In this issue of Blood, Kaiser et al1 pinpoint the glycoprotein IIb (GPIIB)/Gα13/Src tyrosine kinase (c-Src)/14-3-3ζ signaling axis as a requirement for platelet migration and shed light on how the antineoplastic oral tyrosine kinase inhibitor dasatinib increases the risk of inflammation-associated mucosal hemorrhage.
Platelets are highly dynamic, migratory anucleate blood cells that not only are involved in blood clot formation but also play a key role in first-line immune responses. To accomplish both functions, platelets restructure their cytoskeleton when they adhere to matrix molecules following vascular injury.2 This phenomenon mainly involves myosin heavy chain 9 (MYH9) and actin-related protein complex 2/3 (Arp2/3).3,4 So far, the signaling pathways that regulate platelet migration up- and downstream of these mechanisms have remained elusive. In this issue of Blood, Kaiser and colleagues reveal how the sarcoma family kinase c-Src and the Src family kinase-binding protein 14-3-3ζ, situated downstream of the fibrinogen receptor GPIIBIIIA, coupled to Gα13, is required for platelet polarization and migration.1 In addition to further defining this downstream signaling pathway, Kaiser et al elucidate an important side effect of the antineoplastic oral tyrosine kinase inhibitor dasatinib. Dasatinib is an inhibitor of B-cell receptor-ABL-tyrosine kinase and of the sarcoma family kinase c-Src, used clinically to treat chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL). They found that dasatinib-treatment causes mucosal bleeding through the blockade of platelet migration.
The fibrin(ogen) receptor GPIIBIIIA regulates platelet adherence to extracellular matrices. Kaiser and colleagues found that both genetic and pharmacologic targeting of the Arp2/3 complex abrogates the polarized platelet phenotype and the ability of platelets to form lamellipodia and migrate on albumin/fibrin(ogen) hybrid matrices, highlighting the importance of Arp2/3 for platelet polarization and migration.5 Intriguingly, although platelet size and circularity was reduced, inhibition of Arp2/3 did not affect clot retraction. Previous work by Gaertner and coworkers has demonstrated the critical involvement of MYH9 in platelet migration.6 In this issue of Blood, Kaiser et al show that coordinated myosin II function is a requirement for motility (retraction of lamellipodia) but is not necessary for platelet polarization, which is mainly mediated by dynamic actin waves along the leading edge of lamellipodia. Moreover, the authors identified phospholipase C as the upstream regulator of Arp2/3-dependent platelet migration. Inhibitor experiments with pyrazolopyrimidine, which blocks the action of potent platelet agonists, suggested the involvement of Src family kinases in platelet polarization and migration. A subsequent screening of different kinase inhibitors identified c-Src as the most sensitive Src family kinase promoting platelet migration on fibrinogen matrices. In addition to c-Src, the sarcoma family kinase-binding protein 14-3-3ζ is involved in GPIIBIIIA-mediated outside-in signaling. Indeed, colocalization of GPIIB to 14-3-3ζ, and inhibitor screening with the 14-3-3ζ-inhibitor 3′,4′,7′ trihydroxyisoflavone, univocally demonstrated the involvement of the Src family kinase binding protein 14-3-3ζ in platelet migration. Stimulation with soluble agonists such as ADP and thromboxane play a role in platelet recruitment prior to adhesion but have no effect once migration is initiated.
Most importantly, the authors found that platelet migration, as well as polarization and lamellipodium formation,7 was impaired at low doses of the c-Src inhibitor dasatinib, whereas retraction of cross-linked fibrin, platelet degranulation, and thrombus formation were only impaired at higher doses (see figure). Indeed, treatment with low doses of dasatinib reduced platelet area, circularity, and aspect ratio, with an abnormal presence of filopodia, through inhibition of Src tyrosine 418 phosphorylation. Since the occurrence of hemorrhage due to dasatinib treatment is associated with mucosal inflammation8 and platelet GPIIBIIIA function is known to be crucial to prevent bleeding when vascular integrity is jeopardized in the inflamed vasculature,9 a mouse model of acute lung injury was used to better explore the underlying molecular mechanisms. This in vivo model of lipopolysaccharide (LPS)-induced mucosal inflammation confirmed the clinical observation of enhanced inflammatory hemorrhage in patients treated with dasatinib.10 Importantly, neither systemic platelet counts nor the number of recruited platelets or neutrophils were changed. Furthermore, in an LPS-induced sepsis mouse model, 4-dimensional confocal intravital microscopy of mesenterial vessels revealed fewer migrating platelets in the dasatinib-treated group. Importantly, Kaiser et al confirmed the translational relevance of their findings by a pilot study, analyzing the platelets of patients with CML treated with dasatinib or bosutinib compared with those receiving imatinib, which does not inhibit c-Src. Although platelet spreading on fibrinogen matrices was unaffected, the migratory capacity of platelets from patients with CML treated with c-Src inhibitor was vastly reduced.
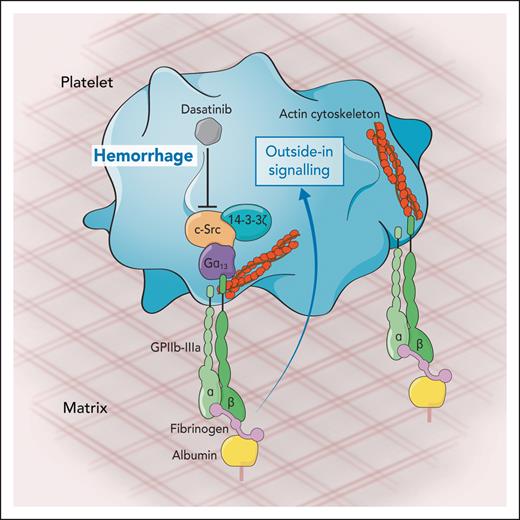
Platelet migration on fibrinogen surfaces measured ex vivo, after derivatization of albumin-coated matrices. The formation of lamellipodia is accomplished through the integrin receptor GPIIbIIIa. Fibrinogen binding to GPIIbIIIa triggers an outside-in signaling mediated by the kinases c-Src and 14-3-3ζ through the Gα13 that results in the reorganization of actin cytoskeleton and subsequent platelet migration. Dasatinib, a kinase inhibitor therapeutically used to treat chronic myeloid leukemia, inhibits platelet migration and is therefore associated with hemorrhage events observed during the treatment of leukemia patients. Professional illustration by Somersault18:24.
Platelet migration on fibrinogen surfaces measured ex vivo, after derivatization of albumin-coated matrices. The formation of lamellipodia is accomplished through the integrin receptor GPIIbIIIa. Fibrinogen binding to GPIIbIIIa triggers an outside-in signaling mediated by the kinases c-Src and 14-3-3ζ through the Gα13 that results in the reorganization of actin cytoskeleton and subsequent platelet migration. Dasatinib, a kinase inhibitor therapeutically used to treat chronic myeloid leukemia, inhibits platelet migration and is therefore associated with hemorrhage events observed during the treatment of leukemia patients. Professional illustration by Somersault18:24.
Altogether, this translational study by Kaiser et al demonstrate that the GPIIB/Gα13/c-Src/14-3-3ζ signaling axis is essential for platelet migration, but in CML and ALL therapies the blockade of this pathway comes with the risk of inflammatory hemorrhage due to impaired platelet migration.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal