Key Points
Malignant T cells orchestrate changes in the microenvironment, which lead to skin barrier defects in cutaneous T-cell lymphoma.
We provide a novel rationale for JAK1 inhibition as an important adjuvant therapy for patients with cutaneous T-cell lymphoma.
Abstract
Cutaneous T-cell lymphoma (CTCL) is a devastating lymphoid malignancy characterized by the accumulation of malignant T cells in the dermis and epidermis. Skin lesions cause serious symptoms that hamper quality of life and are entry sites for bacterial infection, a major cause of morbidity and mortality in advanced diseases. The mechanism driving the pathological processes that compromise the skin barrier remains unknown. Here, we report increased transepidermal water loss and compromised expression of the skin barrier proteins filaggrin and filaggrin-2 in areas adjacent to TOX-positive T cells in CTCL skin lesions. Malignant T cells secrete mediators (including cytokines such as interleukin 13 [IL-13], IL-22, and oncostatin M) that activate STAT3 signaling and downregulate filaggrin and filaggrin-2 expression in human keratinocytes and reconstructed human epithelium. Consequently, the repression of filaggrins can be counteracted by a cocktail of antibodies targeting these cytokines/receptors, small interfering RNA–mediated knockdown of JAK1/STAT3, and JAK1 inhibitors. Notably, we show that treatment with a clinically approved JAK inhibitor, tofacitinib, increases filaggrin expression in lesional skin from patients with mycosis fungoides. Taken together, these findings indicate that malignant T cells secrete cytokines that induce skin barrier defects via a JAK1/STAT3-dependent mechanism. As clinical grade JAK inhibitors largely abrogate the negative effect of malignant T cells on skin barrier proteins, our findings suggest that such inhibitors provide novel treatment options for patients with CTCL with advanced disease and a compromised skin barrier.
Introduction
Cutaneous T-cell lymphoma (CTCL) represents a heterogeneous group of malignant extranodal non-Hodgkin lymphoproliferative disorders arising from neoplastic T cells.1-4 The most prevalent variant, mycosis fungoides (MF), is characterized by epidermotropism of malignant T cells associated with inflammatory skin lesions.5 MF lesions are chronically inflamed, and the tumor microenvironment (TME) is dominated by the presence of T helper cell 2–associated cytokines, including interleukin 13 (IL-13) secreted by malignant T cells.6-8 Early-stage MF presents with scaly patches and plaques, and some patients progress to advanced stages suffering from tumors and extensive erythroderma.2 Sézary syndrome is a rare and aggressive leukemic CTCL variant, characterized by the presence of malignant T cells in the blood and the skin, causing extensive erythroderma.9
Attempts to identify key mutational drivers have suggested that the disease is not caused by a single genetic event.10,11 Instead, dysregulation of JAK/STAT signaling and several other signaling pathways has been linked to malignant T-cell proliferation, apoptosis resistance, and inflammation in CTCL.12,13 Mutations and fusion proteins involving the JAK/STAT pathway have been described in some patients, whereas deficiencies and abnormal activity of regulators of the pathway have been described in other patients.14-17 Notably, STAT3 activation in malignant T cells has long been suspected to play a key role in the pathogenesis, and constitutive STAT3 activation in CD4+ T cells drives disease development in a mouse model of CTCL.18 Moreover, environmental factors, such as enterotoxin-producing Staphylococcus aureus (S. aureus), drive STAT3/5 activation and proliferation of malignant T cells.19-21 Thus, it is likely that genetic, epigenetic, environmental factors, and immune deregulation, are all implicated in the pathogenesis of CTCL.2,11,22-28
The key function of the epidermis is to act as a barrier to the external world. The uppermost layer of the epidermal compartment, stratum corneum, is essential for the barrier function and integrity of the skin.29 Two important components of the stratum corneum are filaggrin and the filaggrin family member 2 (filaggrin-2; FLG2).30,31 Filaggrin is synthesized in granular keratinocytes, and filaggrin monomers aggregate keratin filaments into bundles.30-32 Filaggrin deficiencies have long been associated with impaired stratum corneum properties; decreased levels of natural moisturizing factors, decreased barrier function, and increased susceptibility to bacterial colonization, microbial dysbiosis, and infection, highlighting the importance of filaggrins in maintaining a functional skin barrier.33,34
Recently, it has been reported that skin barrier proteins such as filaggrins are decreased in CTCL lesions, suggesting that the skin barrier is compromised in CTCL.35,36 This may at least partly explain why patients with CTCL are susceptible to cutaneous colonization and infection by bacteria such as enterotoxin-producing S. aureus and Bacillus safensis.37-42 Clinical data indicate that the colonization of skin lesions by S. aureus and other bacteria fuels the disease in CTCL. Consequently, a broken skin barrier is likely to play an important pathogenic role in disease activity and progression.19,37,42-45 However, the mechanisms underlying the development of skin lesions in patients with CTCL remain largely unknown. Here, we provide the first evidence that malignant T cells deteriorate the skin barrier through the cytokine-driven, JAK1/STAT3- mediated repression of filaggrins in keratinocytes. Thus, our findings indicate that malignant T cells orchestrate profound changes in the skin TME, which in turn may pave the way for further disease escalation and bacterial complications.
Materials and methods
Cell line cultures
The malignant MF T-cell lines MF2059 and MF2000 and the nonmalignant T-cell lines MyLa1850 and MySi were cultured as described in detail elsewhere.46-48 Supernatants were obtained from T-cell lines cultured for 24 hours at a cell density of 1 × 106 cells per milliliter.
Epidermal models
Normal human epidermal keratinocytes (NHEKs) were cultured in a medium containing supplements (PromoCell, C20111). Keratinocyte differentiation was induced by adding 1.25 mM calcium chloride for 3 days before the experimental setup. Reconstructed human epidermis (RHE) models (Episkin, SkinEthic RHE, S-17, 0.5 cm2) were applied according to the manufacturer’s instructions. Paired MF biopsies obtained from the same plaque were washed in phosphate-buffered saline supplemented with penicillin-streptomycin (P/S) and gentamycin, and then cultured in Dulbecco’s modified Eagle medium/Ham’s tissue culture F12 (1:1, Thermo Fisher Scientific, #3966047/31966021) media supplemented with 10% fetal bovine serum, 1% P/S, 10 μg/mL insulin, and 10 ng/mL epidermal growth factor for 72 hours with 1μM tofacitinib.
Patient material
Experiments with samples from patients with CTCL were performed in accordance with the Declaration of Helsinki. Biopsies were obtained from the patients after approval by the Regional Ethical Committee, Denmark (1-10-72-151-16, M-20090102, and H-16025331). A table of patients is presented in supplemental Table 1, which is available on the Blood website.
TEWL
Skin barrier function was measured by assessing transepidermal water loss (TEWL) using a DermaLab Evaporimeter (Cortex Technology) according to the guidelines. Skin sites from 12 patients (15 nonlesional and 16 leisonal) were measured (in triplicates) on anatomically paired lesional and nonlesional skin sites. The results are shown as mean (g/m2 per hour). ΔTEWL was calculated by subtracting the site matched nonlesional TEWL from the lesional TEWL.
Inhibitors and blocking antibodies
Cells were treated from 0.001 to 1μM of the pan-JAK inhibitor tofacitinib (InvivoGene, CP6905590) and 0.5 to 1 μM of the JAK1 inhibitor abrocitinib or dimethyl sulfoxide. R&D Systems Blocking Antibodies were applied as follows 1 μg/mL IL-22Rα1 (#AF2770), 10 μg/mL glycoprotein 130 (#MAB228), 30 μg/mL IL-13Rα1 (#AF152), and 250 μg/mL dupilumab.
Lipofectamine transfection
NHEK were transfected with 10 pmol (20 nmol/L) small interfering RNA (siRNA)–targeting JAK1, JAK2, STAT3, or control nontargeting siRNA (Dharmacon, ON-TARGETplus) using Lipofectamine RNAiMAX Transfection Reagent (ThermoFisher Scientific). After 48 hours of transfection, NHEK cells were cultured in the presence of the supernatants.
Quantitative reverse transcription PCR
RNA from skin samples and NHEK/HaCaT cells and biopsies was purified (Qiagen 217004/74134), transcribed to complementary DNA, and subjected to quantitative reverse transcription polymerase chain reaction (RT-qPCR, Lightcycler480) using β-actin as a reference gene for normalization, as described in detail.49 The data were calculated according to the 2ΔΔCT method.
IHC
Immunohistochemistry (IHC) was performed on skin sections using antihuman pY-STAT3, filaggrin, filaggrin-2, and TOX. The method is described in detail in the supplement and elsewhere.19
Enzyme-linked immunosorbent assay
The DuoSet ELISA detection of IL-6, IL-13, IL-22, and oncostatin M (OSM) (R&D Systems) was performed according to the manufacturer’s instructions.
Cytokine expression in skin biopsies using single-cell RNA sequencing (scRNA-seq)
Count matrices from 4 studies were obtained or generated from the Gene Expression Omnibus (accession numbers GSE165623,50 GSE173205,50 and GSE128531),51 or Sequence Read Archive (SRP332550).52 Only skin biopsies from healthy controls or lesional CTCL skin samples containing an identifiable malignant T-cell population were included in the analysis. A detailed description is included in the supplement.
Statistics
All graphs and statistical analyses were performed using the software GraphPad Prism, version 8.0. Student t test and 1-way analysis of variance were applied, followed by post hoc analysis. For the quantitative PCR results, the relative expression of each intervention was compared with that of the control. Error bars represent the standard error of the mean, and the level of statistical significance was set at ∗P < .05.
Results
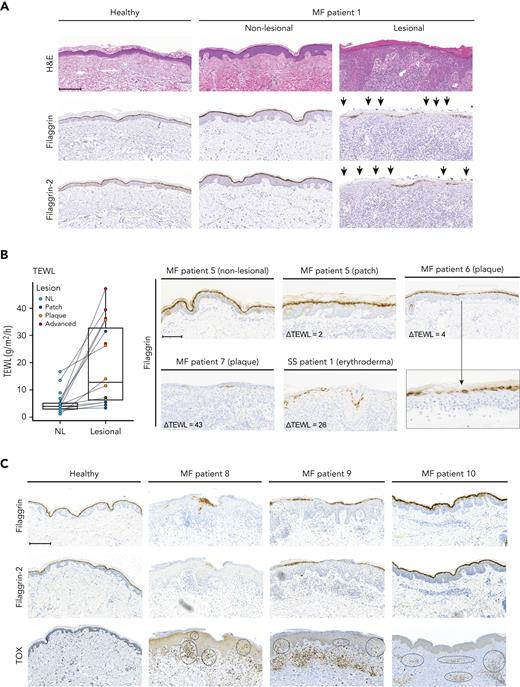
As malignant T cells reside in a unique TME in the epidermis, with keratinocytes as the major cell type, we hypothesized that malignant T cells may interact with adjacent keratinocytes to orchestrate the characteristic pathological changes observed in the CTCL skin. Comparing lesional MF skin (plaques) to nonlesional MF and healthy skin, we found a reduction in filaggrin and filaggrin-2 protein expression in stratum granulosum/stratum corneum (Figure 1A; supplemental Figure 1A). Notably, expression was decreased to varying degrees within patient biopsies and among patients (Figure 1; supplemental Figure 1A). To assess barrier function in patients with CTCL, paired nonlesional and lesional skin sites were evaluated for TEWL in 12 patients. In all patients, TEWL measurements were higher in the lesional skin sites than in the matched nonlesional skin sites (Figure 1B). Importantly, TEWL correlated with the severity of the lesions. Most patch lesions displayed minor increases in TEWL than nonlesion skin. In contrast, advanced lesions (tumor/erythroderma) exhibited a much higher increase in TEWL, indicating a severely compromised barrier (Figure 1B). Increased ΔTEWL values appeared to correlate with decreased expression of filaggrin (Figure 1B) and filaggrin-2 (supplemental Figure 1B-C).
Filaggrin and filaggrin-2 protein expression is reduced in lesional MF skin, TEWL is increased, and epidermal immune infiltrates correlate with the reduced expression levels. (A) Lesional and nonlesional (NL) skin biopsies obtained from a patient with MF (MF1), and a healthy donor, were stained for hematoxylin and eosin stain (H&E), filaggrin, and filaggrin-2. The arrowheads highlight areas of deficient filaggrin and filaggrin-2 protein expression. (B) TEWL (g/m2 per hour) measurements were performed for anatomically matched lesional and nonlesional sites in 12 patients with patches, plaques, or advanced lesions (tumor/erythroderma) (left). Some patients had multiple lesions that were analyzed. Lesional skin biopsies from 3 patients with MF and 1 with Sézary syndrome (MF5, MF6, MF7, and SS1) and nonlesional skin biopsy from patient MF5 were stained for filaggrin (right). Micrograph close-up for patient MF6 illustrates areas that lack filaggrin expression. ΔTEWL values (subtracting nonlesional from lesional values) were given for each of the patients. (C) Lesional skin site biopsies obtained from 3 patients with MF (MF8, MF9, and MF10) and a biopsy from a healthy donor were stained for filaggrin, filaggrin-2, and TOX. Areas of positive TOX staining in the epidermis/dermis are highlighted by black circles. Images are scanned by the Zeiss Axio Scan.Z1 with original magnification ×20 for all panels. Scale bars; 200 μm. Close up; 300%.
Filaggrin and filaggrin-2 protein expression is reduced in lesional MF skin, TEWL is increased, and epidermal immune infiltrates correlate with the reduced expression levels. (A) Lesional and nonlesional (NL) skin biopsies obtained from a patient with MF (MF1), and a healthy donor, were stained for hematoxylin and eosin stain (H&E), filaggrin, and filaggrin-2. The arrowheads highlight areas of deficient filaggrin and filaggrin-2 protein expression. (B) TEWL (g/m2 per hour) measurements were performed for anatomically matched lesional and nonlesional sites in 12 patients with patches, plaques, or advanced lesions (tumor/erythroderma) (left). Some patients had multiple lesions that were analyzed. Lesional skin biopsies from 3 patients with MF and 1 with Sézary syndrome (MF5, MF6, MF7, and SS1) and nonlesional skin biopsy from patient MF5 were stained for filaggrin (right). Micrograph close-up for patient MF6 illustrates areas that lack filaggrin expression. ΔTEWL values (subtracting nonlesional from lesional values) were given for each of the patients. (C) Lesional skin site biopsies obtained from 3 patients with MF (MF8, MF9, and MF10) and a biopsy from a healthy donor were stained for filaggrin, filaggrin-2, and TOX. Areas of positive TOX staining in the epidermis/dermis are highlighted by black circles. Images are scanned by the Zeiss Axio Scan.Z1 with original magnification ×20 for all panels. Scale bars; 200 μm. Close up; 300%.
TOX is highly expressed in malignant T cells from patients with MF and may be weakly expressed in some reactive nonmalignant T cells.53-56 We found TOX-positive cells in lesional biopsies from all investigated patients, whereas TOX expression was absent in the healthy skin (Figure 1C; supplemental Figure 1B-D). Interestingly, strong epidermal staining was observed for TOX (Figure 1C; supplemental Figure 1B-D) in patients with weak or absent filaggrin and filaggrin-2 staining. In contrast, in lesional skin displaying normal or near the normal expression of filaggrins, we did not detect TOX staining in the epidermis (patients MF5 and MF10). In these patients, positive TOX staining was observed in deeper dermal layers (Figure 1C; supplemental Figure 1B). This suggests that the proximity between malignant T cells and epidermal keratinocytes may be associated with deficient filaggrin expression. In support of this, increased numbers of TOX-positive cells in the epidermis were associated with both increased TEWL values and downregulated filaggrin (Figure 1B; supplemental Figure 1B-C).
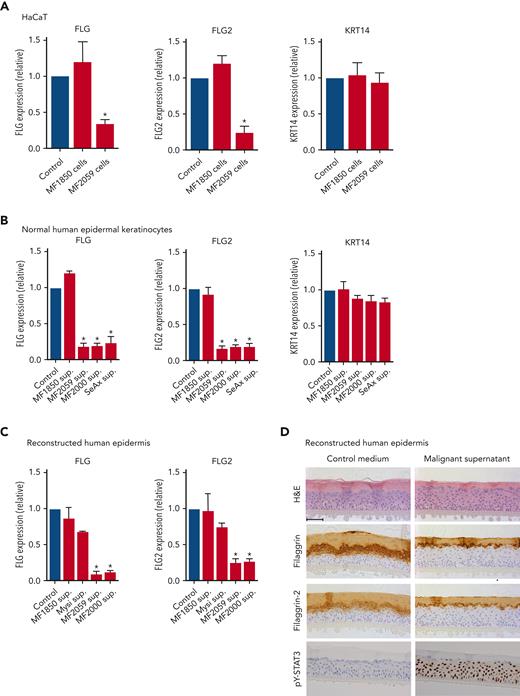
To test the hypothesis, we performed coculture experiments to mimic the close interplay between keratinocytes and malignant T cells. A keratinocyte cell line (HaCaT) was cultured for 24 hours in the presence or absence of the malignant T-cell line MF2059, before quantification of filaggrin (FLG) and filaggrin-2 (FLG2) gene expression. As shown in Figure 2A, FLG and FLG2 expression significantly decreased in the presence of malignant MF2059 T cells. In contrast, FLG and FLG2 expression were not repressed in cocultures of HaCaT cells in the presence of the nonmalignant MF1850 cell line. The gene encoding KRT14, which is expressed in basal keratinocytes, was not affected by coculture with either of the cell lines (Figure 2A). To determine whether cell-cell contact was required for the reduction in filaggrin and FLG2 expression, culture supernatants were collected from malignant T-cell lines and incubated for 24 hours in HaCaT cell cultures. Supernatants from malignant T cells significantly reduced the expression of FLG and FLG2 (supplemental Figure 2A). To validate these observations, NHEKs and RHE were incubated with supernatants from malignant and nonmalignant T-cell lines, as described above. The mRNA expression of FLG and FLG2 was significantly reduced in NHEK cultured for 24 hours with supernatants from the malignant T-cell lines MF2059, MF2000, and SeAx but not in nonmalignant MF1850 cells (Figure 2B). Similar changes in mRNA expression were observed in the RHE samples (Figure 2C). Furthermore, supernatants from malignant T cells reduced the protein expression of filaggrin and filaggrin-2 in the stratum granulosum (Figure 2D). The staining intensity and number of keratinocytes positive for filaggrin and filaggrin-2 decreased, supporting the hypothesis that malignant T cells secrete soluble factors that inhibit the expression of filaggrins.
Malignant T cells induce alterations in the epidermis. Messenger RNA (mRNA) expression was analyzed by quantitative PCR (qPCR), using β-actin as a reference gene in panels A-C. Gene expression was given as relative expression. (A) HaCaT cells were cultured for 24 hours with the nonmalignant MF1850 or malignant MF2059 T-cell line. (B) NHEK were cultured for 24 hours with supernatants obtained from the T-cell lines MF1850, MF2059, MF2000, and SeAx. (C) RHE samples were cultured in the presence of supernatants for 24 hours. (D) Protein levels were analyzed by IHC for RHE samples cultured for 48 hours with supernatants from the malignant T-cell line MF2059. The samples were stained for H&E, filaggrin, filaggrin-2, and pY-STAT3. Images are scanned by the Zeiss Axio Scan.Z1 with original magnification ×20. Scale bar; 50 μm. n = 3. ∗P < .05. KRT14, keratin protein 14; sup, supernatant.
Malignant T cells induce alterations in the epidermis. Messenger RNA (mRNA) expression was analyzed by quantitative PCR (qPCR), using β-actin as a reference gene in panels A-C. Gene expression was given as relative expression. (A) HaCaT cells were cultured for 24 hours with the nonmalignant MF1850 or malignant MF2059 T-cell line. (B) NHEK were cultured for 24 hours with supernatants obtained from the T-cell lines MF1850, MF2059, MF2000, and SeAx. (C) RHE samples were cultured in the presence of supernatants for 24 hours. (D) Protein levels were analyzed by IHC for RHE samples cultured for 48 hours with supernatants from the malignant T-cell line MF2059. The samples were stained for H&E, filaggrin, filaggrin-2, and pY-STAT3. Images are scanned by the Zeiss Axio Scan.Z1 with original magnification ×20. Scale bar; 50 μm. n = 3. ∗P < .05. KRT14, keratin protein 14; sup, supernatant.
The JAK/STAT3 pathway plays a key role in the repression of filaggrin in chronic inflammatory skin conditions, such as atopic dermatitis (AD).57,58 Thus, we examined whether the repression of filaggrins is mediated by STAT3 signaling in CTCL. Staining of RHE cultures for the activated form of STAT3 (pY-STAT3) showed that supernatants from malignant T cells induced profound pY-STAT3 expression in keratinocytes in RHE samples (Figure 2D). Comparable results were observed using western blotting for pY-STAT3 in HaCaT and NHEK cells (supplemental Figure 2C-D).
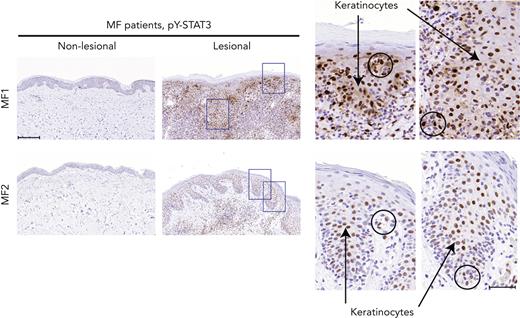
Although constitutive activation of STAT3 has been implicated in malignant T-cell proliferation, apoptosis resistance, and inflammation in CTCL, little is known about STAT3 activation in keratinocytes in the lesional skin of patients with CTCL.12 As shown in Figure 3, staining for pY-STAT3 in patients with MF with deficient filaggrin expression (patients MF1 and MF2) demonstrated strong positive pY-STAT3 staining in the epidermal layers of the lesional skin, whereas nonlesional skin did not exhibit staining. Importantly, STAT3 activation was not only seen in lymphocytes with a neoplastic morphology (circles), but also in a large fraction of keratinocytes (arrows), as shown in the close-up micrographs of the lesional skin. These findings demonstrate that STAT3 activation is indeed observed in keratinocytes in lesional skin with concomitant filaggrin deficiencies.
pY-STAT3 is induced in lesional MF skin. Lesional and nonlesional skin site biopsies obtained from 2 patients with MF (MF1 and MF2) were stained for pY-STAT3. Micrograph close-ups illustrate areas with pY-STAT3–positive keratinocytes (arrows) close to areas with immune infiltrates, potentially pY-STAT3–positive malignant T cells (circles). Images are scanned by the Zeiss Axio Scan.Z1 with original magnification ×20. Scale bars; 200 μm and 50 μm for close-ups.
pY-STAT3 is induced in lesional MF skin. Lesional and nonlesional skin site biopsies obtained from 2 patients with MF (MF1 and MF2) were stained for pY-STAT3. Micrograph close-ups illustrate areas with pY-STAT3–positive keratinocytes (arrows) close to areas with immune infiltrates, potentially pY-STAT3–positive malignant T cells (circles). Images are scanned by the Zeiss Axio Scan.Z1 with original magnification ×20. Scale bars; 200 μm and 50 μm for close-ups.
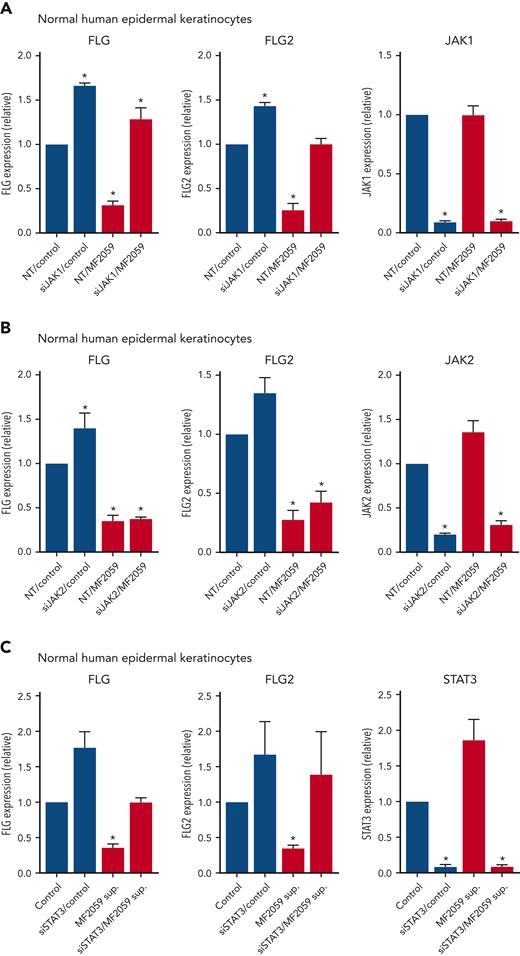
To address the role of JAK/STAT3 signaling in the repression of filaggrins in CTCL, we performed siRNA-mediated JAK1 knockdown in NHEK, following incubation with the supernatant from malignant T cells MF2059. Knockdown of JAK1 triggered an enhanced baseline expression of FLG and FLG2 when compared with nontargeting control siRNA (Figure 4A), suggesting that JAK1 regulates the endogenous level of filaggrins. As expected, the malignant supernatant-induced profound inhibition of FLG and FLG2 expression, but had no effect on JAK1 expression, whereas JAK1 siRNA induced an almost complete knockdown of JAK1 (Figure 4A). Importantly, the inhibition of FLG and FLG2 expression by the malignant T-cell supernatant was largely blocked by siRNA-mediated knockdown of JAK1, indicating that supernatant-induced repression of filaggrins was mediated via JAK1 (Figure 4A). The effect was highly selective, as siRNA-mediated knockdown of JAK2 had little effect (Figure 4B). STAT3 knockdown almost completely blocked STAT3 expression and enhanced the basal level of FLG and FLG2 expression (Figure 4C), which is in line with other findings showing that STAT signaling regulates endogenous filaggrin expression in cultured keratinocytes.57 Notably, knockdown of STAT3 partially blocked the effect of the malignant supernatant on FLG and FLG2 expression (Figure 4C), supporting the hypothesis that malignant T cells repress expression of filaggrins through a JAK1/STAT3-dependent pathway.
JAK1 and pY-STAT3 knockdown blocks tumor cell–induced repression of FLG and FLG2 in the epidermis. NHEK cells were transfected with siRNA targeting either JAK1, JAK2, or STAT3 or a nontargeting siRNA control. After 48 hours, the samples were cultured for 24 hours with the supernatant from the malignant MF2059 T-cell line. The mRNA expression was analyzed by qPCR using β-actin as a reference gene. Gene expression was given as relative expressions. (A) mRNA expression of FLG, FLG-2, and JAK1 was analyzed following siRNA-mediated knockdown of JAK1 in the presence or absence of supernatant from MF2059. mRNA expression of FLG, FLG2, and JAK2 (B) or STAT3 (C) was analyzed following siRNA-mediated knockdown of JAK2 (B) or STAT3 (C) in the presence or absence of supernatant from MF2059. n = 3, ∗P < .05. NT, nontargeting; sup, supernatant.
JAK1 and pY-STAT3 knockdown blocks tumor cell–induced repression of FLG and FLG2 in the epidermis. NHEK cells were transfected with siRNA targeting either JAK1, JAK2, or STAT3 or a nontargeting siRNA control. After 48 hours, the samples were cultured for 24 hours with the supernatant from the malignant MF2059 T-cell line. The mRNA expression was analyzed by qPCR using β-actin as a reference gene. Gene expression was given as relative expressions. (A) mRNA expression of FLG, FLG-2, and JAK1 was analyzed following siRNA-mediated knockdown of JAK1 in the presence or absence of supernatant from MF2059. mRNA expression of FLG, FLG2, and JAK2 (B) or STAT3 (C) was analyzed following siRNA-mediated knockdown of JAK2 (B) or STAT3 (C) in the presence or absence of supernatant from MF2059. n = 3, ∗P < .05. NT, nontargeting; sup, supernatant.
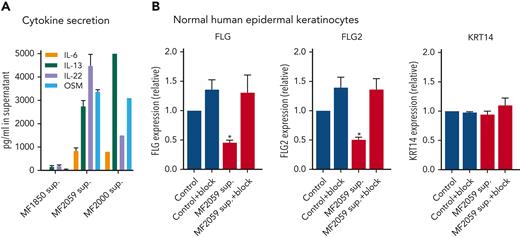
Several cytokines, including IL-4, IL-6, IL-13, IL-22, IL-31, interferon γ (IFN-γ), and OSM, have been reported to induce filaggrin repression via JAK1/STAT3 signaling in keratinocytes.59 High concentrations (>2500 ng/mL) of IL-13, IL-22, and OSM and medium concentrations of IL-6 (∼1000 ng/mL) were detected in the supernatants of malignant T cells (Figure 5A), confirming that these cytokines are upregulated in MF.6,60,61 Very low concentrations of cytokines were detected in the supernatants of nonmalignant T cells. IL-4, IL-17A, IL-17F, IL-31, and IFN-γ were not detected in the supernatants of malignant T cells (supplemental Figure 3A). To evaluate the effect of IL-6, IL-13, IL-22, and OSM on FLG and FLG2 expression, keratinocytes were pretreated with cytokine-receptor blocking antibodies targeting IL-6 and OSM (glycoprotein 130 also blocking IL-11 and leukemia inhibitory factor), IL-13 (IL-13Rα1 and dupilumab), and IL-22 (IL-22Rα1). In combination, these receptor antibodies blocked supernatant-induced repression of FLG and FLG2 (Figure 5B). In contrast, the addition of individual receptor-blocking antibodies was insufficient to completely block FLG and FLG2 repression induced by the malignant supernatant (supplemental Figure 3B). This suggests that the combination of IL-6, IL-13, IL-22, and OSM may play a key role in the repression of filaggrins in malignant T cells.
Malignant T cells produce cytokines that induce alteration of FLG and FLG2 in the epidermis. (A) Supernatants obtained from MF1850, MF2059 or MF2000 cells, which had been cultured for 24 hours were analyzed for the cytokines IL-6, IL-13, IL-22, and OSM by enzyme-linked immunosorbent assay. Concentrations were given as picogram cytokine per milliliter supernatant. (B) NHEK were cultured for 2 hours in the presence or absence of receptor-blocking antibodies; IL-13Rα1 and dupilumab (IL-13), glycoprotein 130 (IL-6 and OSM), and IL22Rα1 (IL-22) to block cytokine signaling. NHEKs were then cultured for 24 hours with supernatants obtained from MF2059. The mRNA expression levels were analyzed by qPCR using β-actin as a reference gene. Gene expression was given as relative expressions. n = 3 for MF2059, MF1850, n = 1 for MF2000; ∗P < .05.
Malignant T cells produce cytokines that induce alteration of FLG and FLG2 in the epidermis. (A) Supernatants obtained from MF1850, MF2059 or MF2000 cells, which had been cultured for 24 hours were analyzed for the cytokines IL-6, IL-13, IL-22, and OSM by enzyme-linked immunosorbent assay. Concentrations were given as picogram cytokine per milliliter supernatant. (B) NHEK were cultured for 2 hours in the presence or absence of receptor-blocking antibodies; IL-13Rα1 and dupilumab (IL-13), glycoprotein 130 (IL-6 and OSM), and IL22Rα1 (IL-22) to block cytokine signaling. NHEKs were then cultured for 24 hours with supernatants obtained from MF2059. The mRNA expression levels were analyzed by qPCR using β-actin as a reference gene. Gene expression was given as relative expressions. n = 3 for MF2059, MF1850, n = 1 for MF2000; ∗P < .05.
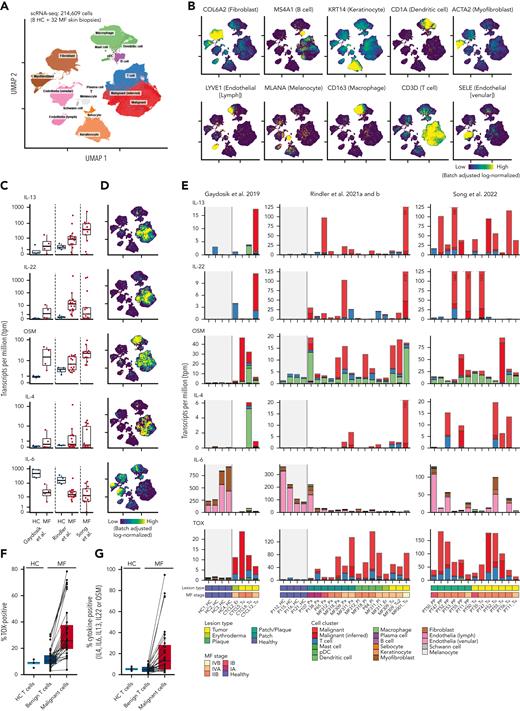
To evaluate the physiological and clinical relevance of the cytokines investigated, we analyzed 4 publicly available scRNA-seq data sets containing a total of 32 lesional skin samples (patch/plaque/tumor) from 21 patients with MF and 8 healthy controls (Figure 6).50-52,62 Cluster analysis showed a clear separation of various cell types, including T cells, dendritic cells, macrophages, and keratinocytes (Figure 6A-B). T-cell receptor clonality and the expression of malignant-associated genes were used to distinguish between malignant and nonmalignant T cells. IL-13, IL-22, OSM, and IL-4 were highly expressed in lesional skin biopsies from patients with MF compared with healthy control skin (Figure 6C-E). Although we found large heterogeneity between patients, and also between different lesions from the same patient, IL-13, IL-22, OSM, and IL-4 were highly expressed by malignant T cells in multiple patients (Figure 6E). IFN-γ was produced in the lesions of some patients; IL-17A/F was expressed in 2 of the 21 included patients, whereas IL-31 was largely absent (supplemental Figure 4A-C). IL-6 was not highly expressed and was primarily expressed by macrophages, fibroblasts, and endothelial cells (Figure 6C-E). TOX expression was higher and more frequent in malignant cells than in nonmalignant cells (Figure 6F). Furthermore, malignant cells were more likely to produce IL-13, IL-22, OSM, IL-4, or IL-6 than their nonmalignant counterparts from the same biopsies (Figure 6G). Taken together, these data confirm that the investigated cytokines, especially IL-13, IL-22, OSM, and IL-4, were highly expressed in the lesional skin from patients with MF and expressed by malignant T cells and, to some extent, by other cells in the TME.
IL-13, IL-22, OSM, and IL-4 are expressed in lesional skin from patients with MF. scRNA-seq samples from 8 healthy controls (HCs) and 32 MF biopsies across 4 publicly available studies50-52,62 were integrated and batch corrected using SCVI. (A,B) Cell-type annotation visualized on uniform manifold approximation and projection (UMAP) showing distinct clustering of major cell types annotated by the expression of lineage markers. (C) Quantification of IL-13, IL-22, OSM, IL-4, and IL-6 transcripts across each sample divided into HC and MF. Values denote the number of unique cytokine transcripts per million total transcripts (tpm). (D) UMAP visualization of which cell clusters express each cytokine. Owing to sparsity of the transcripts, positive cells are plotted on top. (E) Quantification of IL-13, IL-22, OSM, IL-4 IL-6, and TOX transcripts across cell clusters within each sample. Bars depicting samples with extreme expression were truncated to allow visualization, together with samples with lower expression levels. The truncation is indicated by dashed lines, and numbers in bars denote tpm for the given bar at full height. Gray areas mark HC samples. The samples were ordered by study and disease status (HC vs MF), lesion type, and MF stage as shown at the bottom. Multiple biopsies across multiple lesion types are included for some patients and can be identified by the sample names below the plot. Fraction of cells expressing at least 1 transcript of TOX (F) or IL-13, IL-22, OSM, IL-4, or IL-6 (G) within T cells from HC, and benign T cells, or malignant cells from MF biopsies. Lines link values from matched benign T cells and malignant cells from the same sample. pDC, plasmacytoid dendritic cell.
IL-13, IL-22, OSM, and IL-4 are expressed in lesional skin from patients with MF. scRNA-seq samples from 8 healthy controls (HCs) and 32 MF biopsies across 4 publicly available studies50-52,62 were integrated and batch corrected using SCVI. (A,B) Cell-type annotation visualized on uniform manifold approximation and projection (UMAP) showing distinct clustering of major cell types annotated by the expression of lineage markers. (C) Quantification of IL-13, IL-22, OSM, IL-4, and IL-6 transcripts across each sample divided into HC and MF. Values denote the number of unique cytokine transcripts per million total transcripts (tpm). (D) UMAP visualization of which cell clusters express each cytokine. Owing to sparsity of the transcripts, positive cells are plotted on top. (E) Quantification of IL-13, IL-22, OSM, IL-4 IL-6, and TOX transcripts across cell clusters within each sample. Bars depicting samples with extreme expression were truncated to allow visualization, together with samples with lower expression levels. The truncation is indicated by dashed lines, and numbers in bars denote tpm for the given bar at full height. Gray areas mark HC samples. The samples were ordered by study and disease status (HC vs MF), lesion type, and MF stage as shown at the bottom. Multiple biopsies across multiple lesion types are included for some patients and can be identified by the sample names below the plot. Fraction of cells expressing at least 1 transcript of TOX (F) or IL-13, IL-22, OSM, IL-4, or IL-6 (G) within T cells from HC, and benign T cells, or malignant cells from MF biopsies. Lines link values from matched benign T cells and malignant cells from the same sample. pDC, plasmacytoid dendritic cell.
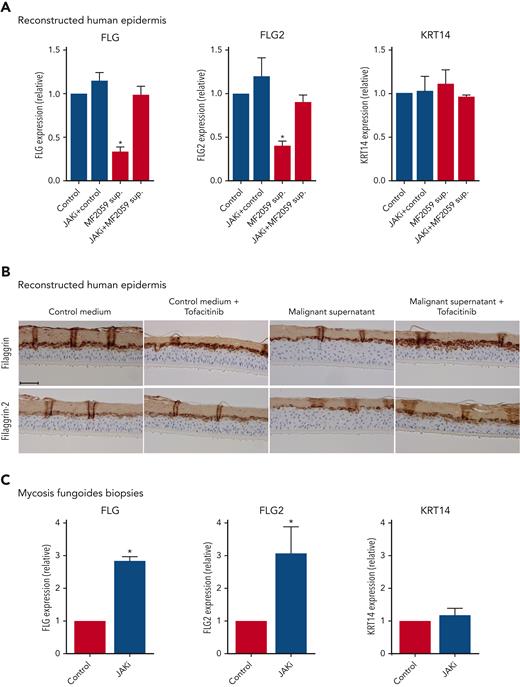
Given our finding that JAK1 knockdown blocked cytokine-mediated repression of filaggrins in keratinocytes, we hypothesized that JAK inhibitors might also block the effects of malignant T-cell supernatants. Accordingly, RHE samples were treated with 1 μM of the pan-JAK inhibitor, tofacitinib, and cultured with the supernatant from malignant MF2059 T cells. Importantly, the supernatant from malignant cells induced a profound downregulation of FLG and FLG2 mRNA expression in RHE, which was reversed in the presence of tofacitinib (Figure 7A). The effect of JAK inhibition was also observed in NHEK treated with supernatants from both malignant MF2059 and MF2000 cells, and was shown to be dependent on the JAK inhibitor concentration (supplemental Figure 5A-B). Furthermore, the effects of JAK inhibition were confirmed using another JAK inhibitor (abrocitinib, 0.5-1 μM) which is more JAK1 selective than tofacitinib (supplemental Figure 5C-D). IHC analysis of RHE samples cultured with malignant supernatants showed reduced protein expression of filaggrin and filaggrin-2, an effect that was strongly inhibited by tofacitinib (Figure 7B). The skin barrier in RHE was functionally compromised after incubation with malignant supernatants, as indicated by the increased permeability of Lucifer yellow (supplemental Figure 5E). Importantly, tofacitinib, which had little effect on its own, appeared to block the effect of the malignant supernatant on the barrier permeability in RHE (supplemental Figure 5E). To evaluate the effect of tofacitinib on MF skin, we cultured biopsies from the lesional skin of patients with MF ex vivo in culture medium in the presence or absence of 1 μM tofacitinib. As illustrated in Figure 7C, FLG and FLG2 expression increased in the lesional skin biopsies cultured with tofacitinib, highlighting the potential of JAK inhibitors to reverse deficient filaggrin and filaggrin-2 expression in the MF skin of patients.
JAK inhibition blocks tumor cell–induced reduction of filaggrin and FLG2 in keratinocytes and in ex vivo MF skin. (A) RHE samples were cultured for 24 hours with the malignant MF2059 T-cell line in the presence of either 1 μM JAK inhibitor (tofacitinib) or dimethyl sulfoxide (DMSO) (control). (B) RHE samples were cultured in the presence of supernatants for 48 hours with 1 μM tofacitinib or DMSO. (C) Skin biopsies from MF plaques were cultured with 1 μM tofacitinib or DMSO for 72 hours. mRNA expression was analyzed by qPCR using β-actin as a reference gene in panels A and C. Gene expression was given as relative expression. Protein expression was analyzed by IHC for RHE samples. The RHE samples were stained for H&E, filaggrin, and filaggrin-2. Images are scanned by the Zeiss Axio Scan.Z1 with original magnification ×20. Scale bar; 50 μm. n = 3, ∗P < .05.
JAK inhibition blocks tumor cell–induced reduction of filaggrin and FLG2 in keratinocytes and in ex vivo MF skin. (A) RHE samples were cultured for 24 hours with the malignant MF2059 T-cell line in the presence of either 1 μM JAK inhibitor (tofacitinib) or dimethyl sulfoxide (DMSO) (control). (B) RHE samples were cultured in the presence of supernatants for 48 hours with 1 μM tofacitinib or DMSO. (C) Skin biopsies from MF plaques were cultured with 1 μM tofacitinib or DMSO for 72 hours. mRNA expression was analyzed by qPCR using β-actin as a reference gene in panels A and C. Gene expression was given as relative expression. Protein expression was analyzed by IHC for RHE samples. The RHE samples were stained for H&E, filaggrin, and filaggrin-2. Images are scanned by the Zeiss Axio Scan.Z1 with original magnification ×20. Scale bar; 50 μm. n = 3, ∗P < .05.
Discussion
Skin lesions are characteristic manifestations of T-cell lymphomas and play an important role in the symptomatology and secondary bacterial colonization, infections, and comorbidity of CTCL.2 However, relatively little is known regarding the pathological processes that drive disease-associated skin changes. Here, we provide the first evidence that malignant T cells, through the secretion of cytokines (IL-13, IL-22, OSM, and potentially IL-6) and JAK1/STAT3 signaling, induce transcriptional repression of FLG and FLG2 in keratinocytes. Thus, supernatants from malignant T cells triggered enhanced STAT3 activation and decreased the expression of filaggrins in human keratinocytes and a human skin equivalent 3-dimensional model. Importantly, the repression of filaggrins was strongly inhibited by a cocktail of relevant cytokine-receptor blocking antibodies and by the knockdown of JAK1 and STAT3. Data from scRNA-seq showed that malignant T cells in lesional skin expressed IL-13, IL-22, and OSM, confirming the pathological relevance of the cytokines identified in the supernatants of malignant T-cell lines. Interestingly, a fraction of nonmalignant T cells derived from the lesional skin also expressed IL-13, IL-22, IFN-γ, and OSM, suggesting that other cell types may also contribute to filaggrin repression in skin lesions.
It is well established that CTCL is a highly heterogeneous disease, displaying large differences in gene expression between malignant cells from different individuals and even between different subpopulations of the malignant T-cell clone in each patient.63,64 Therefore, it was not surprising that the relative abundance of malignant T cells producing the cytokines in question differed between patients. IL-13 is the dominating cytokine-driving repression of filaggrins in AD, and our scRNA-seq analysis showed high expression of IL-13 in the lesional skin of several patients. This suggests that IL-13 may also play an important role in skin barrier defects in patients with CTCL. Thus, blocking IL-13 partially inhibited the effects of malignant supernatants. However, blockage of other cytokines was also required to completely negate the effect of malignant supernatants. This finding supports the notion that multiple cytokines repress the skin barrier proteins in concert. We cannot exclude the possibility that additional cytokines (such as IL-17)59 may also contribute to the repression of filaggrins, but only 2 of the analyzed 21 patients with MF displayed significant expression of IL-17A/F, suggesting that IL-17 may not play a general role in the repression of filaggrins in CTCL. Although IL-4 was not detected in the supernatants of malignant T cells, it was produced by a fraction of malignant T cells from lesional skin samples, which is consistent with previous reports.65 This suggests that IL-4, like IL-13, may contribute to filaggrin repression in CTCL. Interestingly, targeting IL-4 and IL-13 signaling by dupilumab rapidly inhibited itch and TH-2 bias in a patient with Sézary syndrome,66 which may be because of an improvement in the skin barrier following IL-4/IL-13 blockage, as hypothesized in the present study. As dupilumab may unmask or promote CTCL in AD,67,68 it is uncertain whether IL-4/IL-13 blockage and, in turn, JAK1 inhibition are associated with a risk in patients with CTCL. In contrast, disease aggravation was not reported in a clinical phase 2 study on ruxolitinib, a JAK1/JAK2 inhibitor.69,70
TOX is an important but not an exclusive marker for malignant T cells, because it can also be expressed at lower levels in reactive T cells.53,55 However, there is an overall correlation between high TOX expression and accumulation of malignant T cells in CTCL.53,54,56 Indeed, the scRNA-seq analysis showed that malignant cells accounted for the vast majority of the TOX transcripts. Moreover, malignant cells exhibited a consistently higher percentage of TOX-positive cells than their benign T-cell counterparts within the same biopsies. Coupled with the overall higher abundance of malignant cells in most biopsies, TOX-positive cells in histological stainings are much more likely to represent malignant than benign T cells. Similarly, malignant cells were much more likely to be cytokine positive (IL-4, IL-6, IL-13, IL-22, and OSM) than their benign counterparts. Thus, our observations suggest that a high frequency of TOX-positive malignant cells in the epidermis correlates with a deficient filaggrin barrier. Interestingly, we only observed deficient filaggrin expression in lesions where TOX-positive cells were located in the epidermis, whereas the skin barrier was unaffected in lesions where epidermal TOX-positive cells were absent, despite the presence of large clusters of TOX-positive cells in the dermis. This may indicate that the basal membrane could function as a diffusion barrier for cytokines from TOX-positive cells.
Our data suggest that local skin defects were driven by cytokine-producing malignant T cells and other cells in the TME. In addition to cytokines, malignant T cells release galectins, which may enhance the proliferation of keratinocytes.47 Notably, malignant T cells may also regulate other cells in the TME, which in turn stimulate T cells, thereby supporting the concept of malignant inflammation.8,71-73
Bacteria pose an important clinical problem in CTCL.38,74 In advanced disease, most of the patients harbor enterotoxin-producing S. aureus, and more than half of the patients die from infection rather than cancer per se.74 A compromised skin barrier is an important point for bacterial entry, and recent evidence indicates that enterotoxin-producing S. aureus drives changes in the TME that fuel the proliferation of malignant T cells and disease activity.19,21,43,46,75 Thus, novel treatments are warranted that can heal the skin lesions. JAK inhibitors decrease the severity of symptoms and improve epidermal barrier markers in patients with AD, and are also used to treat vitiligo, alopecia areata, and rheumatoid arthritis.57,58,76,77 Accordingly, we examined the effects of JAK inhibitors and demonstrated that tofacitinib reversed the repression of filaggrins, decreased skin permeability in an epidermal model, and enhanced filaggrin expression in freshly isolated specimens from MF skin lesions. These findings suggest that JAK inhibitors have the potential to improve the skin lesions in patients with CTCL. As tofacitinib inhibits ectopic expression of oncomicroRNA-155 and IL-2Rg–driven proliferation in primary malignant T cells,20 it may simultaneously target multiple disease mechanisms in CTCL (in addition to its effect on skin barrier defects, as shown here).
As antitumor immune responses are important in early-stage MF,78 JAK inhibition may pose a risk related to a weakened antitumor defense. Our findings indicate that structural and functional skin barrier defects were predominately seen in plaque, tumor, or erythrodermic lesions, whereas the skin barrier was relatively structurally and functionally intact in patch lesions. Therefore, we propose that therapeutic intervention with JAK inhibition is most relevant in more advanced diseases, where skin defects are most pronounced and antitumor immunity already seems to have failed.
In conclusion, we provide evidence for the existence of cytokine-mediated crosstalk between malignant T cells and keratinocytes, leading to a marked reduction in skin barrier proteins in CTCL. Moreover, our data demonstrated that JAK1 inhibitors largely abrogate the negative effect of malignant T cells on skin barrier proteins, suggesting that they provide novel treatment options for patients with CTCL with compromised skin.
Acknowledgments
K. Rindler, P. M. Brunner, X. Song and P.-L. Chen graciously assisted in the annotation and elaboration of their respective single-cell RNA sequencing data sets.
This research was funded by grants from the LEO Foundation through the LEO Foundation Skin Immunology Research Center and LEO Foundation Grant (LF-OC-20-000351) (S.B.K.), The Danish Cancer Society (Kræftens Bekæmpelse), the Fight Cancer Program (Knæk Cancer), Novo Nordisk Research Foundation, Novo Nordisk Foundation Tandem Program (grant NNF21OC0066950), Lundbeck Foundation (A.W.-O.), and The Danish Council for Independent Research (Danmarks Frie Forskningsfond, 2 project grants [N.Ø.]; Sapera Aude Talent Grant [DFF-4092-00122] [T.K.]). LINAK A/S, Nordborg, Denmark.
The funding source had no influence on the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Authorship
Contribution: M.G. and N.Ø. conceived and designed the study and drafted the manuscript; N.Ø. obtained the funding and supervised the study; E.M.H.P., T.B.B., L.M.R.G., L.M.L., M.B., M.D., R.B., C.K.V., A.L.-V., M.R.K., S.B.K., L.I., T.L., and A.W. provided administrative, technical, or material support; and all authors performed acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content.
Conflict-of-interest disclosure: N.Ø. received consulting honoraria from Mindera Corp, Micreos Human Health, PS Consulting, and Almirall. J.C.B. is receiving speaker’s bureau honoraria from Amgen, Pfizer, Merck Serono, Recordati, and Sanofi; is a paid consultant/advisory board member for Boehringer Ingelheim, eTheRNA, InProTher, Merck Serono, Pfizer, 4SC, Regeneron, and Sanofi, and his group receives research grants from Bristol Myers Squibb, Merck Serono, HTG, IQVIA, and Alcedis. L.M.R.G. receives funding from NanoString Technologies. T.L. is funded by LEO Pharma. L.I. served as a consultant and/or paid speaker for, and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Janssen Cilag, Kyowa, LEO Pharma, Micreos Human Health, MSD, Novartis, Pfizer, Regranion, Samsung, Union Therapeutics, and UCB. The remaining authors declare no competing financial interests.
Correspondence: Niels Ødum, LEO Foundation Skin Immunology Research Center, Department of Immunology and Microbiology, The Maersk Tower 07-12, University of Copenhagen, Blegdamsvej 3, 2200, Copenhagen, Denmark; e-mail: ndum@sund.ku.dk.
References
Author notes
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession numbers GSE165623, GSE173205, GSE128531).
The data sets generated and/or analyzed during the current study are publicly available or on request from the corresponding author, Niels Ødum (ndum@sund.ku.dk).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal