations during the peri- or posttransplant period were not captured by our study design, so the potential impact of acute neurological events cannot be evaluated. We cannot determine the effects of tacrolimus or immunosuppressive agents because most subjects discontinued tacrolimus before post-HSCT imaging.
Patients were imaged on different MRIs during the study. In our previous work, we did not note differences in CBF and OEF among different scanners. Some of the CBF measurements were imputed, but there was a strong correlation between CBF1s and CBF1.5s (r2 = 0.88) in our previous work. Imputed CBF1.5s was lower than CBF1s. CBF1s was primarily in the pre-HSCT time point and control groups, so using the lower imputed value biases toward the null hypothesis, as differences between pre- and post-HSCT participants or post-HSCT and control participants would be smaller when a lower comparison value is used. A two-compartment CBF model may be more accurate than the single-compartment model; however, within- and between-participant comparisons remain valid because the same model was used for all participants.44 Finally, the small sample size prevented evaluation of HSCT effects on regional (gray and white matter) CBF and OEF; however, with global improvements following HSCT and greater improvements after HSCT than after transfusion, we expect that regional hemodynamics would also improve or normalize.
Individuals in our cohort suggest areas for future study. Participant A (Table 2) was the youngest, aged 6.5 years at the pre-HSCT MRI. This individual’s CBF was higher 1 year after HSCT (101.7 mL/100 g/min) than before HSCT after transfusion (93.5 mL/100 g/min). His CBF increase may have been physiologic as CBF peaks at age 7 to 8 years.45 In contrast, the other 3 subjects receiving CRTT had a lower CBF and OEF after HSCT than after a pre-transplant transfusion. Longitudinal studies of younger children with SCD, with or without HSCT, are needed to understand whether CBF increases are expected in this patient group. Participant E had cerebral vasculopathy and prior SCIs and rejected a previous MSD HSCT. She had vasculopathy progression and development of new SCIs in the deep white matter concomitant with graft rejection. Although successful HSCT is neuroprotective, graft rejection or failure may be accompanied by a return of hemolytic anemia, cerebral metabolic stress, and strokes, so strategies to minimize graft loss are needed. In addition, the role of cerebral vasculopathy in HSCT outcomes and the potential neuroprotective effects of cerebral revascularization before HSCT should be explored.
In conclusion, we demonstrate that cerebral hemodynamic and metabolic stress are relieved by successful HSCT in children with SCD, and CBF and OEF normalize after HSCT. Future work should focus on the impacts of stem cell source, preparative regimen, and recipient age on cerebral hemodynamic outcomes, so that more children with SCD can receive curative therapy in a way that maximizes neuroprotection.
Acknowledgments
The authors thank the patients and their families for their participation.
The study was supported by National Institutes of Health grants R01HL129241 (A.L.F.), R01HL157188 and K23HL136904 (M.E.F.), and K23NS099472 (K.P.G.), and Washington University School of Medicine (M.L.H).
Authorship
Contribution: M.L.H., S.S., J.-M.L., and A.L.F. designed the study, enrolled the study subjects, and analyzed and interpreted the data; M.E.F. and K.P.G. enrolled the study subjects and analyzed the data; P.B., A.S.T., and K.V. performed clinical data acquisition and interpretation; and H.A., S.F., M.M.B., R.C.M., J.S.S., Y.C., C.E., and D.K.R. performed imaging data analysis and interpretation.
Conflict-of-interest disclosure: M.L.H. has spouse employed at Pfizer Inc, is a consultant for bluebird bio and for Global Blood Therapeutics, and has received research funding from Global Blood Therapeutics and Forma Therapeutics. M.E.F. owns equity at Proclara Biociences, participated in a scientific advisory board with compensation at bluebird bio, and is a consultant for Global Blood Therapeutics. S.S. is a consultant for Aruvant Sciences and participated in scientific advisory boards with compensation at Bristol Myers Squibb, Graphite Bio, and Janssen Pharmaceuticals. M.B.B. is employed at Open Cell Technologies Inc. The remaining authors declare no competing financial interests.
Correspondence: Monica L. Hulbert, Department of Pediatrics, Washington University in St. Louis, One Children's Pl, Campus Box 8116, Saint Louis, MO 63110; e-mail: monicahulbert@wustl.edu.
References
Author notes
Data are available on request from the corresponding author, Monica L. Hulbert (monicahulbert@wustl.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal