Key Points
Tet2 knockout in mice induces heterogeneous clonal expansion associated with an aberrant expression of RNA splicing factors.
Repression of RNA splicing factor Rbm25 triggers clonal expansion of Tet2 knockout hematopoietic cells in vitro and in vivo.
Abstract
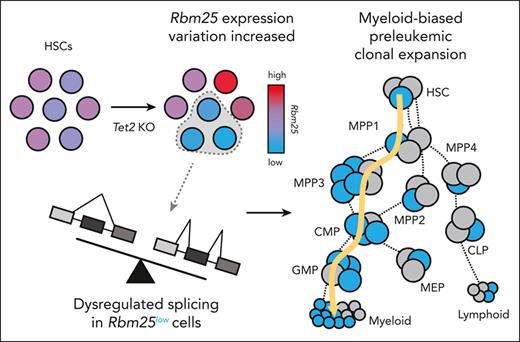
Clonal expansion sets the stage for cancer genesis by allowing for the accumulation of molecular alterations. Although genetic mutations such as Tet2 that induce clonal expansion and malignancy have been identified, these mutations are also frequently found in healthy individuals. Here, we tracked preleukemic clonal expansion using genetic barcoding in an inducible Tet2 knockout mouse model and found that only a small fraction of hematopoietic stem cells (HSCs) expanded excessively upon Tet2 knockout. These overexpanded HSCs expressed significantly lower levels of genes associated with leukemia and RNA splicing than nonoverexpanded Tet2 knockout HSCs. Knocking down Rbm25, an identified RNA splicing factor, accelerated the expansion of Tet2-knockout hematopoietic cells in vitro and in vivo. Our data suggest that mutations of an epigenetic factor Tet2 induce variability in the expression of an RNA splicing factor Rbm25, which subsequently drives heterogeneous preleukemic clonal expansion. This heterogeneous clonal expansion could contribute to the variable disease risks across individuals.
Introduction
Clonal expansion is the process by which the number of progenies of a cell excessively increases.1-4 It plays a crucial role during the early phases of oncogenesis. Clonal expansion is often initiated by sporadic genetic mutations or epigenetic dysregulations, which in turn provide greater opportunities for accumulating the additional genetic and epigenetic changes that may eventually lead to malignancy.4-7 In the hematopoietic system, clonal expansion, named clonal hematopoiesis, can be easily monitored by sampling the peripheral blood. Clonal hematopoiesis is monitored in clinical settings as an indicator of leukemia genesis6,7 and provides an easily accessible experimental model for studying the initial phase of cancer genesis.
Several commonly mutated genes, including TET2, DNMT3A, ASXL1, IDH2, and NPM11,5,8,9 have been identified to be associated with clonal hematopoiesis, many of which encode epigenetic factors. Mutations of these genes are also found frequently in patients with leukemia and preleukemic hematopoietic disorders.5,10-13 The knockout of these genes in mouse models drives cellular expansion and hematologic malignancies.14-17 With technical advances in high throughput sequencing that allow for the identification of clonal hematopoiesis marked by sporadic genetic mutations, recent studies show individuals carrying preleukemic mutations have an increased risk of developing leukemia, although most remain healthy and disease-free.1,5,8,18 For example, mutations of the DNA demethylase TET2 induce preleukemic clonal expansion in both humans and mice and are frequently found in patients with hematopoietic malignancies12,19-25 and in people without any sign of hematologic disease.5,18,26 The variable outcomes in humans may arise from the different responses of individual cells to the same sporadic mutation. Many studies to date analyze bulk cell populations and are unable to address cell-to-cell variation. Understanding cellular heterogeneity can help improve cancer prognosis by further stratifying individuals at high risk and may provide new therapeutic targets.
Hematopoietic stem cells (HSCs) sustain life-long hematopoietic regeneration and provide unique opportunities for accumulating genetic mutations that initiate clonal expansion and disease genesis. Recent studies show that HSCs are heterogeneous in their proliferation, differentiation, and gene expression.27,28 We hypothesized that variation among individual HSCs might generate variable responses to the same preleukemic mutation and produce heterogeneous levels of clonal expansion. To test this hypothesis, we applied genetic barcoding technology29,30 to track the clonal expansion of hundreds of individual HSCs with Tet2 knockout (KO) in a mouse model. In addition, we integrated single-cell RNA sequencing with clonal tracking31 to identify genes that were differentially expressed between overly and normally expanded Tet2 KO clones. Our results suggest that only a small fraction of Tet2 KO HSCs exhibits excessive clonal expansion and that their expansion is associated with the reduced expression of RNA splicing factors.
Materials and methods
Mice
Mice were purchased from Jackson Laboratories. Inducible Tet2 KO mice were generated by crossing B6;129S-Tet2tm1.1Iaai/J (Tet2fl/fl, CD45.2, stock No. 017573) and B6.129- Gt(ROSA)26Sortm1(Cre/ERT2)Tyj/J mice (Rosa26Cre+/+, CD45.2, stock No. 008463). Their offspring, Rosa26Cre+/−Tet2fl/fl mice, were used for inducing Tet2 deletion, and their offspring Rosa26Cre–/–Tet2fl/fl from the same litter were used as controls. Tet2+/− dead Cas9 (dCas9)-KRAB mice were generated by crossing B6(Cg)-Tet2tm1.2Rao/J (Tet2+/−, CD45.2, stock No. 023359) and B6.Cg-Igs2tm1(CAG-mCherry,-cas9/ZNF10∗)Mtm/J mice (dCas9-KRAB+/+, CD45.2, stock No. 030000). Donor mice were 12- or 16-week-old. Recipients were 12- or 16-week-old B6.SJL-Ptprca Pepcb/BoyJ mice (CD45.1, stock no. 002014). Flushed femur bone marrow (BM) cells from C57BL/6J and B6.SJL-Ptprca Pepcb/BoyJ (CD45.1/CD45.2, stock nos. 000664 and 002014) offspring were also used in the transplantation. To induce excision of floxed alleles, 4 mg tamoxifen per 40 g body weight was intraperitoneally injected into each mouse every other day for one week. All transplantation experiments were independently conducted twice. Data from all mice were combined and reported. All animal procedures were approved by the institutional animal care and use committee of University of Southern California.
HSC isolation, transduction, and transplantation
HSCs (lineage [TCR, CD4, CD8, B220, Gr1, Mac1, and Ter119]−/cKit+/Sca1+/Flk2−/CD34−/CD150+]) were obtained from the crushed bones and enriched using CD117 microbeads (Miltenyi Biotec, Auburn, CA). HSCs were then sorted with a fluorescence-activated cell sorter using the Aria II (BD Biosciences, San Jose, CA), transduced for 15 hours with barcode/single guide RNA (sgRNA) lentivirus, and washed 3 times before the transplantation. Recipient mice were preconditioned with 950 cGy X-ray irradiation before undergoing transplantation. Each recipient mouse received from 4000 to 5000 HSCs along with 200 000 whole BM cells via retro-orbital injection.
scRNA-seq and analysis
Single-cell RNA sequencing (scRNA-seq) was performed following the manufacturer’s protocol for the Chromium Single Cell 3′ Library (10x Genomics, version 3.1) and sequenced using an Illumina Novaseq and HiSeq 4000, with 50 000 raw reads per cell coverage. Data were processed using the Cell Ranger pipeline (10x Genomics, version 2.1.0). Cells were excluded if more than 10% unique molecular identifiers (UMIs) were mapped to mitochondrial genes or if total gene/UMI counts exceeded 3 deviations from the median. Molecules containing both the clonal tracking barcodes and the 10x chromium cellular barcodes were polymerase chain reaction–amplified from the complementary DNA library and sequenced using the SMRT sequencing platform. The data was analyzed using SMRT Analysis software with default parameters (Sequel II, SMRT Link version 5.1.0, Pacific Biosciences). Mapping between scRNA-seq and clonal tracking was established when 1 or more molecules contained an exact match to a tracking barcode and a chromium cellular barcode. Genes with 3 or more UMIs in at least 5% of the cells were used for downstream analyses. To compare single-cell gene expression data, P values were calculated using the one-sided Mann-Whitney U test.
Results
Tet2 deletion induces cellular expansion specifically in the myeloid lineage
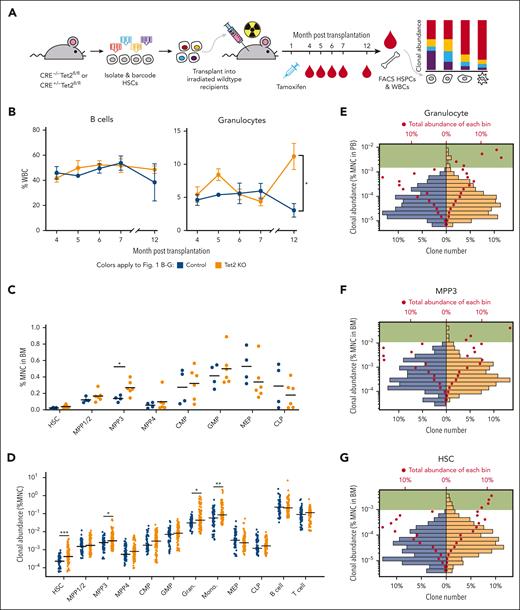
To investigate the heterogeneity of Tet2 KO–induced clonal expansion, we isolated and barcoded HSCs from Rosa26-CreERT2+Tet2fl/fl mice and their littermates of Rosa26-CreERT2−Tet2fl/fl mice as controls. The barcoded HSCs were transplanted into lethally irradiated wild-type recipients that subsequently received tamoxifen injection 1 month after the transplantation to induce Tet2 deletion. Tet2 KO mice herein refer to the mice carrying Tet2-deleted HSCs (Figure 1A; supplemental Figure 1A, available on the Blood website). We found that myeloid cells (eg, granulocytes), but not lymphoid cells (eg, B cells), expanded significantly in the peripheral blood of Tet2 KO mice compared with that in control mice 12 months after thetransplantation (Figure 1B; supplemental Figure 2). We set the experimental end time point at 13 months after transplantation when all mice remained alive and exhibited no detectable physiological changes. Our end time point analysis showed that 2 out of 6 mice from the Tet2 KO group had enlarged spleens (supplemental Figure 1B). In addition, significant expansion was detected specifically in the multipotent progenitor 3 (MPP3) population isolated from the BM (Figure 1C; supplemental Figure 3), which is known as a myeloid-biased multipotent progenitor.32 The specificity of clonal expansion in the myeloid lineage is consistent with the prevalence of TET2 induced–myeloid leukemia22 and other studies of Tet2 mutant mouse models.14,33
Heterogeneous cellular expansion induced by Tet2 KO. (A) Experimental workflow. HSCs from Rosa26-CreERT2+/−Tet2fl/fl mice and Rosa26-CreERT2–/–Tet2fl/fl littermate mice were barcoded and transplanted into wild-type recipients. Tet2 KO was induced 1 month after transplantation via tamoxifen injection. The experiment was performed twice. The combined results of 4 control mice and 6 Tet2 KO mice are shown. (B) Peripheral blood analysis via fluorescence-activated cell sorting (FACS). The abundance of B cells and granulocytes in control and Tet2 KO mice are shown. Error bars represent SEM. Two-tailed t test. (C) FACS analysis of HSPCs at the end point, 13 months after transplantation. Bars show the mean of control and Tet2 KO groups. Two-tailed t test. (D) The clonal abundance of the top 15 most abundant clones in each mouse. Each dot represents 1 clone. Bars show the median of the control and Tet2 KO groups. Control clones n = 60 and Tet2 KO clones n = 90. Two-tailed Mann-Whitney U test. (E-G) Distribution of granulocyte clonal abundance in the peripheral blood (PB) (E) and distribution of MPP3 (F) and HSC (G) clonal abundance in the BM. The bottom x-axis denotes the percent of unique clones in respective groups shown as bars. The top x-axis denotes the total abundance as a percent of the corresponding cell type for each bar shown as red dots. Green highlights bars that represent high-abundance clones are only found in the Tet2 KO group. Shown are clones from all mice in each group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. BM, bone marrow; CMP, common myeloid progenitor; GMP, granulocyte-monocyte progenitor; MEP, megakaryocyte–erythroid progenitor; MNC, mononuclear cell; SEM, standard error of mean; WBCs, white blood cells.
Heterogeneous cellular expansion induced by Tet2 KO. (A) Experimental workflow. HSCs from Rosa26-CreERT2+/−Tet2fl/fl mice and Rosa26-CreERT2–/–Tet2fl/fl littermate mice were barcoded and transplanted into wild-type recipients. Tet2 KO was induced 1 month after transplantation via tamoxifen injection. The experiment was performed twice. The combined results of 4 control mice and 6 Tet2 KO mice are shown. (B) Peripheral blood analysis via fluorescence-activated cell sorting (FACS). The abundance of B cells and granulocytes in control and Tet2 KO mice are shown. Error bars represent SEM. Two-tailed t test. (C) FACS analysis of HSPCs at the end point, 13 months after transplantation. Bars show the mean of control and Tet2 KO groups. Two-tailed t test. (D) The clonal abundance of the top 15 most abundant clones in each mouse. Each dot represents 1 clone. Bars show the median of the control and Tet2 KO groups. Control clones n = 60 and Tet2 KO clones n = 90. Two-tailed Mann-Whitney U test. (E-G) Distribution of granulocyte clonal abundance in the peripheral blood (PB) (E) and distribution of MPP3 (F) and HSC (G) clonal abundance in the BM. The bottom x-axis denotes the percent of unique clones in respective groups shown as bars. The top x-axis denotes the total abundance as a percent of the corresponding cell type for each bar shown as red dots. Green highlights bars that represent high-abundance clones are only found in the Tet2 KO group. Shown are clones from all mice in each group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. BM, bone marrow; CMP, common myeloid progenitor; GMP, granulocyte-monocyte progenitor; MEP, megakaryocyte–erythroid progenitor; MNC, mononuclear cell; SEM, standard error of mean; WBCs, white blood cells.
Preleukemic clonal expansion is initiated at the HSC level without measurable population expansion
At the clonal level, although the number of unique barcodes detected from the peripheral blood was slightly higher in Tet2 KO mice at early time points, the barcode numbers from the peripheral blood and from the BM were similar at the end time point between Tet2 KO and control mice (supplemental Figure 4), suggesting that similar number of HSC clones were producing blood in both groups of mice. However, the abundances of the most abundant clones in HSCs, MPP3s, granulocytes, and monocytes of the Tet2 KO mice were significantly higher than in the controls (Figure 1D,G; supplemental Figure 5). The high-abundance clones were few but contributed higher amounts of cells than the low- abundance clones in Tet2 KO mice (Figure 1E-F). This suggested that the highly expanded clones played an important role in driving the cellular expansion of granulocyte and MPP3 populations (Figure 1B-C). Notably, HSCs did not exhibit gross population expansion even though their clonal expansion was evident (Figure 1C,D,G). The high-abundance clones in Tet2 KO mice contributed a substantial number of HSCs, whereas the low-abundance clones in these mice contributed fewer HSCs compared with those in control mice (Figure 1G). These data suggest that clonal expansion can occur in the absence of gross population expansion when high-abundance clones outcompete low-abundance clones while the overall cell number is constrained.
Overexpanded clones in Tet2 KO mice produced more hematopoietic cells than any other clones in control mice
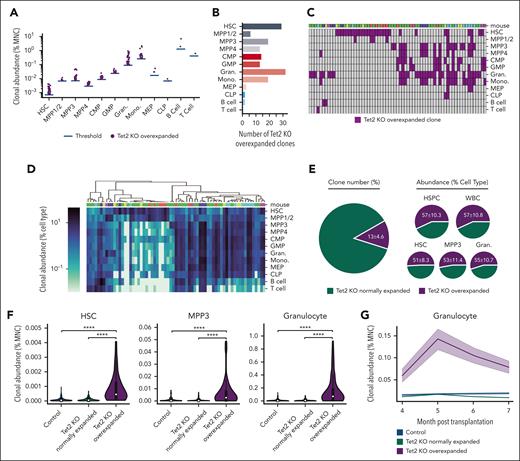
To identify clones that excessively and abnormally expanded upon Tet2 deletion, we defined overexpanded clones as the Tet2 KO clones whose abundances were greater than the most abundant clone from the control mice. The average abundance of the most abundant clone from each of the 4 control mice was used as the threshold. Consistent with the aforementioned myeloid expansion at the population and clonal levels (Figure 1), most overexpanded clones were found in the myeloid lineage and myeloid-biased hematopoietic stem and progenitor cells (HSPCs) and were very rarely found in megakaryocyte–erythroid progenitors, common lymphoid progenitors (CLPs), B cells, and T cells (Figure 2A-B). We identified 142 overexpanded clones across 12 hematopoietic cell types among all 6 Tet2 KO mice that belonged to 66 unique clones because some clones were identified as overexpanded in multiple cell types (Figure 2C-D).
Preleukemic clonal expansion in Tet2 KO mice is driven by rare, overexpanded clones. (A) Tet2 KO overexpanded clones are defined as clones whose abundances are greater than the most abundant clone in control mice. The blue bar shows the average abundance of the most abundant clone from each of the 4 control mice, which was used as the threshold to identify overexpanded clones. Each dot represents 1 overexpanded clone. (B) The number of Tet2 KO overexpanded clones in each cell type. (C) Tet2 KO overexpanded clones across cell types. Each column represents 1 unique clone. Some clones are identified as overexpanded in multiple cell types. (D) Hierarchical clustering of Tet2 KO overexpanded clones based on their abundance in different cell types. (C-D) Clones identified from the same mouse are indicated as a unique color (top). (E) Numbers and abundances of Tet2 KO overexpanded clones among all Tet2 KO clones. (F) Comparing HSC and MPP3 clonal abundance in the BM and granulocyte clonal abundance in the peripheral blood of control, Tet2 KO normally expanded and overly expanded clones. White dots represent the median. Two-tailed Mann-Whitney U test. ∗∗∗∗P < .0001. (G) The clonal abundance of granulocytes in the peripheral blood before population-level expansion is detected. Shaded regions represent SEM.
Preleukemic clonal expansion in Tet2 KO mice is driven by rare, overexpanded clones. (A) Tet2 KO overexpanded clones are defined as clones whose abundances are greater than the most abundant clone in control mice. The blue bar shows the average abundance of the most abundant clone from each of the 4 control mice, which was used as the threshold to identify overexpanded clones. Each dot represents 1 overexpanded clone. (B) The number of Tet2 KO overexpanded clones in each cell type. (C) Tet2 KO overexpanded clones across cell types. Each column represents 1 unique clone. Some clones are identified as overexpanded in multiple cell types. (D) Hierarchical clustering of Tet2 KO overexpanded clones based on their abundance in different cell types. (C-D) Clones identified from the same mouse are indicated as a unique color (top). (E) Numbers and abundances of Tet2 KO overexpanded clones among all Tet2 KO clones. (F) Comparing HSC and MPP3 clonal abundance in the BM and granulocyte clonal abundance in the peripheral blood of control, Tet2 KO normally expanded and overly expanded clones. White dots represent the median. Two-tailed Mann-Whitney U test. ∗∗∗∗P < .0001. (G) The clonal abundance of granulocytes in the peripheral blood before population-level expansion is detected. Shaded regions represent SEM.
The overexpanded clones represented ∼13% of all Tet2 KO clones but produced more than half of the HSPCs in the BM and mature blood cells in the peripheral blood (Figure 2E). Moreover, the overexpanded clones in Tet2 KO mice, on an average, produced significantly more MPP3s and granulocytes than normally expanded clones (those that did not overexpand) in Tet2 KO mice and all the clones in control mice (Figure 2F). These results suggest that the identified overexpanded clones are few but play a major role in hematopoietic regeneration and in driving the population-level expansion of MPP3s and granulocytes (Figure 1B-C).
Overexpanded clones initiated cellular expansion at the HSC level that is unmeasurable at the population level
Although the population-level expansion was not significant among HSCs (Figure 1C), some HSC clones exhibited overexpansion (Figures 1D,G and 2A). These clones produced significantly more HSCs than normally expanded clones in Tet2 KO mice as well as all clones in control mice (Figure 2F). This suggested that the abnormal clonal expansion was initiated at the HSC level, which is the uppermost level of the hematopoietic hierarchy. Furthermore, to determine whether the overexpanded clones had already started expansion before any population-level expansion was detected (Figure 1B), we retrospectively analyzed the peripheral blood collected at 4, 5, 6, and 7 months after transplantation. Although the normally expanded clones in Tet2 KO mice continuously produced similar amounts of granulocytes as that of the clones in control mice, the overexpanded clones produced significantly more granulocytes as early as 4 months after the transplantation (Figure 2G). These results suggest that clonal expansion is initiated at the apex of the hematopoietic hierarchy.
Overexpanded myeloid-biased clones drive cellular expansion upon Tet2 deletion
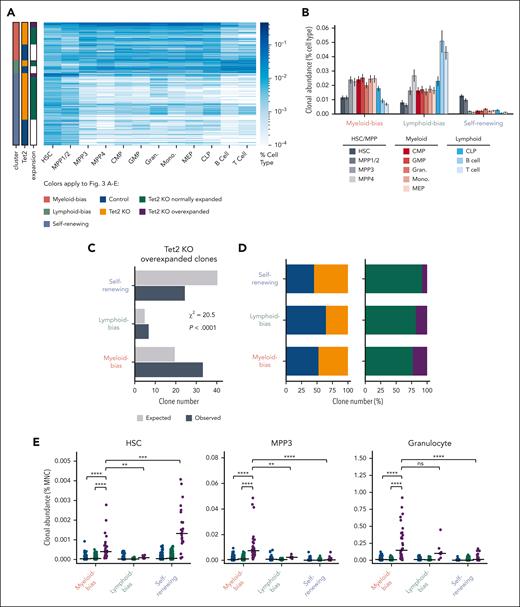
Because some of the overexpanded clones appeared to expand only at the HSC and MPP stages (Figure 2D-E), we next analyzed the heterogeneity of overexpanded clones to identify those that contributed to the observed myeloid expansion. We performed hierarchal clustering of all the clones from control and Tet2 KO mice and identified 3 major clusters that represented myeloid-biased clones, lymphoid-biased clones, and self- renewing clones (Figure 3A; supplemental Figure 6). Myeloid-biased clones were highly abundant in myeloid cell types, such as MPP3, common myeloid progenitor, granulocyte-monocyte progenitor, granulocyte, and monocyte populations, and had low abundance in lymphoid cells, including CLPs, B cells, and T cells (Figure 3A-B). Lymphoid-biased clones were highly abundant in CLP, B-cell, and T-cell populations as well as in MPP4, known as lymphoid-biased MPPs32 (Figure 3A-B). Self-renewing clones were highly abundant in HSC and MPP1/2 populations, the most primitive MPPs immediately downstream of HSCs32 and had low abundance in all downstream cell types (Figure 3A-B). Among all Tet2 KO clones, overexpanded clones were more likely to be myeloid-biased clones and less likely to be self-renewing clones compared with a random distribution (Figure 3C).
Overexpanded myeloid-biased Tet2 KO clones drive MPP3 and granulocyte expansion. (A) Hierarchical clustering of all clones from control and Tet2 KO mice identifies 3 major clusters. Clones in each cluster are grouped by Tet2 genotype and expansion profiles. Overexpanded clones were determined as shown in Figure 2A. (B) The average clonal abundance of each cell type in the 3 major clusters from (A). Error bars represent standard deviation. (C) Expected and observed number of Tet2 KO overexpanded clones in each cluster. The expected clone numbers are calculated based on the frequency in each cluster of all detected clones from the control and KO groups. χ2 = 20.5; P < .0001. (D) Number of unique clones across the 3 clusters. (E) HSC and MPP3 clonal abundance in the BM and granulocyte clonal abundance in the peripheral blood of control and Tet2 KO normally expanded and Tet2 KO overexpanded clones across the 3 clusters. Bars represent the median. Two-tailed Mann-Whitney U test. ns: not significant. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Overexpanded myeloid-biased Tet2 KO clones drive MPP3 and granulocyte expansion. (A) Hierarchical clustering of all clones from control and Tet2 KO mice identifies 3 major clusters. Clones in each cluster are grouped by Tet2 genotype and expansion profiles. Overexpanded clones were determined as shown in Figure 2A. (B) The average clonal abundance of each cell type in the 3 major clusters from (A). Error bars represent standard deviation. (C) Expected and observed number of Tet2 KO overexpanded clones in each cluster. The expected clone numbers are calculated based on the frequency in each cluster of all detected clones from the control and KO groups. χ2 = 20.5; P < .0001. (D) Number of unique clones across the 3 clusters. (E) HSC and MPP3 clonal abundance in the BM and granulocyte clonal abundance in the peripheral blood of control and Tet2 KO normally expanded and Tet2 KO overexpanded clones across the 3 clusters. Bars represent the median. Two-tailed Mann-Whitney U test. ns: not significant. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Conversely, we also found that myeloid-biased Tet2 KO clones were more likely to overexpand than lymphoid-biased and self-renewing Tet2 KO clones (Figure 3D). These data suggest that myeloid-biased HSC clones are more prone to develop excessive expansion upon Tet2 deletion.
Although overexpanded clones on average significantly contributed more to HSCs, MPP3s, and granulocytes than normally expanded Tet2 KO clones and clones in control mice (Figure 2F-G), we found that different subsets of the overexpanded clones made significantly different contributions (Figure 3E). Although both myeloid-biased and self-renewing overexpanded clones contributed substantially to HSCs (Figure 3E), expansion at the MPP3 and granulocyte stages were specifically attributed to myeloid-biased overexpanded clones. In particular, myeloid-biased overexpanded clones contributed significantly more to MPP3s and granulocytes than normally expanded Tet2 KO clones and clones in control mice; they also contributed more than lymphoid-biased overexpanded clones and self-renewing overexpanded clones (Figure 3E). Therefore, myeloid-biased overexpanded clones play a major role in driving the observed population-level expansion at the MPP3 and granulocyte stages.
Myeloid-biased overexpanded and normally expanded Tet2 KO clones express genes differently
Because overexpanded myeloid-biased clones constituted only a small subset of all Tet2 KO clones but played a dominant role in driving the expansion of HSC, MPP3, and granulocyte populations (Figure 3), we sought to determine whether distinct gene expression characteristics underlie their expansion by performing scRNA-seq analysis at the end time point of the clonal tracking experiments (Figures 1-3). We used 10x chromium single-cell RNA sequencing to profile single-cell transcriptomes and recovered the clonal tracking barcodes from the complementary DNAs of individual cells, as previously described31 (Figure 4A; supplemental Figure 7). By mapping the clonal tracking barcodes with single-cell complementary DNA barcodes (Figure 4A), we were able to compare HSCs and MPP3s derived from overexpanded Tet2 KO myeloid-biased clones and normally expanded Tet2 KO myeloid-biased clones to identify genes specifically associated with different levels of clonal expansion (Figure 4B-G; supplemental Table 2). We also identified genes associated with clonal expansion among Tet2 KO self-renewing clones (supplemental Table 2). Although the overexpansion of self-renewing clones could have long-term effects on leukemia genesis (Figure 3E), we focused on the genes identified from myeloid-biased clones because these clones drove expansion at the cell population level (Figure 1).
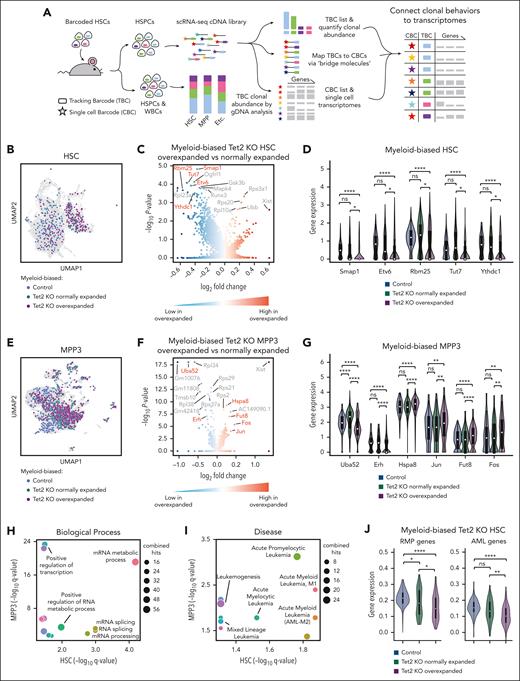
Overexpanded myeloid-biased Tet2 KO clones exhibit significantly reduced expression of genes associated with RNA splicing and AML. (A) Workflow to connect clonal tracking data to single-cell gene expression profiles. (B) Uniform manifold approximation and projection (UMAP) visualization of myeloid-biased HSCs. (C) Differentially expressed genes comparing overly expanded and normally expanded HSCs that are myeloid-biased and Tet2 KO. The complete gene list is available in supplemental Table 2. (D) Examples of significantly differentially expressed genes are highlighted in red font in panel C. (E) UMAP visualization of myeloid-biased MPP3s. (B,E) Colors highlight cells identified as derived from myeloid-biased clones. The rest of the cells are shown in gray. (F) Differentially expressed genes comparing overly expanded and normally expanded MPP3s that are myeloid-biased and Tet2 KO. The complete gene list is available in supplemental Table 2. (G) Examples of significantly differentially expressed genes are highlighted in red font in (F). (D,G) One-side Mann-Whitney U test. The adjusted P values (Benjamini–Hochberg correction) are shown. (H,I) Gene ontology (GO) analysis of significantly differentially expressed genes (adjusted P < .1) from panels C and F. All the GO terms associated with biological processes (H) and diseases (I) shared by the GO analysis of HSC and MPP3 data are shown. The complete GO list is available in supplemental Table 2. (J) Comparing averaged expression of all genes associated with the mRNA metabolic process GO terms (GO:0016071) and AML (C0026998). Two-tailed Mann-Whitney U test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Overexpanded myeloid-biased Tet2 KO clones exhibit significantly reduced expression of genes associated with RNA splicing and AML. (A) Workflow to connect clonal tracking data to single-cell gene expression profiles. (B) Uniform manifold approximation and projection (UMAP) visualization of myeloid-biased HSCs. (C) Differentially expressed genes comparing overly expanded and normally expanded HSCs that are myeloid-biased and Tet2 KO. The complete gene list is available in supplemental Table 2. (D) Examples of significantly differentially expressed genes are highlighted in red font in panel C. (E) UMAP visualization of myeloid-biased MPP3s. (B,E) Colors highlight cells identified as derived from myeloid-biased clones. The rest of the cells are shown in gray. (F) Differentially expressed genes comparing overly expanded and normally expanded MPP3s that are myeloid-biased and Tet2 KO. The complete gene list is available in supplemental Table 2. (G) Examples of significantly differentially expressed genes are highlighted in red font in (F). (D,G) One-side Mann-Whitney U test. The adjusted P values (Benjamini–Hochberg correction) are shown. (H,I) Gene ontology (GO) analysis of significantly differentially expressed genes (adjusted P < .1) from panels C and F. All the GO terms associated with biological processes (H) and diseases (I) shared by the GO analysis of HSC and MPP3 data are shown. The complete GO list is available in supplemental Table 2. (J) Comparing averaged expression of all genes associated with the mRNA metabolic process GO terms (GO:0016071) and AML (C0026998). Two-tailed Mann-Whitney U test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Our results show that the most significantly differentially expressed genes were downregulated in myeloid-biased overexpanded clones compared with those in myeloid-biased normally expanded clones (Figure 4C,F; supplemental Table 2), consistent with reports that Tet2 KO HSCs undergo DNA hypermethylation.33,34 Critically, many genes differentially expressed between overexpanded and normally expanded Tet2 KO myeloid-biased clones exhibited little or no difference in their expression between normally expanded Tet2 KO clones and control clones (Figure 4D,G), indicating their specific association with excessive clonal expansion instead of with the Tet2 mutation.
Many differentially expressed genes we identified had already been shown to play essential roles in cancer genesis. For example, Smap1, Etv6, Rbm25, Tut7, and Ythdc1 genes identified from myeloid-biased overexpanded HSCs (Figure 4D) have been implicated in cancer-related pathways and leukemia.35-39 Similarly, genes identified from myeloid-biased overexpanded MPP3s, such as Uba52, Erh, Hspa8, Jun, Fut8, and Fos (Figure 4G), have also been implicated in cancer.40-45 In particular, Jun and Fos have been identified as proto-oncogenes that cause healthy cells to become malignant when mutated. Therefore, our approach of comparing cells carrying the same Tet2 genetic mutation but expanding at different levels identified genes underlying cancer genesis. These results highlight the disease relevance of the molecular differences between clones exhibiting different levels of preleukemic clonal expansion.
Reduced expression of RNA splicing factors is associated with Tet2 KO–induced preleukemic clonal expansion
To identify the biological processes underlying Tet2 KO–induced clonal expansion, we performed gene ontology analysis of differentially expressed genes between myeloid-biased overexpanded Tet2 KO clones and normally expanded Tet2 KO clones (Figure 4H, I). At both HSC and MPP3 levels, these genes were significantly enriched for biological processes related to RNA processing, particularly messenger RNA (mRNA) splicing (Figure 4H; supplemental Figure 8; supplemental Table 3). Moreover, these genes were also significantly relevant to acute myeloid leukemia (AML; Figure 4I; supplemental Figure 8; supplemental Table 3), a major disease associated with TET2 mutations.24,25,34,46
To further verify the relevance of RNA processing and AML in Tet2 KO–induced excessive clonal expansion, we compared the average expression levels of all RNA processing and AML-related genes in overexpanded clones and normally expanded clones. We found that these genes were expressed at significantly lower levels in overexpanded Tet2 KO myeloid-biased HSCs than in normally expanded Tet2 KO myeloid-biased clones and myeloid-biased control clones without the Tet2 mutation (Figure 4J). These data suggest that RNA processing plays a role in driving preleukemic clonal expansion upon Tet2 mutation.
Rbm25 modulates the expansion of Tet2 KO HSPC clones
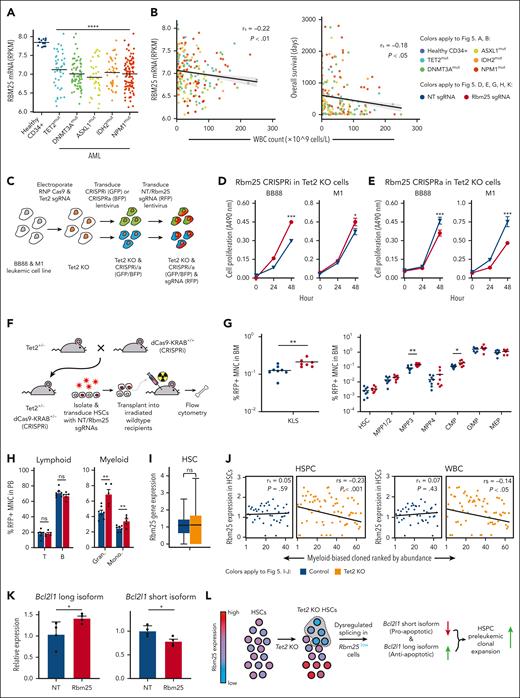
To determine the functional role of the RNA processing genes we had identified, we further investigated Rbm25, an RNA splicing factor that was significantly downregulated in Tet2 KO myeloid-biased overexpanded HSCs compared with that in Tet2 KO myeloid-biased normally expanded HSCs and control myeloid-biased HSCs without Tet2 mutation (Figure 4D). Rbm25 was selected because it is among the most significantly differentially expressed RNA splicing factors in our analysis (Figure 4C-D; supplemental Table 2). Moreover, RBM25 is expressed at significantly low levels in human AML blasts that carry mutations in TET2 and other AML-associated genes encoding epigenetic factors, such as DNMT3A, ASXL1, IDH2, and NPM147,48 (Figure 5A). In addition, reduced RBM25 expression in these AML blasts is significantly associated with increased white blood cell count, and the white blood cell count is significantly negatively correlated with the overall survival of the patients with AML. These data highlight the clinical relevance of Rbm25 in AML (Figure 5B).
Repression of the RNA splicing factor Rbm25 accelerates the expansion of Tet2 KO HSPCs. (A) Rbm25 expression in AML with preleukemic mutations from patients. Controls are CD34+ BM cells from healthy individuals. Healthy CD34+ n = 16, TET2mut n = 38, DNMT3Amut n = 72, ASXL1mut n = 29, IDH2mut n = 41, and NPM1mut n = 93. Two-tailed t test. (B) Spearman correlation (rs) of WBC count with Rbm25 expression (left) or with overall survival (right) of patients with AML carrying preleukemic mutations as shown in panel A. (A-B) Data derived from BEAT AML ELN2017 cohort.47,48 (C) Workflow for engineering leukemic cell lines to carry Tet2 KO and Rbm25 expression knockdown (CRISPRi) or Rbm25 expression activation (CRISPRa). Transduced cells were sorted after each transduction. (D) 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell proliferation assay of Tet2 KO leukemic cell lines with CRISPRi of Rbm25. (E) MTS cell proliferation assay of Tet2 KO leukemic cell lines with CRISPRa of Rbm25. (D-E) Cells were transduced with either nongenomic targeting (NT; gray) or Rbm25-targeting (red) sgRNAs. Error bars represent SEM. Two-tailed t test. (F) Experimental workflow to determine the effect of Rbm25 expression on the expansion of Tet2 KO HSPCs. HSCs carrying heterozygous Tet2 KO and dCas9-KRAB were transduced with either NT sgRNAs or Rbm25-targeting sgRNAs. (G) Fraction of various types of HSPCs among all red fluorescent protein (RFP)+ MNCs in the BM 3 months after transplantation. RFP indicates successful transduction of sgRNAs. The experiment was performed twice. The combined results of 8 mice in the NT sgRNA group and 7 mice in the Rbm25 sgRNA group are shown. (H) Fraction of various types of blood cells among all RFP+ MNCs in the peripheral blood 6 months after the transplantation. The experiment was performed twice. The combined results of 7 mice in the NT sgRNA group and 5 mice in the Rbm25 sgRNA group are shown. (G-H) Each dot depicts data from 1 mouse. One-tailed t test. (I) Rbm25 expression in control and Tet2 KO HSCs. (J) Spearman correlation (rs) of clonal abundance and averaged expression level of Rbm25 in each myeloid-biased clone. Clonal abundance ranked from least to greatest, with rank 1 being the least abundant. (I-J) Data derived from the scRNA-seq experiment is shown in Figure 4. (K) Bcl2l1 mRNA isoform abundance in Tet2+/− Cas9-KRAB+/− HSPCs transduced with NT sgRNAs or Rbm25-targeting sgRNAs. One-tailed t test. (L) Model depicting how Tet2 KO and Rbm25 repression drive hematopoietic expansion. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .001.
Repression of the RNA splicing factor Rbm25 accelerates the expansion of Tet2 KO HSPCs. (A) Rbm25 expression in AML with preleukemic mutations from patients. Controls are CD34+ BM cells from healthy individuals. Healthy CD34+ n = 16, TET2mut n = 38, DNMT3Amut n = 72, ASXL1mut n = 29, IDH2mut n = 41, and NPM1mut n = 93. Two-tailed t test. (B) Spearman correlation (rs) of WBC count with Rbm25 expression (left) or with overall survival (right) of patients with AML carrying preleukemic mutations as shown in panel A. (A-B) Data derived from BEAT AML ELN2017 cohort.47,48 (C) Workflow for engineering leukemic cell lines to carry Tet2 KO and Rbm25 expression knockdown (CRISPRi) or Rbm25 expression activation (CRISPRa). Transduced cells were sorted after each transduction. (D) 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell proliferation assay of Tet2 KO leukemic cell lines with CRISPRi of Rbm25. (E) MTS cell proliferation assay of Tet2 KO leukemic cell lines with CRISPRa of Rbm25. (D-E) Cells were transduced with either nongenomic targeting (NT; gray) or Rbm25-targeting (red) sgRNAs. Error bars represent SEM. Two-tailed t test. (F) Experimental workflow to determine the effect of Rbm25 expression on the expansion of Tet2 KO HSPCs. HSCs carrying heterozygous Tet2 KO and dCas9-KRAB were transduced with either NT sgRNAs or Rbm25-targeting sgRNAs. (G) Fraction of various types of HSPCs among all red fluorescent protein (RFP)+ MNCs in the BM 3 months after transplantation. RFP indicates successful transduction of sgRNAs. The experiment was performed twice. The combined results of 8 mice in the NT sgRNA group and 7 mice in the Rbm25 sgRNA group are shown. (H) Fraction of various types of blood cells among all RFP+ MNCs in the peripheral blood 6 months after the transplantation. The experiment was performed twice. The combined results of 7 mice in the NT sgRNA group and 5 mice in the Rbm25 sgRNA group are shown. (G-H) Each dot depicts data from 1 mouse. One-tailed t test. (I) Rbm25 expression in control and Tet2 KO HSCs. (J) Spearman correlation (rs) of clonal abundance and averaged expression level of Rbm25 in each myeloid-biased clone. Clonal abundance ranked from least to greatest, with rank 1 being the least abundant. (I-J) Data derived from the scRNA-seq experiment is shown in Figure 4. (K) Bcl2l1 mRNA isoform abundance in Tet2+/− Cas9-KRAB+/− HSPCs transduced with NT sgRNAs or Rbm25-targeting sgRNAs. One-tailed t test. (L) Model depicting how Tet2 KO and Rbm25 repression drive hematopoietic expansion. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .001.
To determine whether Rbm25 expression plays a role in regulating the expansion of Tet2 KO hematopoietic cells, we used the CRISPRi and CRISPRa systems to change Rbm25 expression in mouse leukemic cell lines (BB88 and M1) that were genetically modified to be Tet2 KO and express dCas9–Krüppel associated box domain (KRAB) or dCas9–VP64, p65 and Rta (VPR), respectively (Figure 5C; supplemental Figure 9). We found that both Tet2 KO cell lines proliferated significantly faster when Rbm25 expression was downregulated (Figure 5D) and significantly slower when Rbm25 expression was upregulated (Figure 5E). These results suggest Rbm25 regulates the expansion of Tet2 KO hematopoietic cells.
To determine whether Rbm25 repression triggers the expansion of Tet2 KO HSPCs in vivo, we isolated HSCs from mice carrying heterozygous Tet2 KO and dCas9-KRAB and transduced them with Rbm25 targeting or nongenome targeting sgRNAs. These HSCs were then transplanted into lethally irradiated wild-type recipient mice (Figure 5F; supplemental Figure 9). Three months after transplantation, Rbm25 downregulation had already induced significantly increased expansion of cKit+Sca1+lineage–progenitors, specifically the MPP3 subpopulation (Figure 5G). Additionally, common myeloid progenitors also significantly expanded (Figure 5G), further demonstrating the myeloid-specific expansion. Moreover, myeloid-specific expansion in the peripheral blood cells became evident at 6 months after transplantation (Figure 5H). This result suggests that Tet2 KO myeloid progenitors undergo increased expansion when Rbm25 expression is suppressed.
Tet2 KO induces variable expression of Rbm25, subsequently altering the splicing of Bcl2l1 mRNA
Previous bulk RNA-seq analyses suggest that Rbm25 expression is downregulated upon Tet2 KO.16 Our single-cell analysis showed Rbm25 downregulation took place specifically in overexpanded clones (Figure 4D). We found that Tet2 KO resulted in an increased variability of Rbm25 expression among individual HSCs (Figure 5I). The variable expression of Rbm25 could be directly regulated by Tet2 because previous studies that used chromatin immunoprecipitation sequencing of Tet2 in mouse hematopoietic cells49 suggest that Tet2 binds to Rbm25 within 10kb of the target 5-hydroxy-methyl cytosine (5hmc) site. Moreover, the loss of Tet2 results in reduced 5hmc at the Rbm25 gene locus in mouse HSCs (supplemental Figure 10).16 Altogether, these data suggest that Tet2 binds to the Rbm25 gene locus and regulates the 5hmc level and Rbm25 expression and that the loss of Tet2 results in the decrease of 5hmc and increase of Rbm25 expression variation among individual HSCs.
The variability of Rbm25 expression is associated with the variable clonal expansion of individual HSCs (Figure 5J). In particular, HSCs with lower levels of Rbm25 expression exhibited higher levels of clonal expansion upon Tet2 KO (Figure 5J). This correlation only existed among Tet2 KO clones and not among control clones (Figure 5J), indicating that the Tet2 mutation was a prerequisite for Rbm25 to modulate clonal expansion. The clonal expansion of HSCs with reduced Rbm25 expression may allow these cells to eventually dominate the entire cell population and result in the apparent downregulation of Rbm25 expression upon Tet2 KO, as identified in the previous bulk RNA-seq analyses.16
To determine how Rbm25 repression induced clonal expansion in conjunction with Tet2 KO, we examined RNA splicing in primary mouse cKit+Sca1+lineage– progenitors that were transduced with Rbm25 targeting sgRNAs and carried heterozygous Tet2 KO and dCas9-KRAB. We found that Rbm25 repression changed the splicing of Bcl2l1 mRNA (Figure 5K). Specifically, HSPCs with the downregulation of Rbm25 expressed a significantly less short splicing isoform and significantly more long splicing isoform of Bcl2l1. The former is known to promote apoptosis, and the latter is known to inhibit apoptosis.50 Reduced apoptosis can promote clonal expansion, although its functional effect and the roles of other Rbm25 targets remain to be completely investigated. Together, these data suggest that Tet2 KO results in the variable expression of Rbm25, which dysregulates the splicing of Bcl2l1 mRNA and promotes clonal expansion (Figure 5L).
Discussion
Although the heterogeneity in the prognosis of leukemic mutations across individuals has been well recognized,1,51,52 little is known about the underlying cellular and molecular mechanisms. In this study, we provide original experimental evidence that this may be attributed to different expansion levels induced by a single mutation among individual cell clones. Although our clonal tracking study uses a limited number of mice and may require further investigation and validation, our findings suggest that sporadic preleukemic mutations in humans do not always induce excessive clonal expansion. Additional factors, such as reduced expression of RNA splicing factors, may be involved. Although preleukemic mutations may have other effects that increase leukemia risk, such as altering gene expression and genetic stability, clonal expansion is considered an initial step of cancer genesis.2-4,53 The variability in clonal expansion induced by a single preleukemic mutation could explain the risk and rarity of disease genesis in humans.
Genes involved in RNA splicing are often mutated in hematopoietic malignancies and clonal hematopoiesis.5,54-56 Moreover, mutations of RNA splicing factors have been found to drive preleukemic diseases such as myelodysplastic syndromes.57,58 Repression of the RNA splicing factor Rbm25 has been shown to increase the proliferation of AML cell lines but does not affect the expansion of primary wild-type HSPCs.37 This is in line with our data that reveal that Rbm25 expression is negatively correlated with clonal expansion only in Tet2 KO HSCs and not in control HSCs (Figure 5J). Our data further reveal a new role of Rbm25 in driving Tet2 KO–associated clonal expansion in primary mouse HSPCs. Using Tet2 and Rbm25 as examples, our data suggest a potential general mechanism that splicing alterations may act as a secondary driver following epigenetic alterations during the multiple steps of leukemia genesis, which requires further experimental studies. This discovery suggests that RNA splicing could be used to further stratify patients at high risk and as therapeutic targets to prevent clonal hematopoiesis and cancer genesis. Moreover, our data suggest that maintaining Rbm25 expression may prevent leukemic expansion in the presence of preleukemic mutations (Figure 5).
Our study reveals cell-to-cell variability in clonal expansion during the preleukemic stage. Cellular heterogeneity has been increasingly recognized in cancer progression and remains a major challenge in developing viable treatments.59 We show that the deletion of Tet2 in HSCs induces variable levels of clonal expansion. The overexpanded clones expressed significantly lower levels of AML-related genes (Figure 4J), including tumor suppressors such as Etv6, Runx1, Ezh2, and Suz1236,60,61 whose repression may contribute to the observed clonal expansion in addition to Rbm25 (Figure 5). At the molecular level, Tet2 KO induces variability in Rbm25 expression (Figure 5I), consistent with previous reports that Tet2 binds to the Rbm25 gene locus and that the loss of Tet2 results in the reduction of 5hmc at Rbm25 (supplemental Figure 10).16,49 Our unique approach of comparing overly expanded and normally expanded clones with the same genetic mutation brings new insights into the molecular events underlying preleukemic clonal expansion, which is different from previous studies relying on population-level comparisons between Tet2 mutant and healthy cells. This approach can be extended to studying other types of cancer and diseases driven by cellular expansion.
Acknowledgments
The authors thank the USC Stem Cell Flow Cytometry Core, CHLA Sequencing Core, and UCI Genomics High Throughput Facility for their technical support. The authors also thank Jasper Rubin-Sigler and Miller Huang for providing dCas9-KRAB–green fluorescent protein and dCas9-VPR–blue fluorescent protein lentivirus used for in vitro experiments. This manuscript was edited by Life Science Editors.
R.L. is a Scholar of the Leukemia & Lymphoma Society (LLS-1370-20) and was a Richard N. Merkin Associate Professor, supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) grants R35HL150826, R01HL138225, R01HL135292, and K99/R00HL113104. C.B. was supported by NIH NHLBI grant 1F31HL149278-01A1. J.E. and I.G. were supported by California Institute for Regenerative Medicine grant EDUC4-12756 and EDUC2-12607, respectively. The project described was supported in part by award number P30CA014089 from the NIH National Cancer Institute.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Authorship
Contribution: R.L. conceptualized the project, designed the experiments, and wrote the manuscript; C.B. conceptualized the project, designed and performed the experiments, performed data analysis, and wrote the manuscript; J.E., D.J., and P.C. performed data analysis and generated figures; Y.L., M.V.-R., and I.G. assisted with experiments and data collection; and A.N. assisted in writing and editing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rong Lu, 1425 San Pablo St, BCC 306, Los Angeles, CA 90033; e-mail: ronglu@usc.edu.
References
Author notes
Gene expression data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE212534).
Protocols presented in the study are available on request from the corresponding author, Rong Lu (ronglu@usc.edu).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal