In this issue of Blood, Xiao et al1 demonstrate that the hemochromatosis protein HFE is required for the participation of hepatocellular transferrin receptor 1 (TFR1) in the co-regulation of iron metabolism and erythropoiesis. Such co-regulation is essential to maintain iron homeostasis and normal red blood cell production, and its disruption contributes to the pathology of the iron-loading anemias and multiple conditions of erythropoietin resistance. Hepatocytes couple iron availability to iron demand by sensing extracellular signals reflective of erythropoietic activity (erythroferrone [ERFE]), iron utilization (iron-bound transferrin [FeTF]), and liver iron stores (bone morphogenetic proteins [BMP6/2]) to influence hepcidin production. Hepcidin then regulates the release of iron into the circulation from sites of storage and from the diet to TF for utilization, primarily in hemoglobin production. Identified hepatocellular sensors of these extracellular signals include the BMP receptor complex and the 2 transferrin receptors (TFR1 and TFR2). Identification of the individual and interrelated contributions of these “iron sensors” has provided insights into novel therapeutic approaches to correct their dysregulation.
Each of the hepatocellular TFRs participate in hepcidin regulation, but by different mechanisms. Mice with knockout of hepatocyte Tfr1 have a modest decrease in hepatocellular iron, low plasma iron, and inappropriately high hepcidin.2 Mice with knockout of hepatocellular Tfr2, by contrast, have hepatocellular iron overload, excess plasma iron, and inappropriately low hepcidin.3 In each of these situations, the primary defect appears to be the hepcidin dysregulation, with the changes in liver and plasma iron status being secondary. Identifying the mechanisms responsible for hepcidin dysregulation in these settings has been informed by combining knockouts of each TFR with other molecular participants in hepcidin regulation. Here, Xiao et al use this approach to assess the role of the hemochromatosis protein HFE in the regulation of hepcidin by hepatocellular TFR1.
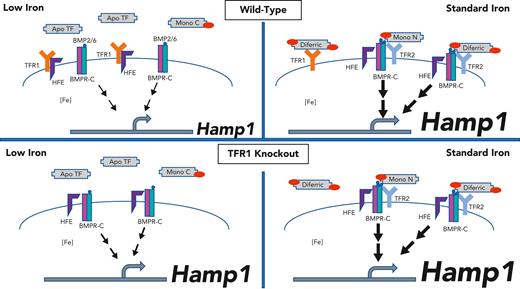
The discovery that HFE, the gene mutated in most cases of hereditary hemochromatosis, interacts with TFR1 provided a plausible link between this major histocompatibility complex class 1 molecule and iron metabolism. Studies examining the consequences of HFE on TFR1-mediated iron uptake, however, have not provided a plausible explanation for hepcidin dysregulation with loss of HFE. Studies directed instead to examining the functional consequences of this interaction on HFE rather than TFR1 have been more informative. These studies support a model by which the interaction of HFE with TFR1 prevents HFE from otherwise exerting an upregulatory effect on hepcidin expression. Such a model explains the relative hepcidin excess in mice with hepatocellular HFE overexpression,4 mice with a mutated TFR1 incapable of interacting with HFE,5 and mice with hepatocellular Tfr1 knockout.2 Such studies, however, leave undetermined whether TFR1 can regulate hepcidin independently of HFE. Xiao et al address this question by examining the effect of dietary iron manipulation in mice with or without knockout of hepatocellular Tfr1, and with or without knockout of Hfe. The investigators observed the expected relative hepcidin excess and consequent iron-restrictive erythropoiesis in mice with knockout of hepatocellular Tfr1. With the caveat that some outcomes had considerable scatter (possibly related to the mixed strain backgrounds), knockout of hepatocyte Tfr1 had no appreciable impact on the phenotype of the Hfe knockout mice, either on a basal or an iron-limited diet. These findings evidence the investigators’ conclusion that the contribution of TFR1 to the regulation of hepcidin expression requires HFE (see figure).
Proposed model. Top panels: wild-type mice. Bottom panels: Tfr1 hepatocellular knockout mice. Left: low plasma iron conditions. Right: standard iron conditions. Under low iron conditions (left), plasma monoferric C and apo TF predominate. TFR1 is highly expressed. HFE is sequestered by TFR1, preventing HFE from interacting with the BMP receptor complex (BMPR-C). TFR2 is destabilized. Hamp expression is low. Under standard iron conditions (right), plasma diferric TF and monoN TF are relatively increased. TFR1 expression is low. HFE is freed from TFR1 to interact with the BMPR-C. TFR2 is stabilized and also contributes to Hamp expression. In the hepatocellular TfR1 knockout model, the absence of TFR1 prevents the sequestration of HFE as a function of diferric transferrin, resulting in excess hepcidin in both low and standard iron states. Regulation by TFR2 remains intact.
Proposed model. Top panels: wild-type mice. Bottom panels: Tfr1 hepatocellular knockout mice. Left: low plasma iron conditions. Right: standard iron conditions. Under low iron conditions (left), plasma monoferric C and apo TF predominate. TFR1 is highly expressed. HFE is sequestered by TFR1, preventing HFE from interacting with the BMP receptor complex (BMPR-C). TFR2 is destabilized. Hamp expression is low. Under standard iron conditions (right), plasma diferric TF and monoN TF are relatively increased. TFR1 expression is low. HFE is freed from TFR1 to interact with the BMPR-C. TFR2 is stabilized and also contributes to Hamp expression. In the hepatocellular TfR1 knockout model, the absence of TFR1 prevents the sequestration of HFE as a function of diferric transferrin, resulting in excess hepcidin in both low and standard iron states. Regulation by TFR2 remains intact.
An HFE/TFR1 complex could serve as an “iron sensor” by 2 mechanisms: 1 mechanism reflecting intracellular and the other extracellular iron. Intracellularly, a fall in cellular iron would stabilize the TFR1 transcript, increase TFR1 protein, increase HFE sequestering, and dampen hepcidin expression. Extracellularly, Fe2TF can outcompete HFE for binding to TFR1,5 thus freeing it to upregulate hepcidin. A fall in plasma iron would decrease Fe2TF concentrations and shift FeTF distribution to the monoferric forms. The monoferric TFs have substantially weaker affinities for TFR1 and are thus less able to displace HFE from TFR1. Empirical support for this mechanism comes from mice in which transferrin has been mutated to prevent iron binding in 1 or the other of the 2 (N or C) transferrin lobes. Although they are not equivalent to each other, each of the mutant “monotransferrinemic” mouse models demonstrates inappropriately low hepcidin.6
The studies by Xiao et al demonstrate that neither HFE nor hepatocellular TFR1 is required for iron-mediated regulation of hepcidin. Administration of enteral iron to mice with knockout of each nonetheless acutely increased hepcidin expression. It did so without an increase in Bmp6 expression, suggesting mediation by plasma rather than tissue iron. This regulation is reasonably attributable to TFR2. TFR2 does not require HFE to affect hepcidin expression, as combined Tfr2 and Hfe knockout results in lower hepcidin expression than does Hfe knockout alone.7 Both TFRs appear to regulate hepcidin in the same direction by plasma iron, even though they produce opposite phenotypes when knocked out. This is explicable if TFR1 mediates suppression of an upregulatory signal (HFE-mediated) as plasma iron falls, whereas TFR2 mediates augmentation an upregulatory signal (likely BMP-mediated) as plasma iron rises.
Additional studies by Xiao et al demonstrate the relevance of TFR1-mediated hepcidin regulation in a disease state. Improvements in markers of iron status and of erythropoiesis were seen in β-thalassemic mice when crossed to TFR1 hepatocellular knockout mice. Similar improvements in β-thalassemic mice have been observed using other approaches that increase hepcidin. Plausibly, high erythroid iron utilization in the β-thalassemic mice lowers plasma diferric TF, increases TFR1-mediated sequestering of HFE, and decreases hepcidin. However, the contribution of TFR1-mediated hepcidin suppression appears low compared with ERFE, and would be expected to dissipate over time as plasma iron, and thus diferric TF increases.
How HFE upregulates hepcidin expression once “freed” is not entirely clear. Evidence supports the interaction of HFE with the BMP receptor complex, possibly via TFR28 or the BMP receptor ALK3.9 It would be informative to characterize these molecular participants in the murine models used by Xiao et al. It also appears that HFE can influence cellular iron status independently of hepcidin.10 Regardless of the downstream events, these studies support a role for hepatocellular HFE/TFR1 as an iron sensor in the co-regulation of iron homeostasis and erythropoiesis.
Conflict-of-interest disclosure: R.E.F. and N.L.P. received funding from Ultragenyx Pharmaceutical, Inc. to test the consequences of HFE expression in HFE knockout mice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal