Abstract
Patients with lymphoproliferative disorders such as chronic lymphocytic leukemia and mantle cell lymphoma (MCL) who are resistant to covalent Bruton tyrosine kinase inhibitors (cBTKis), especially if also venetoclax refractory, have an unmet therapeutic need. Pirtobrutinib, a noncovalent BTKi, achieves high response rates in patients who are refractory to cBTKi, regardless of mechanism of cBTKi resistance. This led to recent accelerated US Food and Drug Administration approval in MCL. The toxicity profile in early studies suggests suitability for use in combination approaches. We summarize existing preclinical and clinical data for pirtobrutinib.
Introduction
Bruton tyrosine kinase inhibitors (BTKis) are standard-of-care for multiple B-cell malignancies.1 The first 3 BTKis to be approved (ibrutinib, acalabrutinib, and zanubrutinib) bind covalently to the cysteine 481 (C481) residue of BTK and are collectively known as covalent BTKis (cBTKis).2-4 All cBTKis are susceptible to resistance mutations at C481, most commonly C481S. The functional characteristics of this mutation are best characterized for ibrutinib, in which it reduces binding avidity, rendering the interaction reversible.5,6 Thus, given the short half-life of ibrutinib, C481S mutation results in inadequate 24-hour BTK coverage (Table 1).2-4BTK C481S mutations are also frequent in patients with acquired acalabrutinib and zanubrutinib resistance,16 although functional consequences are less well characterized than for ibrutinib. Additional mechanisms of resistance to cBTKis in chronic lymphocytic leukemia (CLL) include other mutations in BTK, reviewed here21 and gain-of-function mutations in the downstream kinase PLCG2.6 However, in up to 20% of patients with CLL, the resistance mechanism is unknown. In Waldenstrom macroglobulinemia (WM), ∼50% of patients have BTK C481S mutations at progression,22 but these mutations are infrequent in mantle cell lymphoma (MCL) and marginal zone lymphoma, for which mechanisms of resistance are less well characterized.21,23
Key differences between available cBTKis and pirtobrutinib
| . | Ibrutinib . | Acalabrutinib . | Zanubrutinib . | Pirtobrutinib . |
|---|---|---|---|---|
| BTK binding | Covalent | Covalent | Covalent | Reversible |
| C481 | C481 | C481 | ATP pocket | |
| Distant from C481 | ||||
| Half-life | 6 hours | 1 hour | 4 hours | 20 hours >90% BTK inhibition |
| BTK Y223 autophosphorylation | Inhibited | Inhibited | Inhibited | Inhibited |
| BTK Y551 phosphorylation | No effect | No effect | No effect | Inhibited (maintenance of closed conformation) |
| BTK C481S mutation | Common | Reported | Reported | Not described Effective against C481S |
| Kinase-dead mutations | Uncommon and restricted to C481∗ (active against HCK) | Not reported to date | Reported: L528W > C481Y | Reported: L528W > V416L, A428D, C481R, M477I, and M437R |
| T474I/T474L gatekeeper mutation | Uncommon∗; active against T474I and T474L | Reported | Not reported to date | Reported |
| Off-target hits† | BLK | HER4 | BLK | HER4 |
| BMX | BMX | BRK | ||
| BRK | BRK | |||
| EGFR | EGFR | |||
| HER2 | HER4 | |||
| HER4 | RLK | |||
| ITK | ||||
| JAK3 | ||||
| RLK | ||||
| TEC | ||||
| References | 2,6-12 | 3,13-15 | 4,7,16 | 8,17-19 |
| . | Ibrutinib . | Acalabrutinib . | Zanubrutinib . | Pirtobrutinib . |
|---|---|---|---|---|
| BTK binding | Covalent | Covalent | Covalent | Reversible |
| C481 | C481 | C481 | ATP pocket | |
| Distant from C481 | ||||
| Half-life | 6 hours | 1 hour | 4 hours | 20 hours >90% BTK inhibition |
| BTK Y223 autophosphorylation | Inhibited | Inhibited | Inhibited | Inhibited |
| BTK Y551 phosphorylation | No effect | No effect | No effect | Inhibited (maintenance of closed conformation) |
| BTK C481S mutation | Common | Reported | Reported | Not described Effective against C481S |
| Kinase-dead mutations | Uncommon and restricted to C481∗ (active against HCK) | Not reported to date | Reported: L528W > C481Y | Reported: L528W > V416L, A428D, C481R, M477I, and M437R |
| T474I/T474L gatekeeper mutation | Uncommon∗; active against T474I and T474L | Reported | Not reported to date | Reported |
| Off-target hits† | BLK | HER4 | BLK | HER4 |
| BMX | BMX | BRK | ||
| BRK | BRK | |||
| EGFR | EGFR | |||
| HER2 | HER4 | |||
| HER4 | RLK | |||
| ITK | ||||
| JAK3 | ||||
| RLK | ||||
| TEC | ||||
| References | 2,6-12 | 3,13-15 | 4,7,16 | 8,17-19 |
ATP, adenosine triphosphate; HCK, hematopoietic cell kinase.
With the exception of RT, in which T474I, T474S, and L528W have been reported at progression on ibrutinib.20
Pirtobrutinib (LOXO-305) is the leading member of a new generation of BTKi that noncovalently binds BTK, distant from the C481 residue.17 Emerging data from clinical trials of pirtobrutinib,17 nemtabrutinib (MK1026, ARQ531),24 and vecabrutinib (SNS-062)25 have shown clear clinical activity in patients whose disease carries the C481S mutant but also in patients without C481S mutation. In this review, we discuss the preclinical and early-phase clinical trial results of pirtobrutinib that supported its recent accelerated approval.
Pirtobrutinib mechanism-of-action and pharmacology
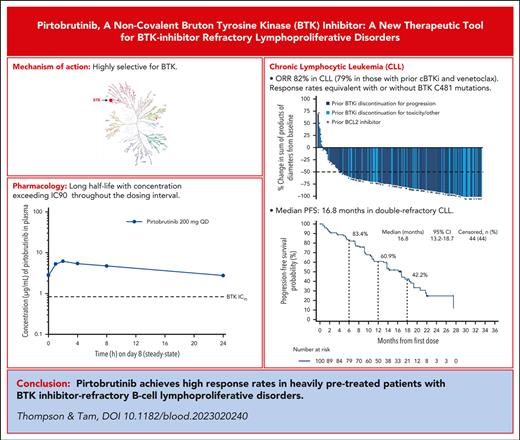
X-ray crystal structures of BTK (both wildtype and C481S) revealed pirtobruinib complexing in the adenosine triphosphate–binding site distant from C481.19 Biochemical and cell-based assays confirmed equipotent binding and kinase inhibition in both wildtype and C481S-mutated BTK.19 Accordingly, autophosphorylation of Y223 on BTK is potently inhibited by pirtobrutinib. Unexpectedly for a back-pocket binder, pirtobrutinib also inhibited upstream BTK phosphorylation at Y551, possibly due to pirtobrutinib stabilization of BTK in a closed, inactive conformation.19 This structural consideration is important because activation of Y551 is important for recruitment of hematopoeitic cell kinase (HCK) to “kinase-dead” BTK mutants, a potential explanation for how these mutants manage to signal despite having an inactive kinase site.9
Biochemical and cell-based assays confirmed high selectivity of pirtobrutinib for BTK (Table 1). The only kinases affected by pirtobrutinib with <20-fold selectivity are HER4 and BRK.19 Studies of cBTKis have linked selectivity with reduced cardiovascular and other adverse events (AEs).3,26,27
In the first-in-human study, pirtobrutinib demonstrated linear dose-proportion exposures throughout the tested dosing range of 25 mg to 300 mg daily, with a half-life of 20 hours.28 No dose-limiting toxicities were observed and the recommended phase 2 dose (200 mg daily) was selected based on a trough plasma concentration resulting in 96% BTK inhibition.28 Thus, continuous BTK coverage is provided by pirtobrutinib’s long half-life, which is distinct from cBTKis for which irreversible binding compensates for short half-lives (Table 1). A theoretical advantage of a long half-life is that BTK molecules that are newly synthesized within the 24-hour window are also inhibited.
Clinical data for pirtobrutinib
BRUIN trial
The phase 1/2 BRUIN trial enrolled 773 patients with relapsed/refractory (R/R) B-cell malignancies. Most AEs were of low-grade. Neutropenia (20.4% of patients) was the most common grade ≥3 treatment-emergent AE. Only 2.6% of patients discontinued treatment because of an adverse effect. Regarding AEs of special interest, grade ≥3 hemorrhage occurred in 1.8% of patients, any grade hypertension in 2.3% of patients, and atrial fibrillation/flutter in 2.8%.29
Expansion cohorts were enrolled in cBTKi-pretreated CLL/small lymphocytic lymphoma (SLL), MCL, WM, and marginal zone lymphoma, and in patients with Richter transformation (RT). Supplemental Table 1 (available on the Blood website) shows pretreatment patient characteristics across key cohorts. Table 2 shows efficacy metrics in response-evaluable populations reported at the 2022 annual meeting of the American Society of Hematology.
Efficacy metrics from most recent publicly reported data in key response-evaluable populations
| Response . | CLL/SLL, n = 276 (previous cBTKi)1 . | MCL, n = 90 (previous cBTKi)2 . | RT, n = 753 . | WM (previous cBTKi),4 n = 55 . |
|---|---|---|---|---|
| ORR/major response (for WM) | 74 | 57.8 | 52.0 | 64 |
| VGPR (WM)/CR (others) | 1 | 20.0 | 13.3 | 24 |
| PR | 64 | 37.8 | 38.7 | 40 |
| PR-L (CLL)/minor response (WM) | 8 | N/A | N/A | Not reported |
| SD | Not reported | 15.6 | 13.3 | Not reported |
| Median DOR (mo) | Not reported | 22 | 5.6 | 83% at 6 mo |
| Median PFS (mo) | 19.4 | 7.4 | 3.7 | Not reported |
| Median OS (mo) | NE | NE | 13.1 | NE |
| Response . | CLL/SLL, n = 276 (previous cBTKi)1 . | MCL, n = 90 (previous cBTKi)2 . | RT, n = 753 . | WM (previous cBTKi),4 n = 55 . |
|---|---|---|---|---|
| ORR/major response (for WM) | 74 | 57.8 | 52.0 | 64 |
| VGPR (WM)/CR (others) | 1 | 20.0 | 13.3 | 24 |
| PR | 64 | 37.8 | 38.7 | 40 |
| PR-L (CLL)/minor response (WM) | 8 | N/A | N/A | Not reported |
| SD | Not reported | 15.6 | 13.3 | Not reported |
| Median DOR (mo) | Not reported | 22 | 5.6 | 83% at 6 mo |
| Median PFS (mo) | 19.4 | 7.4 | 3.7 | Not reported |
| Median OS (mo) | NE | NE | 13.1 | NE |
CR, complete remission; N/A, not available; NE, not estimable; PR, partial remission; PR-L, partial remission with lymphocytosis; SD, stable disease; VGPR, very good partial remission.
MCL
Patients with MCL who progressed during, or who were intolerant to, cBTKi treatement had survival of 3 to 10 months in the era preceeding chimeric antigen receptor T-cell therapies.30,31 Brexucabtagene ciloleucel is US Food and Drug Administration (FDA) approved for this population based on the ZUMA2 study, with durable remissions in 40% to 50% of patients,32 but it is toxic and impractical for many patients. In addition, real-world data show a <6 months median progression free survival (PFS) for patients with high-risk simplified mantle cell international prognostic index or TP53 mutation.32 Thus, novel strategies are needed for this population. The last update of the BRUIN MCL data focused on 90 patients previously exposed to cBTKis33 with a median of 3 prior therapies; 82% discontinued cBTKi use because of progression. Overall response rate (ORR) was 58% (complete remission, 20%). Median duration of response (DOR) among 52 patients who responded to treatment was 22 months. Among the patients who discontinued BTKi use because of progression (rather than intolerance), ORR and DOR were lower (ORR, 50%; median DOR, 14.8 months). These data led to accelerated FDA approval of pirtobrutinib for patients with MCL, after ≥2 previous therapies, including a cBTKi.34
CLL/SLL
Patients refractory to both a cBTKi and venetoclax (double-refractory), particularly patients who are penta-refractory (previous cBTKi, chemotherapy, CD20 antibody, BCL2i, and PI3Ki) have no accepted standard-of-care therapy available. In the primary efficacy population of 247 cBTKi-treated patients, presented at the 2022 American Society of Hematology Annual Meeting, pirtobrutinib demonstrated an ORR of 82% in patients with CLL29; all had received previous cBTKi treatment. ORR was 79%, (median PFS, 16.8 months) in patients who were double-refractory (median, 5 previous therapies), and 77.8% in patients who were penta-refractory. Median PFS in the whole cohort was 19.4 months (16.8 months for double-refractory). As predicted by preclinical data,35 pirtobrutinib was efficacious in patients with BTK C481S mutation; furthermore, ORR was 73.9% in patients without BTK C481 mutations and 55.6% in patients with downstream activating mutations in PLCG2, which renders the disease less dependent on BTK.5,6
RT
Chemoimmunotherapy is ineffective and toxic in most patients with RT.36 ORR with pirtobrutinib in 75 patients who were evaluable (median of 4 previous therapies for CLL and RT), was 52%, including 13.3% complete remission. Median DOR was 5.6 months. Six patients were bridged to curative intent allogeneic stem cell transplantation. Evaluation in larger cohorts and combination approaches are indicated.
WM
A total of 78 patients with WM were treated: 78% had received prior cBTKis and 64% had received chemotherapy, CD20 antibody, and cBTKis. Among patients who had received a previous cBTKi (ceased for progression in 66%), ORR was 64%, with 6-month PFS of 83% in responders.37
Patients intolerant of prior BTKi
Among the 773 patients in the BRUIN phase 1/2 trial, 127 were intolerant of previous cBTKis (95% ibrutinib), most commonly because of atrial fibrillation/flutter (24%).38 Pirtobrutinib was well tolerated, with 6% (7 of 127) discontinuations for pirtobrutinib-related AEs and no discontinuations for recurrence of the previous cBTKi-associated AE. In this subgroup, ORR was 77% in CLL/SLL and 82% in MCL, with median DOR of 28.4 months in CLL/SLL and not estimable in MCL.
Mechanisms of resistance to pirtobrutinib
Two recent reports have highlighted a spectrum of non-C481S BTK mutants in patients who progressed on pirtobrutinib (Table 1)8,39 Surprisingly, many of these BTK mutants are kinase-dead mutants that have inactive kinase sites and cannot autophosphorylate Y223 yet show active downstream signaling.8,39 These kinase-dead mutants are rare in patients with ibrutinib resistance but relatively common in patients treated with zanubrutinib7 or pirtobrutinib.8,39 These differences may be because of ibrutinib’s off-target effects on HCK340; recruitment of HCK may be an explanation for how kinase-dead BTK mutants continue to signal.9 Gatekeeper mutations at T474 have also been reported in patients with progressive disease after acalabrutinib and pirtobrutinib treatment.8,12,39
Future directions
Pirtobrutinib is FDA approved for patients with MCL who have previously received a cBTKi. Country-specific expanded access programs are also available.
Phase 3 studies evaluating pirtobrutinib in earlier lines of therapy and in direct comparison to cBTK inhibitors are underway (supplemental Table 1). A first-line (1L) approval strategy is only being pursued in CLL. In R/R MCL, the sole phase 3 trial compares pirtobrutinib to investigator’s choice of cBTKi. The phase 3 development program in CLL comprises 4 studies: 2 evaluate pirtobrutinib compared with standard-of-care 1L (BR) and R/R regimens (BR or idelalisib + rituximab); 1 compares pirtobrutinib with ibrutinib in both 1L and R/R CLL; the fourth compares a triplet of pirtobrutinib + venetoclax and rituximab vs venetoclax + rituximab (VR) in R/R CLL. The VR vs pirtobrutinib + venetoclax and rituximab study represents the first major trial of time-limited venetoclax in patients with progression after covalent BTKi therapy; 80% of the patients enrolled will be BTKi pretreated. In contrast, in the MURANO trial, which led to the approval of VR, only 2.6% of patients had received previous B-cell receptor–signaling inhibitors.
Several investigator-initiated phase 2 studies are evaluating novel combination strategies in 1L and R/R CLL, RT, and in R/R MCL with venetoclax with or without CD20 monoclonal antibodies (supplemental Table 2).
Conclusions
Pirtobrutinib is an important new agent for multirefractory lymphoid malignancies. Across CLL and low-grade lymphomas, the majority of patients refractory to previous cBTKis responded. The reasons for the high ORR in cBTKi-refractory lymphoproliferative disorders without BTK mutations are unclear. However, a point of difference between pirtobrutinib and cBTKis is the markedly longer half-life and potential for superior 24-hour target coverage under conditions of accelerated BTK synthesis.
Pirtobrutinib will be initially prescribed for patients who are heavily pretreated after other standard therapeutic options, including a cBTKi, have been exhausted. However, the ongoing phase 3 program seeks to establish superiority of pirtobrutinib compared with cBTKis in CLL (1L and R/R) and MCL (R/R). The durability of responses to pirtobrutinib compared with those to cBTKis in earlier lines of therapy will be determined by these phase 3 trials. In addition to the efficacy seen in patients who are refractory to cBTKis, pirtobrutinib’s toxicity profile in early-phase trials has been favorable (potentially because of high selectivity for BTKs relative to other kinases).35 Similarly to studies of acalabrutinib13 and zanubrutinib41 in patients intolerant of ibrutinib, 94% of patients intolerant of previous cBTKis (95% ibrutinib) tolerated pirtobrutinib. Pirtobrutinib could therefore potentially be used after discontinuation of ibrutinib because of toxicity, although the data for pirtobrutinib use after intolerance of acalabrutinib and zanubrutinib remain limited. The relative tolerability in head-to-head comparisons will be determined by the phase 3 program. Notably, although the CLL trials compare ibrutinib with pirtobrutinib, in the MCL study, the comparator is investigator’s choice of cBTKi, where a significant proportion patients will likely receive a second-generation cBTKi. Thus, we will have data for relative tolerability of pirtobrutinib compared with both ibrutinib and second-generation cBTKis.26,27,42
In CLL, resistance to pirtobrutinib occurs because of development of 1 of several mutations in BTK. The pattern of BTK mutations seen in patients resistant to pirtobrutinib is important because several of these mutations (for example, A428D, T474I, and L528W) may produce cross-resistance with some or all cBTKis.8 These data may have implications for sequencing of therapies in CLL in the future, but at present, there is insufficient knowledge surrounding mutations to sway prescribing patterns.
Optimal treatment of patients who develop pirtobrutinib resistance is unknown. As our available treatment options and understanding of molecular mechanisms of relapse expand, personalizing therapy by selecting subsequent treatments based on molecularly predicted sensitivity becomes critical. Early data from studies of BTK degrader molecules suggest that these may have activity in disease with mutated BTK (including kinase-dead mutations resulting in panresistance to BTKis).43 Novel immunotherapies such as bispecific antibodies and chimeric antigen receptor T-cell therapies may also offer a mutation-agnostic approach to treatment of patients who are multirefractory, and could also potentially be combined with pirtobrutinib, similarly to the use of ibrutinib in combination with lisocabtagene maraleucel in CLL.44
The occurrence of BTK mutations during cBTKi therapy are most frequent in patients with high-risk genomic features such as del(17p) and complex karyotype.6 The BRUIN trial enrolled patients who are multirefractory with disease already known to be prone to developing resistance mutations. Evaluation of pirtobrutinib in earlier phases of treatment and lower-risk populations will be important to understand the potential for long-term remissions with pirtobrutinib monotherapy, as have been seen in 1L studies of cBTKis.45 The favorable toxicity profile of pirtobrutinib also lends itself to combination with other agents; we look forward to seeing data from the ongoing investigator-initiated studies with pirtobrutinib and venetoclax in CLL, RT, and MCL. Finally, the data from patients with RT who are heavily pretreated are encouraging, but pirtobrutinib monotherapy will likely be inadequate for most patients with RT and we therefore look forward to data from studies evaluating pirtobrutinib in combination with other agents in RT.
Authorship
Contribution: P.A.T. and C.S.T. contributed equally to the writing the manuscript.
Conflict-of-interest disclosure: P.A.T. has consulted for Adaptive Biotechnologies, AbbVie, AstraZeneca, BeiGene, Genentech, LOXO/Lilly, Janssen, and Pharmacyclics, outside the submitted work; and has received research funding from Adaptive Biotechnologies, AbbVie, Genentech, LOXO/Lilly, and Pharmacyclics, outside the submitted work. C.S.T. has received research funding from Janssen, AbbVie, and BeiGene; and has received honoraria from Janssen, AbbVie, BeiGene, Loxo Oncology, and AstraZeneca.
Correspondence: Constantine S. Tam, The Alfred Hospital, Melbourne 3004, Australia; e-mail: constantine.tam@alfred.org.au.
References
Author notes
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal