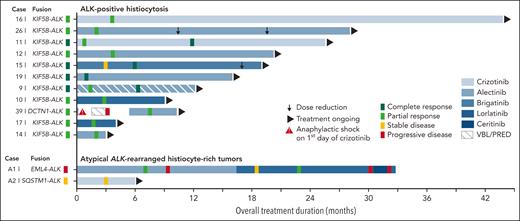
Swimmer plot of outcomes in patients with ALK-positive histiocytosis (n = 11) or atypical ALK-rearranged histiocyte-rich tumors (n = 2) treated with ALK inhibition. ALK inhibition was initiated at timepoint zero. Median time on ALK inhibition was 16 months in ALK-positive histiocytosis patients (range 3-43 months). Responses were measured by CT, MRI, and/or PET-CT in all patients. Dose reductions were 67% (90 mg brigatinib/d → 30 mg/d) and 50% (1200 mg alectinib/d → 900 mg/d → 600 mg/d) in Case 15 and Case 26, respectively. Case 39 developed a severe (grade 3) anaphylactic shock on the first day of crizotinib administration, requiring the patient to be resuscitated. The patient subsequently received vinblastine/prednisone-based chemotherapy with progressive disease and then switched to alectinib with objective response after 2 months. Case A1 developed a subcutaneous gluteal metastasis during treatment with alectinib (supplemental Figure 3C), which was found to harbor an ALK p.I1171N mutation, a mutation known to confer secondary resistance to alectinib.78,79 Therefore, the patient switched to lorlatinib and later to ceritinib after repeated progressive disease. Due to continuing progressive disease during treatment with ceritinib, the patient recently stopped ceritinib, received 3 weeks of bridging therapy with lorlatinib during antalgic radiotherapy of 2 metastases, and subsequently started vinblastine/prednisone-based chemotherapy. VBL/PRED, vinblastine and prednisone-based chemotherapy.
Swimmer plot of outcomes in patients with ALK-positive histiocytosis (n = 11) or atypical ALK-rearranged histiocyte-rich tumors (n = 2) treated with ALK inhibition. ALK inhibition was initiated at timepoint zero. Median time on ALK inhibition was 16 months in ALK-positive histiocytosis patients (range 3-43 months). Responses were measured by CT, MRI, and/or PET-CT in all patients. Dose reductions were 67% (90 mg brigatinib/d → 30 mg/d) and 50% (1200 mg alectinib/d → 900 mg/d → 600 mg/d) in Case 15 and Case 26, respectively. Case 39 developed a severe (grade 3) anaphylactic shock on the first day of crizotinib administration, requiring the patient to be resuscitated. The patient subsequently received vinblastine/prednisone-based chemotherapy with progressive disease and then switched to alectinib with objective response after 2 months. Case A1 developed a subcutaneous gluteal metastasis during treatment with alectinib (supplemental Figure 3C), which was found to harbor an ALK p.I1171N mutation, a mutation known to confer secondary resistance to alectinib.78,79 Therefore, the patient switched to lorlatinib and later to ceritinib after repeated progressive disease. Due to continuing progressive disease during treatment with ceritinib, the patient recently stopped ceritinib, received 3 weeks of bridging therapy with lorlatinib during antalgic radiotherapy of 2 metastases, and subsequently started vinblastine/prednisone-based chemotherapy. VBL/PRED, vinblastine and prednisone-based chemotherapy.
The publisher apologizes for these errors, which have been corrected in the online version of the article.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal