Key Points
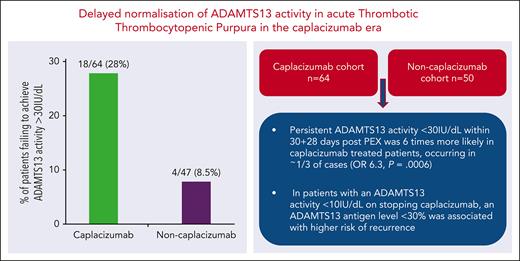
Twenty-eight percent of patients treated with caplacizumab had persistent ADAMTS13 activity <30% within 30 + 28 days after PEX.
In patients with ADAMTS13 activity <10% on stopping caplacizumab, an ADAMTS13 antigen level <30% had higher risk of recurrence.
Abstract
The benefits of caplacizumab in acute immune-mediated thrombotic thrombocytopenic purpura (iTTP) are well established. We identified a delayed normalization of a disintegrin and metalloprotease with thrombospondin type 1 motif, member 13 (ADAMTS13) activity (>30%) in a subgroup treated with caplacizumab, not evident in the precaplacizumab era. Patients treated with caplacizumab (n = 64) achieved ADAMTS13 activity >30% at median 31 days after plasma exchange (PEX), compared with 11.5 days in the noncaplacizumab group (n = 50, P = .0004). Eighteen of 64 (28%) patients treated with caplacizumab had ADAMTS13 activity <10% at stopping caplacizumab with a longer time to ADAMTS13 activity >30% (median, 139 days after completing PEX). Eighteen of 64 (28%) patients receiving extended caplacizumab (31-58 days) failed to achieve ADAMTS13 activity >30% at the time of caplacizumab cessation, compared with 4 of 47 (8.5%) historical controls at a similar timepoint (30 + 28 days, P < .0001). Failure to achieve ADAMTS13 activity >30% within 30 + 28 days was 6 times more likely with caplacizumab (odds ratio, 6.3; P = .0006). ADAMTS13 antigen <30% at caplacizumab cessation was associated with increased iTTP recurrence (4/10 vs 0/9 in patients with ADAMTS13 antigen ≥30%). Admission anti-ADAMTS13 immunoglobulin G (IgG) antibody level did not predict recurrence. Anti-ADAMTS13 IgG antibody levels, immunosuppression, and ethnicity did not account for differences in ADAMTS13 activity response. ADAMTS13 antigen levels ≥30% may be useful to guide stopping caplacizumab therapy after extended use with ADAMTS13 activity <10%. The reason for delayed ADAMTS13 normalization is unclear and requires further investigation.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare microangiopathic hemolytic anemia associated with microvascular thrombosis and thrombocytopenia, with potential for end organ damage. It is confirmed by a severe deficiency of the von Willebrand factor (VWF) cleaving protease called a disintegrin and metalloprotease with thrombospondin type 1 motif, member 13 (ADAMTS13) to <10%. The presence of autoantibodies, usually immunoglobulin G (IgG), against ADAMTS13 distinguishes immune from congenital TTP. Current established management of immune TTP (iTTP) consists of daily plasma exchange (PEX) at 1.5 plasma volume initially followed by a reduction to 1.0 plasma volume with an increasing platelet count. This has reduced untreated mortality from 90% to 10% to 20%.1,2 PEX aims to replenish ADAMTS13 as well as reduce the autoantibodies. Immunosuppression is key in the management of iTTP and primarily consists of administration of steroids and rituximab.3,4 In recent years, caplacizumab has become an integral part of the acute management of iTTP.5,6
Caplacizumab (Cablivi) is an anti-VWF–humanized single-domain immunoglobulin, which binds with high affinity to the A1 domain of VWF, thereby preventing interaction with the platelet glycoprotein Ib-IX-V receptor. The single blind placebo-controlled phase 2 TITAN study, and the double-blind randomized controlled phase 3 HERCULES trial have shown therapeutic efficacy of caplacizumab.5-7 Approval from European Medicines Agency8 and Food and Drug Administration9 were granted in 2018 and 2019, respectively. In 2020, caplacizumab received National Institute for Health and Care Excellence approval for patients who were >12-years old with acute TTP, to be used in conjunction with PEX and immunosuppression for at least 30 days after completion of PEX, or longer if required until ADAMTS13 normalizes.10
Using FRETS VWF73 method,11 we investigated the time to achieve ADAMTS13 activity >30% in patients receiving caplacizumab therapy compared with patients in the precaplacizumab era and factors which may contribute to this. The International TTP Working Group recommendation is to continue caplacizumab until ADAMTS13 activity >20%, but in the United Kingdom, we initially opted for confirmed ADAMTS13 activity levels >30% as a guide to caplacizumab discontinuation. This provided time to ensure that adequate control on autoimmunity is achieved and to reduce risk of recurrence associated with lower ADAMTS13 activities. This protocol was initiated before the International Working Group suggested a threshold of 20% to safely stop caplacizumab.12 We also investigated the role of ADAMTS13 antigen levels to guide stopping caplacizumab in those who have ongoing severely deficient ADAMTS13 activity.
Methods
Patient population
Sixty-four patients who received caplacizumab in conjunction with PEX and immunosuppression for a confirmed diagnosis of acute iTTP between January 2016 and October 2021 at a single TTP referral center were investigated (Medical Research Ethics Committee, numbers 08/H0810/54 and 08/H0716/72).
The HERCULES trial protocol6 allowed use of caplacizumab for 30 days after PEX, and then a further 28 days if there is ongoing severe ADAMTS13 deficiency (<10%), hence termed “30 + 28” days. Caplacizumab use is approved by the National Institute for Health and Care Excellence in the United Kingdom for 30 days after completion of PEX, with the option to extend the treatment further, guided by risk factors for recurrence of TTP such as persistent ADAMTS13 deficiency.
Citrated plasma samples for ADAMTS13 analysis were taken at presentation, the time of stopping caplacizumab and the closest timepoint to 30 + 28 days.
For comparison, data from 50 sequential patients with iTTP who did not receive caplacizumab as part of the management for acute TTP between January 2013 and December 2015 were analyzed from presentation and 30 + 28 days following completion of PEX. All patients received standard therapy: PEX, steroids (either methylprednisolone and/or high dose prednisolone), and rituximab 375 mg/m2 × 4 infusions.
Median number of days of IV and/or oral (PO) steroids, and days taken to wean PO steroids have been analyzed. Owing to the wide weight range in patients, we have not given a particular steroid dose threshold to signify high dose steroids. Switching from IV to PO steroids, as well as duration of wean for oral steroids were determined by clinical and laboratory markers. Further immunosuppression was given depending on laboratory parameters, specifically platelet counts and ADAMTS13 activity, aiming for normalization of levels.
Informed consent was obtained from all the patients.
ADAMTS13 assays
Treatment definitions
Time to achieve a normal platelet count ≥150 × 109/L was calculated in days following admission for treatment of acute TTP. Time to ADAMTS13 activity >30% was defined as a sustained rise in ADAMTS13 activity >30% with no subsequent drop in its activity. This ADAMTS13 activity level is used to stop caplacizumab in the United Kingdom. Increasing familiarity in using caplacizumab following completion of HERCULES trial, and subsequent routine ADAMTS13 antigen analysis in these patients allowed us to adapt our practice further. ADAMTS13 antigen levels, in addition to ADAMTS13 activity level and anti-ADAMTS13 IgG antibody level, at time of caplacizumab cessation is taken into consideration to determine when to stop caplacizumab. ADAMTS13 activity at the 30- + 28-day timepoint6 was taken as the result available 58 days after completion of PEX or the closest date available.
The International Working Group consensus report on redefining outcomes in iTTP, updated its definitions to take into account ADAMTS13 activity and the effects of caplacizumab on platelet count.12 Clinical remission is defined as sustained clinical response (platelet count ≥150 × 109/L and lactate dehydrogenase <1.5 × upper limit of normal) with either no PEX and no anti-VWF therapy for ≥30 days, or ADAMTS13 remission, partial remission is defined as ADAMTS13 activity ≥20% and complete remission ADAMTS13 activity above the lower limit of the normal laboratory range. Exacerbation is defined as a fall in platelet count following clinical response within 30 days of stopping PEX or anti-VWF therapy, whereas a fall in platelet count following clinical remission is defined as relapse.
In this analysis, any recurrence (exacerbation/relapse) has been defined in terms of days after completion of PEX and/or days after stopping caplacizumab therapy. Recommencement of caplacizumab treatment owing to exacerbation or relapse after previous discontinuation was analyzed as a separate episode of caplacizumab use. Relapses within 1 year of the acute iTTP episode were assessed.
Statistical analysis
Statistical analysis was performed using Graphpad Prism 9. Continuous data were summarized as median and range/interquartile range (IQR) with use of the Mann-Whitney U test to compare ranks across 2 groups and Kruskal-Wallis test for >2 groups. Gaussian distribution was not assumed for comparison between groups. Number and percentage were used to summarize categorical data. A χ2/Fisher exact test was used to assess statistical significance in categorical variables. The odds ratio and 95% confidence interval were estimated using logistic regression models. P < .05 was considered statistically significant.
Results
Demographics
Patient demographics were analyzed (Table 1). Patient groups were matched for age; however, there was a slight preponderance for females in the non-caplacizumab–treated patients.
Demographics of patients treated with and without caplacizumab
| . | Caplacizumab . | Noncaplacizumab . |
|---|---|---|
| Number of patients analyzed | 64 | 50 |
| Male:female (%) | 22:42 (34:66) | 13:37 (26:74) |
| Median age (range), y | 47 (14-78) | 45 (15-89) |
| Ethnicity, n (%) | ||
| Asian | 7 (11) | 9 (18) |
| Black | 26 (41) | 13 (26) |
| White | 31 (48) | 28 (56) |
| De novo:recurrence | 59:5 | 46:4 |
| Mortality, n (%) | 0 | 3 (6) |
| . | Caplacizumab . | Noncaplacizumab . |
|---|---|---|
| Number of patients analyzed | 64 | 50 |
| Male:female (%) | 22:42 (34:66) | 13:37 (26:74) |
| Median age (range), y | 47 (14-78) | 45 (15-89) |
| Ethnicity, n (%) | ||
| Asian | 7 (11) | 9 (18) |
| Black | 26 (41) | 13 (26) |
| White | 31 (48) | 28 (56) |
| De novo:recurrence | 59:5 | 46:4 |
| Mortality, n (%) | 0 | 3 (6) |
Sixty-four patients received caplacizumab as part of their management for iTTP. Ten received caplacizumab as part of the HERCULES trial and 54 received the drug for compassionate use and subsequently following UK regulatory approval.
Patients in whom caplacizumab therapy was stopped early for clinical reasons (eg, development of venous thromboembolism) and those who started caplacizumab later than standard practice (>48 hours from admission) were not included in the analysis.
Clinical and ADAMTS13 response in caplacizumab- and non-caplacizumab–treated patients
Caplacizumab was used for a median of 35 days (range, 15-130), corresponding to a median of 29.5 days (range, 9-121) after completion of PEX. Patients treated with caplacizumab achieved a sustained normal platelet count after a median of 4 days (range, 2-16) following initiation of acute treatment, compared with median of 9 days (range, 2-39; P < .0001) in non-caplacizumab–treated patients. Patients receiving caplacizumab required fewer days of PEX with a median of 6 days (range, 3-17) compared with median 12.5 days (range, 5-36; P < .0001) in the noncaplacizumab group.
A confirmed ADAMTS13 activity >30% was achieved at median 31 days (IQR, 14-83) following completion of PEX in patients treated with caplacizumab compared with 11.5 days (IQR, 7.25-27; P = .0004) in the noncaplacizumab group. Race (Asian/Black/Caucasian) did not affect time taken to achieve an ADAMTS13 activity >30% in patients treated with caplacizumab- and those not treated with caplacizumab (P = .6 and P = .4, respectively).
ADAMTS13 activity at caplacizumab discontinuation
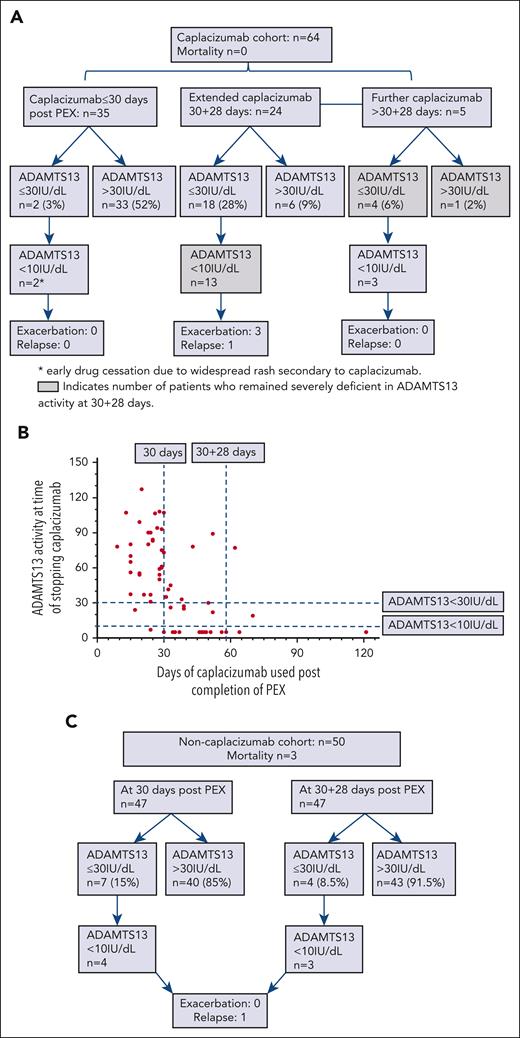
ADAMTS13 activity at time of stopping caplacizumab was analyzed against days of caplacizumab used following completion of PEX (Figure 1). The proportion of patients failing to achieve an ADAMTS13 activity >30% as well as those who remained severely deficient in ADAMTS13 activity are shown. Thirty-five of 64 patients (55%) stopped caplacizumab within 30 days following completion of PEX, median 24 days (range, 9-30). Two patients stopped caplacizumab with an ADAMTS13 activity <10% because of widespread maculopapular rash attributable to caplacizumab, which resolved promptly on cessation of the drug.
Caplacizumab compared to noncaplacizumab patients in acute TTP. (A) Duration of caplacizumab use after completion of PEX, ADAMTS13 activity at point of stopping caplacizumab and clinical outcomes. Failure to achieve an ADAMTS13 activity >30 IU/dL was 6 times more likely in patients treated with caplacizumab (odds ratio, 6.3; 95% confidence interval, 2.15-17.94; P = .0006). This was calculated based on the 18 patients with an ADAMTS13 activity ≤30 IU/dL in those who received extended caplacizumab, and additionally 5 patients who continued to receive capalcizumab after 30 + 28 days owing to ongoing ADAMTS13 activity <30 IU/dL. This was compared with the 33 patients who received ≤30 days of caplacizumab and had an ADAMTS13 activity >30 IU/dL at time of stopping caplacizumab, in addition to the 6 patients with an ADAMTS13 activity >30 IU/dL in those who received extended caplacizumab. The 2 patients with early caplacizumab cessation were not included in this analysis. (B) ADAMTS13 activity at time of stopping caplacizumab and duration of caplacizumab used after completion of PEX. (C) ADAMTS13 activity at 30 days and 30 + 28 days in noncaplacizumab cohort and clinical outcomes.
Caplacizumab compared to noncaplacizumab patients in acute TTP. (A) Duration of caplacizumab use after completion of PEX, ADAMTS13 activity at point of stopping caplacizumab and clinical outcomes. Failure to achieve an ADAMTS13 activity >30 IU/dL was 6 times more likely in patients treated with caplacizumab (odds ratio, 6.3; 95% confidence interval, 2.15-17.94; P = .0006). This was calculated based on the 18 patients with an ADAMTS13 activity ≤30 IU/dL in those who received extended caplacizumab, and additionally 5 patients who continued to receive capalcizumab after 30 + 28 days owing to ongoing ADAMTS13 activity <30 IU/dL. This was compared with the 33 patients who received ≤30 days of caplacizumab and had an ADAMTS13 activity >30 IU/dL at time of stopping caplacizumab, in addition to the 6 patients with an ADAMTS13 activity >30 IU/dL in those who received extended caplacizumab. The 2 patients with early caplacizumab cessation were not included in this analysis. (B) ADAMTS13 activity at time of stopping caplacizumab and duration of caplacizumab used after completion of PEX. (C) ADAMTS13 activity at 30 days and 30 + 28 days in noncaplacizumab cohort and clinical outcomes.
Twenty-four of 64 patients (38%) received caplacizumab up to a further 28 days after completion of the initial 30 days post PEX course (days 31-58), stopping a median of 46.5 days (range, 31-58). Following extended therapy (30 + 28 days), 13 cases had ongoing severe deficiency in ADAMTS13 activity.
A further 5 patients received caplacizumab beyond 30 + 28 days (>58 days) post PEX (range, 62-121 days). Three patients had persistent severe ADAMTS13 deficiency at 30 + 28 days.
For comparison, the proportion of patients not treated with caplacizumab failing to achieve an ADAMTS13 activity >30% at 30 and 30 + 28 days are shown in Figure 1C. Three of 50 patients in this group did not survive the acute admission. A smaller number of patients remained severely deficient in ADAMTS13 activity at 30 days and 30 + 28 days. Patients with an ADAMTS13 activity <30% at 30 + 28 days took a median of 84 days (range, 33-135) to achieve an ADAMTS13 activity >30%.
In summary, following extended use of caplacizumab (31-58 days), 18 of 64 patients (28%) failed to achieve an ADAMTS13 activity >30% at the time of caplacizumab cessation. This was significantly lower at 4 of 47 (8.5%) at 30 + 28 days following PEX in the noncaplacizumab group (P < .0001). The risk of failing to achieve an ADAMTS13 activity >30% within 2 months post PEX was 6 times more likely in patients treated with caplacizumab (odds ratio, 6.3; 95% confidence interval, 2.15-17.94; P = .0006).
ADAMTS13 activity <10% at time of caplacizumab discontinuation
Eighteen of 64 patients (28%) had an ADAMTS13 activity <10% at time of caplacizumab discontinuation, taking a median of 139 days (IQR, 83.5-213) after completing PEX to achieve a sustained ADAMTS13 activity >30%. In contrast, in the remaining 46 of 64 responders who had rising ADAMTS13 activity levels at time of caplacizumab discontinuation, it took a median of 22 days (IQR, 12-33; P < .0001). The delay in achieving an ADAMTS13 activity >30% in the caplacizumab cohort prompted further analysis of ADAMTS13 parameters in this subgroup in relation to subsequent clinical outcomes.
Anti-ADAMTS13 IgG antibody and ADAMTS13 antigen at caplacizumab discontinuation
The potential role of anti-ADAMTS13 IgG antibody level and ADAMTS13 antigen in predicting delayed normalization of ADAMTS13 activity was investigated in patients with an ADAMTS13 activity <10% at the time of stopping caplacizumab (n = 18), compared with those cases in whom ADAMTS13 activity increased (n = 46). There was no difference in presenting anti-ADAMTS13 IgG antibody level (at time of acute TTP diagnosis), median of 41% (IQR, 19%-83.5%) vs 39% (IQR, 14.5%-72.25%; P = .95) or presenting ADAMTS13 antigen, median of 2% (IQR, 1%-4.75%) vs 4% (IQR, 2%-13%; P = .08).
Anti-ADAMTS13 IgG antibody and ADAMTS13 antigen levels at time of stopping caplacizumab were significantly different as expected, with higher anti-ADAMTS13 IgG levels and lower ADAMTS13 antigen levels in patients who were severely deficient in ADAMTS13 activity on stopping caplacizumab (Table 2). Patients who were severely deficient in ADAMTS13 activity at time of caplacizumab discontinuation were also more likely to have a rising anti-ADAMTS13 IgG antibody level from the time of diagnosis to when they stopped caplacizumab, compared with the responders. Conversely, most of the responders had a falling anti-ADAMTS13 IgG level at cessation of caplacizumab (39/46 vs 4/18 in patients with ADAMTS13 activity <10% at time of stopping caplacizumab). Race did not influence time taken to achieve an ADAMTS13 activity >30% in these groups either (P = .6).
Median anti-ADAMTS13 IgG antibody and ADAMTS13 antigen levels in patients who remained severely deficient in ADAMTS13 activity at time of caplacizumab cessation, compared with patients who showed an increase in ADAMTS13 activity >10 IU/dL
| Levels at time of stopping caplacizumab . | Severely deficient in ADAMTS13 activity (n = 18) . | Responders: ADAMTS13 activity >10 IU/dL (n = 46) . | P value . |
|---|---|---|---|
| Median anti-ADAMTS13 IgG antibody (IQR), % | 72 (32-107) | 6.5 (3.75-18) | <.0001 |
| Rising anti-ADAMTS13 IgG antibody (%) | 12/18 (67) | 3/46 (6.5) | <.0001 |
| Median ADAMTS13 antigen (IQR), % | 31 (10.75-51) | 77.5% (46.5-99) | <.0001 |
| Levels at time of stopping caplacizumab . | Severely deficient in ADAMTS13 activity (n = 18) . | Responders: ADAMTS13 activity >10 IU/dL (n = 46) . | P value . |
|---|---|---|---|
| Median anti-ADAMTS13 IgG antibody (IQR), % | 72 (32-107) | 6.5 (3.75-18) | <.0001 |
| Rising anti-ADAMTS13 IgG antibody (%) | 12/18 (67) | 3/46 (6.5) | <.0001 |
| Median ADAMTS13 antigen (IQR), % | 31 (10.75-51) | 77.5% (46.5-99) | <.0001 |
Noncaplacizumab group ADAMTS13 assays
In the 4 of 47 patients who had an ADAMTS13 activity <10% at 30 days post PEX, the median anti-ADAMTS13 IgG antibody levels at presentation and 30 + 28 days were 44% (IQR, 27.5%-86%) and comparable with the caplacizumab cohort, and 40% (IQR, 34.5%-86%) respectively. In these 4 cases, the IgG antibody level increased in 2 patients, decreased in another, and was stable in the third patient.
TTP recurrence
Three patients experienced exacerbation(s) in the caplacizumab cohort. Two patients had an exacerbation 7 days after stopping caplacizumab (42 days after completion of PEX) and 10 days after stopping caplacizumab (60 days after completion of PEX). Both had an ADAMTS13 activity <10% at the time of exacerbation. A third patient had 2 exacerbations occurring 14 and 11 days after stopping caplacizumab, respectively. ADAMTS13 activities were <10% for both exacerbations. The second exacerbation in the third patient was treated with caplacizumab and further immunosuppression (2 additional rituximab and mycophenolate [MMF]) alone without PEX owing to mild thrombocytopenia (platelet count, 139 × 109/L), and therefore, was not included in the analysis. There were no deaths in the caplacizumab-treated group.
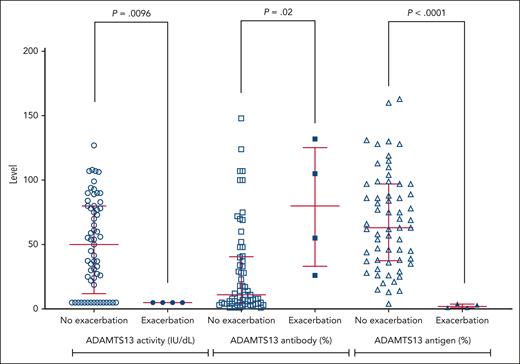
ADAMTS13 activity, anti-ADAMTS13 IgG antibody, and ADAMTS13 antigen levels in TTP exacerbation and nonexacerbation cases are shown in Figure 2 and Table 3.
Graphic representation of comparison of ADAMTS13 activity IgG antibody and ADAMTS13 antigen levels at time of stopping caplacizumab in patients with exacerbation and no exacerbation. ADAMTS13 activity <10 IU/dL has been depicted at a level of 5 IU/dL for clarity on Figure 2. Patients with exacerbation of TTP had a lower ADAMTS13 activity, higher anti-ADAMTS13 antibody level, and lower ADAMTS13 antigen level.
Graphic representation of comparison of ADAMTS13 activity IgG antibody and ADAMTS13 antigen levels at time of stopping caplacizumab in patients with exacerbation and no exacerbation. ADAMTS13 activity <10 IU/dL has been depicted at a level of 5 IU/dL for clarity on Figure 2. Patients with exacerbation of TTP had a lower ADAMTS13 activity, higher anti-ADAMTS13 antibody level, and lower ADAMTS13 antigen level.
Differences in ADAMTS13 activity, anti-ADAMTS13 IgG antibody, and ADAMTS13 antigen levels between patients who experienced exacerbation and no exacerbation
| . | No exacerbation (n = 61) . | Exacerbation (n = 4) . |
|---|---|---|
| Median ADAMTS13 activity (IQR), IU/dL | 50 (9.35-80) | <10 |
| Median anti-ADAMTS13 IgG antibody (IQR), % | 11 (4-40.5) | 80 (33.25-125.3) |
| Median ADAMTS13 antigen (IQR), % | 63 (37.5-97) | 2 (1-3.75) |
| . | No exacerbation (n = 61) . | Exacerbation (n = 4) . |
|---|---|---|
| Median ADAMTS13 activity (IQR), IU/dL | 50 (9.35-80) | <10 |
| Median anti-ADAMTS13 IgG antibody (IQR), % | 11 (4-40.5) | 80 (33.25-125.3) |
| Median ADAMTS13 antigen (IQR), % | 63 (37.5-97) | 2 (1-3.75) |
In the noncaplacizumab patient cohort, 4 patients had an exacerbation within 30 days of stopping PEX and had an ADAMTS13 activity <10%. One patient had a clinical relapse within 1 year and this occurred at 37 days following previous exacerbation of TTP following a nonsustained partial rise in ADAMTS13 activity.
ADAMTS13 antigen level as a predictor for recurrence and to guide safe discontinuation of caplacizumab in patients with ADAMTS13 activity <10%
ADAMTS13 antigen thresholds were investigated in patients who were severely deficient in ADAMTS13 activity at the time of stopping caplacizumab (supplemental Table 1, available on the Blood website). A higher recurrence rate was observed in patients with an antigen level <30%.
ADAMTS13 antigen levels above 30% in this cohort did not experience a recurrence despite high IgG antibody levels. Even at high levels, anti ADAMTS 13 IgG antibody did not predict recurrence.
Immunosuppression used during acute TTP
To exclude confounders to the delayed normalization seen in patients treated with caplacizumab, immunosuppression/immunomodulatory therapy including steroid use and anti-CD20 treatment with rituximab was analyzed (Table 4). More doses of rituximab were required in the caplacizumab cohort and although not statistically significant, more cases relatively required additional immunosuppression such as MMF or bortezomib.
Comparison of immunosuppression and anti-CD20 treatment in caplacizumab and noncaplacizumab cohorts
| . | Caplacizumab . | Noncaplacizumab . | P value . |
|---|---|---|---|
| Median days of IV methylprednisolone only (IQR) | 2 (1-2) | 2 (2-3.5) | .1 |
| Median dose of IV methylprednisolone (IQR), g | 1.5 (0.875-2) | 2 (1.25-2.25) | .2 |
| Number of patients receiving steroids∗ | 62/64 | 47/50 | .65 |
| Days of prednisolone wean (IQR) | 13 (9-19) | 16 (10-18) | .4 |
| Number of patients receiving rituximab | 61/64 | 43/50† | .1 |
| Median number of rituximab doses (range) | 6 (4-8) | 4 (1-8) | .05 |
| Number of patients receiving additional immunosuppression (%)‡ | 21/64 (33) | 12/50 (24) | .5 |
| . | Caplacizumab . | Noncaplacizumab . | P value . |
|---|---|---|---|
| Median days of IV methylprednisolone only (IQR) | 2 (1-2) | 2 (2-3.5) | .1 |
| Median dose of IV methylprednisolone (IQR), g | 1.5 (0.875-2) | 2 (1.25-2.25) | .2 |
| Number of patients receiving steroids∗ | 62/64 | 47/50 | .65 |
| Days of prednisolone wean (IQR) | 13 (9-19) | 16 (10-18) | .4 |
| Number of patients receiving rituximab | 61/64 | 43/50† | .1 |
| Median number of rituximab doses (range) | 6 (4-8) | 4 (1-8) | .05 |
| Number of patients receiving additional immunosuppression (%)‡ | 21/64 (33) | 12/50 (24) | .5 |
Methylprednisolone and/or prednisolone.
Includes 2 patients who died (1 patient received 2 doses and another patient received 1 dose). Five patients had antiretroviral disease, 1 patient had viral encephalitis, and clinical information was lacking in 1 patient.
Caplacizumab-treated cohort: received MMF and 2 patients additionally received bortezomib. Non-caplacizumab-treated cohort: majority received MMF except 3 patients who received bortezomib.
Discussion
Caplacizumab therapy is now an integral part of the management of patients with acute iTTP. The benefits of this additional agent to standard of care has been shown via the TITAN5 and HERCULES6 clinical trials which focused on time to platelet normalization as the primary outcome. Our analysis of patients treated with caplacizumab confirms the shorter time to platelet normalization (4 vs 9 days for noncaplacizumab group, P < .0001) and associated fewer days of PEX (6 vs 13 days for noncaplacizumab group, P < .0001) comparable with other real-world data reported.13,14 Volker et al15 reported a median of 3 days to achieve a normal platelet count after starting caplacizumab, with a median 9 days of PEX required to achieve this. However, caplacizumab was started with a median of 3 days after diagnosis of TTP in their patients, whereas our patients receive caplacizumab once the diagnosis is confirmed within 24 hours of presentation and this is likely to explain this observed difference.
With increasing use and familiarity of caplacizumab, it became apparent that in patients treated with caplacizumab, there is a delay in a proportion of patients achieving an ADAMTS13 activity >30%. Coppo et al14 reported a median of 28 days to achieve ADAMTS13 activity ≥20% in patients treated with the triplet regime (PEX, rituximab and steroids, and caplacizumab). We evaluated patients treated with PEX, steroids, and rituximab in the precaplacizumab era, as a comparator to show the delayed normalization in a subgroup of patients in the caplacizumab era. In our cohort, the time taken to achieve an ADAMTS13 activity >30% from completion of PEX was nearly 3 times longer when analyzing all patients treated with caplacizumab compared with historical acute TTP cases not treated with caplacizumab. Furthermore, nearly one-third of our cohort had ongoing severe ADAMTS13 activity deficiency at the time of caplacizumab cessation, despite extended use. In this subgroup of patients, a median of 139 days was required to achieve an ADAMTS13 activity >30%. Significantly more patients treated with caplacizumab failed to achieve an ADAMTS13 activity >30% at 30 + 28 days (2 months post PEX) than in the precaplacizumab era.
Presenting anti-ADAMTS13 IgG levels were not predictive of the delayed normalization of ADAMTS13 activity, yet a rise in antibody level from diagnosis to the time of stopping caplacizumab does seem relevant. Median ADAMTS13 antigen level was significantly lower at the time of stopping caplacizumab in patients with severe ADAMTS13 activity levels. Concurrent ADAMTS13 antigen levels <30% at time of caplacizumab discontinuation were associated with a greater risk of recurrence. However, raised anti-ADAMTS13 IgG levels was not predictive of TTP recurrence.
Although the TITAN trial showed that early relapses occurred in a subgroup of patients with ADAMTS13 activity <10% when caplacizumab was stopped, the HERCULES trial design was adapted to allow continuation of caplacizumab if ADAMTS13 activity remained severely deficient. These changes led to fewer relapses in the HERCULES trial, and the relapses that did occur were in patients who still had ADAMTS13 activity <10%. Of the 120 patients who had ADAMTS13 activity levels available at the time of caplacizumab/placebo discontinuation (60 at the end of the period of double-blind administration of caplacizumab, 34 at the end of the period of double-blind administration of placebo, and 26 at the end of the period of open-label administration of caplacizumab), 29 patients (24%) had an ADAMTS13 activity <10%. Nine patients from this group had a relapse, of which 3 received open-label caplacizumab. We identified 28% of patients treated with caplacizumab who had severely deficient ADAMTS13 activity at the time of caplacizumab cessation in our cohort.
In the HERCULES trial, ADAMTS13 antigen levels were not examined. ADAMTS13 antigen levels have been shown to be variable at iTTP presentation despite an ADAMTS13 activity <10%16-18 and to have prognostic significance.18,19 Here, we show the use of ADAMTS13 antigen levels to guide caplacizumab therapy cessation.
Volker et al20 have suggested that an ADAMTS13-guided approach can be used to guide caplacizumab therapy and prevent overtreatment and undertreatment. Although anti-ADAMTS13 IgG antibody and low ADAMTS13 activity levels were associated with greater risk of TTP recurrence, a severe deficiency of ADAMTS13 antigen was not.21 As suggested by the authors, this may be explained by the small sample size. Importantly, our analysis further adds insight to guide caplacizumab discontinuation as well as highlighting risk of exacerbations/relapses in those patients with ongoing severe ADAMTS13 deficiency. In patients with ADAMTS13 activity <10%, a severely deficient ADAMTS13 antigen level <30% was predictive of a higher risk of recurrence.
Steroid use was investigated as a possible confounder for the delayed normalization of ADAMTS13 activity in patients treated with caplacizumab. No difference in overall dose of IV methylprednisolone or prednisolone use was demonstrated. However, there was an increased use of rituximab and other immunosuppression (MMF and bortezomib) since the introduction of caplacizumab, reflecting the delayed normalization of ADAMTS13 activity levels, a finding significantly different from the precaplacizumab era. Chaturvedi et al22 have recently shown that race affects response to rituximab in iTTP. They showed that patients of Black race were associated with shorter relapse-free survival and although the addition of rituximab to steroids improved relapse-free survival in White patients, this was not evident in patients of Black race. Furthermore, they showed that there was no effect of race related to ADAMTS13 activity recovery at 90 days. Similarly, our findings suggest that race does not influence time to achieving ADAMTS13 activity >30%.
Ongoing analysis of ADAMTS13 assays in future cases of recurrence would help to support the association, given that this was a retrospective analysis. A prospective analysis to confirm that stopping caplacizumab once an ADAMTS13 antigen level >30% has been achieved in patients who remain severely deficient in ADAMTS13 activity, would support our finding. Whether the smaller number of patients treated with caplacizumab who have also had PEX procedures play a role in the delayed normalization of ADAMTS13 activity, requires investigating too.
In summary, the use of caplacizumab in acute TTP has had a very positive impact in patient care. In a large cohort of iTTP patients treated with caplacizumab, a delay in ADAMTS13 activity level increment (>30%) in 28% of cases was associated with increasing anti-ADAMTS13 IgG antibody levels and the need for further immunosuppressive therapy, when compared with consecutive cases in the precaplacizumab era. The reason for this observation is not clear and does not seem to clearly attribute to the differences in early immunosuppression, although patients receiving caplacizumab had fewer PEX procedures. However, the timing of PEX in relation to analysis of ADAMTS13 activity levels (30 days or 30 + 28 days) would make this less likely to be contributory. Our data suggest that ADAMTS13 antigen levels may provide a further laboratory marker to guide when caplacizumab therapy can be safely stopped. The precise mechanism causing delayed normalization of ADAMTS13 activity in a proportion of patients treated in the caplacizumab era remains to be characterized and requires further investigation.
Acknowledgment
The authors thank Karen Vanhoorelbeke for supplying antibodies required to perform the ADAMTS13 antigen assay.
Authorship
Contribution: N.P. designed the research, conducted laboratory testing, collected data, analyzed data, and wrote the manuscript; M.T. designed research and reviewed the manuscript; M. Stubbs collected data and reviewed the manuscript; J.-P.W. and R.d.G. reviewed the manuscript; D.S. conducted laboratory testing and reviewed the manuscript; and M. Scully is senior author, conceived the research, designed the research, and reviewed the manuscript.
Conflict-of-interest disclosure: M. Scully has received speaker fees and has served on advisory boards for Alexion, Novartis, Takeda, Sanofi, and Octapharma and has received research grants from Shire and Alexion. M.T. received speaker fees/honoraria/served on advisory boards for Sanofi, Bayer, and Anthos. J.-P.W. received speaker fees and has served on advisory boards for Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Nithya Prasannan, Department of Haematology, University College London Hospitals NHS Foundation Trust, 250 Euston Rd, London NW1 2PG; United Kingdom; e-mail: n.prasannan@nhs.net.
References
Author notes
Data are available on request from the corresponding author, Nithya Prasannan (n.prasannan@nhs.net).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal