TO THE EDITOR:
Ectopic infection of T and natural killer (NK) cells by Epstein-Barr virus (EBV) has been associated with lymphoproliferative disorders known as chronic active EBV (CAEBV) disease. CAEBV disease has predominantly been reported in East Asian countries, with sporadic cases in Latin America and the Mediterranean.1-4 Thirty percent to 70% of patients with CAEBV disease comprise the NK type and first present with severe mosquito bite allergy (SMBA), the local form of the disease.5-7 SMBA can evolve into the systemic form of CAEBV disease and then progress suddenly to aggressive malignancy or hemophagocytic lymphohistiocytosis (HLH) without identifiable biomarkers for onset.8 The only known curative treatment is hematopoietic stem cell transplantation; otherwise, 50% to 58% of patients die within 10 to 15 years of diagnosis.6,8,9
Clinical and immunological studies have revealed that both chronic EBV infection and the stimulation of immune cells from patients with CAEBV disease with mosquito salivary gland extracts (SGEs) may play key roles in the development of SMBA and life-threatening HLH.10 However, there are no clear biomarkers for disease severity or for progression to HLH. Here, we performed deep immunophenotyping on a pair of monozygotic (MZ) twins who were both seropositive for EBV but clinically discordant for SMBA and CAEBV disease and identified NK cell memory–associated cytokines as crucial mechanisms and biomarkers of disease progression.
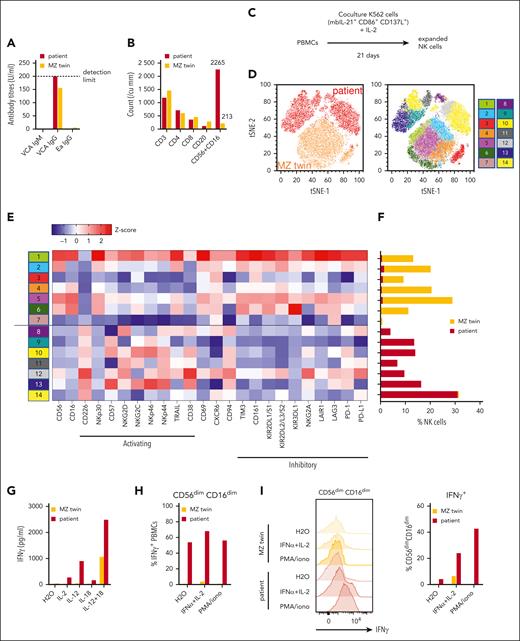
The patient and her MZ twin were born full-term to nonconsanguineous Chinese Singaporean parents. She was healthy apart from SMBA, starting at the age of 3. Unfortunately, the patient progressed to fulminant, treatment-resistant HLH 9 months after referral at age 13 and succumbed to multiorgan failure 2 months later (supplemental Figure 1, available on the Blood website). Targeted next-generation sequencing panels for HLH and cancer did not reveal any candidate mutations, and the family declined comprehensive whole-exome or genome sequencing. Therefore, we screened for clinical and immunological discordance 6 months after referral, between 2 SMBA reactions at 4 and 6.5 months after referral, but before fulfilling the HLH criteria. Informed consent was obtained from the parents and the assent of the twins. Both twins had high serum levels of EBV viral capsid antigen immunoglobulin G, indicating prior EBV exposure (Figure 1A). Despite similar counts of CD4+ and CD8+ T cells and CD20+ B cells, the patient had 10.6 times more NK cells than her healthy twin (Figure 1B).
Patient with SMBA and CAEBV disease progressing to lethal HLH shows expansion of hyperinflammatory memory NK cells absent in her MZ twin. (A) Titers of reactive immunoglobulin G (IgG) antibodies against EBV viral capsid antigen and early antibody (Ea) antigens in sera from patient and MZ twin 6 months after referral. (B) CD3+, CD4+, CD8+, and CD56+CD16 lymphocyte counts from the patient and her twin at 6 months after referral. (C) Procedure for in vitro expansion of NK cells from PBMCs. (D) t-distributed stochastic neighbor embedding (t-SNE) clustering of expanded NK cells from the patient and her MZ twin based on the expression of 24 NK cell activating and inhibitory receptors. Clusters labeled by donor (red, patient and orange, MZ twin) (left), and phenograph of further subpopulations (right), are shown. (E) Surface expression of NK cell receptors in each phenograph subpopulation as a heat map of z scores of the mean fluorescence intensity of each receptor. (F) Frequency of NK cells from the MZ twin and patient in each phenograph subpopulation. (G) Concentration of IFN-γ produced by PBMCs after stimulation with IL-2 (80 IU/mL), IL-12 (100 μg/mL), IL-18 (100 μg/mL), or IL-12 with IL-18. (H) Percentage of CD56dimCD16dim NK cells among the total IFN-γ+ PBMCs from the patient and her MZ twin when unstimulated (H2O), or when stimulated with IFN-α (1000 IU/mL) + IL-2 or PMA (phorbol 12-myristate 13-acetate; 5 ng/mL)/ionomycin (iono; 1 μg/mL). (I) Histogram of intracellular IFN-γ staining among CD56dimCD16dim NK cells from the patient and her MZ twin when unstimulated (H2O) or stimulated with IFNa + IL-2 or PMA/ionomycin, normalized to the mode of cell counts (left) and percentage of cells gated IFN-γ+ among CD56dimCD16dim NK cells (right) are shown. Data are shown from single replicates due to the limited amount of primary sample.
Patient with SMBA and CAEBV disease progressing to lethal HLH shows expansion of hyperinflammatory memory NK cells absent in her MZ twin. (A) Titers of reactive immunoglobulin G (IgG) antibodies against EBV viral capsid antigen and early antibody (Ea) antigens in sera from patient and MZ twin 6 months after referral. (B) CD3+, CD4+, CD8+, and CD56+CD16 lymphocyte counts from the patient and her twin at 6 months after referral. (C) Procedure for in vitro expansion of NK cells from PBMCs. (D) t-distributed stochastic neighbor embedding (t-SNE) clustering of expanded NK cells from the patient and her MZ twin based on the expression of 24 NK cell activating and inhibitory receptors. Clusters labeled by donor (red, patient and orange, MZ twin) (left), and phenograph of further subpopulations (right), are shown. (E) Surface expression of NK cell receptors in each phenograph subpopulation as a heat map of z scores of the mean fluorescence intensity of each receptor. (F) Frequency of NK cells from the MZ twin and patient in each phenograph subpopulation. (G) Concentration of IFN-γ produced by PBMCs after stimulation with IL-2 (80 IU/mL), IL-12 (100 μg/mL), IL-18 (100 μg/mL), or IL-12 with IL-18. (H) Percentage of CD56dimCD16dim NK cells among the total IFN-γ+ PBMCs from the patient and her MZ twin when unstimulated (H2O), or when stimulated with IFN-α (1000 IU/mL) + IL-2 or PMA (phorbol 12-myristate 13-acetate; 5 ng/mL)/ionomycin (iono; 1 μg/mL). (I) Histogram of intracellular IFN-γ staining among CD56dimCD16dim NK cells from the patient and her MZ twin when unstimulated (H2O) or stimulated with IFNa + IL-2 or PMA/ionomycin, normalized to the mode of cell counts (left) and percentage of cells gated IFN-γ+ among CD56dimCD16dim NK cells (right) are shown. Data are shown from single replicates due to the limited amount of primary sample.
We expanded NK cells from both twins in vitro to measure further phenotypic differences in 24 immunomodulatory receptors (Figure 1C; supplemental Table 1).11 Dimensionality reduction by t-distributed stochastic neighbor embedding and cluster identification by phenograph showed nearly complete separation between the patient and her twin (Figure 1D). The patient’s NK cells presented a hyperinflammatory phenotype; over 90% of them were CD226+ and expressed significantly lower levels of the inhibitory receptors CD161, KIRs, and TIM3 (Figure 1E-F), all phenotypes previously ascribed to memory-like NK cells.12-18 Thus, we examined whether the patient’s peripheral blood mononuclear cells (PBMCs) also showed memory-like interferon gamma (IFN-γ) hypersecretion when stimulated by memory-priming cytokines interleukin 2 (IL-2), IL-12, and IL-18.16-18 IL-2 and IL-12 alone were sufficient to induce much higher levels of IFN-γ secretion from the patient’s PBMCs than the MZ twin, whereas IL-18 had only a small effect (Figure 1G). Intracellular cytokine staining of the patient’s PBMC indicated that these IFN-γ–producing cells were nearly all CD56dim CD16dim and contained preformed IFN-γ protein before stimulation, which was absent in her twin’s NK cells (Figure 1H). In addition, the intracellular levels of IFN-γ in NK cells further increased when the cells were stimulated with IFN-α and IL-2, or phorbol 12-myristate 13-acetate/ionomycin (Figure 1I).
Previous studies have shown that mosquito species differ in their ability to prime both NK cell lymphocytosis and CD4+ T cell proliferation in patients with SMBA.19 Therefore, we hypothesized that they also differ in their ability to prime IFN-γ production by the patient’s memory NK cells. We stimulated the patient’s PBMCs with mosquito SGEs from 2 common mosquitoes in Singapore, Aedes aegypti and Aedes albopictus.20 Either SGE alone induced high amounts of IFN-γ from the patient's PBMCs, but we found a significantly stronger effect by A albopictus (Figure 2A). Intracellular cytokine staining showed that IFN-γ was produced mainly by NK and CD4+ T cells of both twins; however, although IFN-γ in the MZ twin was principally expressed by CD4+ T cells, the majority of the patient’s IFN-γ+ PBMCs after stimulation with A albopictus SGE were NK cells (Figure 2B). Because either IL-2 or IL-12 can cause IFN-γ hyperproduction in the patient’s PBMCs (Figure 1G), we hypothesized that they were induced by Aedes SGEs and were responsible for IFN-γ production by the patient’s NK cells. Stimulation of PBMCs from patients with SGE from either species produced higher levels of IL-2 and A albopictus SGE than those from A aegypti. Meanwhile, IL-12 was uniquely induced by A albopictus SGE (Figure 2C). The patient’s NK cells also showed increased CD25 expression and sensitivity to IL-2 in response to A albopictus SGE, but not A aegypti (Figure 2D).
A albopictus SGE glands induce proinflammatory cytokine production by PBMCs from patients with CAEBV disease, which can be suppressed by STAT3. (A) Concentration of IFN-γ in the supernatant of PBMCs from the patient and her MZ twin after 16-hour stimulation with SGE from A aegypti (A. aeg) or A albopictus (A. albo) (25 μg/mL). (B) Percentage of each leukocyte population among IFN-γ+ PBMCs from the patient and her MZ twin stained after 4-hour stimulation with SGE from A aegypti or A albopictus. (C) Concentrations of IL-2 and IL-12 in the supernatant of PBMCs from the patient and her MZ twin after 16-hour stimulation with SGE from A aegypti or A albopictus. (D) Expression of CD25 in NK cells from the patient and her MZ twin after 16-hour stimulation with SGE from A aegypti or A albopictus. (E) Heat maps showing relative concentrations of IFN-γ, IL-12p70, IL-2, soluble IL-2R (sIL-2R), IL-6, IL-8, tumor necrosis factor α (TNFα), CCL3, CXCL10, granulocyte-monocyte colony-stimulating factor (GM-CSF), IL-5, and IL-13 after 16-hour stimulation with SGE from A aegypti or A albopictus, from the patient and her MZ twin at 6 months after referral (left), and patient and 2 unrelated healthy controls (URHCs) at 9 months after referral (right). Data are presented as a heat map scaled relative to the maximum concentration of each cytokine. Concentrations of IL-2 (F), IL-12 (G), and IFN-γ (H) in the supernatants of PBMCs from the patient and her MZ twin, preincubated with S3I-201 (200 μM), STAT5 inhibitor (200 μM), or ML-120B (10 μM), and then stimulated with Aedes SGEs for 16 hours. (I) Relative concentrations of cytokines in the supernatant of patient PBMCs preincubated with S3I-201, STAT5 inhibitor, or ML-120B; then stimulated with Aedes SGEs for 16 hours. Data are presented as a heat map, scaled relative to the maximum concentration of each cytokine. Data are shown from single replicates due to the limited amount of primary sample. DMSO, dimethyl sulfoxide.
A albopictus SGE glands induce proinflammatory cytokine production by PBMCs from patients with CAEBV disease, which can be suppressed by STAT3. (A) Concentration of IFN-γ in the supernatant of PBMCs from the patient and her MZ twin after 16-hour stimulation with SGE from A aegypti (A. aeg) or A albopictus (A. albo) (25 μg/mL). (B) Percentage of each leukocyte population among IFN-γ+ PBMCs from the patient and her MZ twin stained after 4-hour stimulation with SGE from A aegypti or A albopictus. (C) Concentrations of IL-2 and IL-12 in the supernatant of PBMCs from the patient and her MZ twin after 16-hour stimulation with SGE from A aegypti or A albopictus. (D) Expression of CD25 in NK cells from the patient and her MZ twin after 16-hour stimulation with SGE from A aegypti or A albopictus. (E) Heat maps showing relative concentrations of IFN-γ, IL-12p70, IL-2, soluble IL-2R (sIL-2R), IL-6, IL-8, tumor necrosis factor α (TNFα), CCL3, CXCL10, granulocyte-monocyte colony-stimulating factor (GM-CSF), IL-5, and IL-13 after 16-hour stimulation with SGE from A aegypti or A albopictus, from the patient and her MZ twin at 6 months after referral (left), and patient and 2 unrelated healthy controls (URHCs) at 9 months after referral (right). Data are presented as a heat map scaled relative to the maximum concentration of each cytokine. Concentrations of IL-2 (F), IL-12 (G), and IFN-γ (H) in the supernatants of PBMCs from the patient and her MZ twin, preincubated with S3I-201 (200 μM), STAT5 inhibitor (200 μM), or ML-120B (10 μM), and then stimulated with Aedes SGEs for 16 hours. (I) Relative concentrations of cytokines in the supernatant of patient PBMCs preincubated with S3I-201, STAT5 inhibitor, or ML-120B; then stimulated with Aedes SGEs for 16 hours. Data are presented as a heat map, scaled relative to the maximum concentration of each cytokine. Data are shown from single replicates due to the limited amount of primary sample. DMSO, dimethyl sulfoxide.
Fulminant HLH is accompanied by a proinflammatory cytokine storm.21 To assess whether there are further similarities in cytokine production between SMBA and fulminant HLH, we performed multiplexed enzyme-linked immunosorbent assays on patient PBMCs stimulated with SGEs. A albopictus SGE universally induced higher levels of secreted IL-2Ra, a diagnostic criterion for HLH, proinflammatory cytokines and chemokines, and type 2 immunity-associated cytokines, with increasing responsiveness over time (Figure 2E). Because these cytokines are common in HLH-associated cytokine storms, they may have heralded the patient’s progression to HLH.21
The above analyses suggest that persistent induction of IL-2 and IL-12 by Aedes SGEs drives SMBA and CAEBV disease progression by promoting functional memory in the patient’s infected NK cells. Thus, blocking IL-2 and IL-12 production may present new therapeutic opportunities to ameliorate this disease. We examined whether we could abolish the production of these cytokines by inhibiting STAT3, STAT5, and NF-κB, which have been shown to promote the production of these important cytokines. The patient’s PBMCs were stimulated with Aedes SGE after preincubation with the IKK2 inhibitor ML-120B, STAT3 inhibitor S3I-201, and STAT5 inhibitor. S3I-201 abolished the production of IL-2 and also fully blocked IL-12 and IFN-γ production, whereas only partial suppressive effects were observed when using ML-120B or the STAT5 inhibitor (Figure 2F-I).
This discordance in infection and immunophenotype between MZ twins supports the notion that SMBA is not predominantly driven by inborn errors of immunity. Given that the patient’s SMBA started when she was a young child and became more severe over time, with larger lesions and higher fevers, it is likely that NK memory priming began at a young age and developed gradually throughout her life, amplified by multiple rounds of stimulation with IL-12 and IL-2 during SMBA reactions. A albopictus SGE induced both IL-2 and IL-12 in the patient’s PBMCs, whereas A aegypti only induced IL-2 (Figure 2C). Mechanistically, this suggests that A albopictus is a stronger and more versatile agonist of the patient’s NK memory response, as both cytokines prime robust IFN-γ production and NK memory (Figure 2A).16,17,22 Such differences in cytokine induction may also underlie the species selectivity of reactions reported in other patients with CAEBV disease with SMBA.19 Supporting the dependence of memory NK responses on IL-2 and IL-12, STAT3 inhibition completely abrogated IFN-γ, together with IL-2 and IL-12, from patient PBMCs stimulated with A albopictus SGE (Figure 2F-I). STAT3 underlies many complex mechanisms of both tumorigenesis and NK cell immune function; our findings demonstrate it may also be a suitable target for prevention of NK memory priming as a third pathogenic aspect to CAEBV disease.23 Our results highlight that it is crucial to initiate early treatment of CAEBV disease with hematopoietic stem cell transplantation or cytokine inhibitors, before EBV infected NK cells establish a memory population, and suggest that further investigation into the effects of memory-priming cytokines and STAT3 inhibitors may present new therapeutic opportunities.
Acknowledgments
The authors thank the patient and her family for their patience and support of our study, and the Japan chronic active Epstein-Barr virus working group and Bianca Chan Su-Wan for valuable insight into further chronic active Epstein-Barr virus cases, as well as Seah Ching Ching, Liang Ai Wei, and Chai Chean Nee for write-up and technical help with the clinical Epstein-Barr virus titer measurements. The authors also thank Abrar Al-Mahmood Siddiquee and Najwa BinTalib for their technical assistance with in vitro experiments. Mosquitoes were reared, and salivary glands were dissected for use by Duke University–National University of Singapore Insectary Service, which is managed by Milly Choy and Menchie Casayuran Manuel.
This work was supported by core funding from the Agency for Science, Technology and Research (A∗STAR), Singapore.
Authorship
Contribution: C.K.L. and Y.Z. are joint first authors and responsible for writing the initial draft of the manuscript; C.K.L. coordinated scientific studies, collated experimental results, and wrote the scientific portions of the manuscript; Y.Z. was the patient’s primary care physician who made initial observations, diagnosed, and managed care of the patient; P.-L.T. was the physician who closely monitored the patient’s condition and managed conditioning and preparation for hematopoietic stem cell transplantation; Y.Z., C.-H.H., M.L.O., F.Y., M.V., P.P.L.N., S.-B.N., T.C.Q., and P.-L.T. were involved in the clinical care of the patient and contributed to the interpretation of clinical data; C.K.L., R.H., W.-X.S., B.V.A., S.N., C.Y.C.L., A.C., B.W., J.P., and K.-C.C. contributed to in vitro experiments and data analysis and interpretation; C.K.L., Y.Z., R.H., W.-X.S., S.N., C.-H.H., M.L.O., and S.-B.N. contributed to the provision and editing of tables and figures; P.-L.T., K.-C.C., and J.E.C. were responsible for critical appraisal of the final manuscript; and all joint authors reviewed and approved the final version of the submitted manuscript.
The current affiliation for C.K.L. is Gilead Sciences, Oxford, United Kingdom.
Conflict-of-interest disclosure: J.E.C. is a Chief Scientific Officer at the Parker Institute of Cancer Immunotherapy, San Francisco, CA. The remaining authors declare no competing financial interests.
Correspondence: John E. Connolly, Institute of Molecular and Cell Biology, Agency for Science, Technology and Research, 61 Biopolis Drive, #07-17, Singapore 138673; e-mail: jeconnolly@imcb.a-star.edu.sg.
References
Author notes
∗C.K.L. and Y.Z. contributed equally to this study.
†P.-L.T., K.-C.C., and J.E.C. are joint corresponding authors.
Data are available on request from the corresponding author, John E. Connolly (jeconnolly@imcb.a-star.edu.sg).
The online version of this article contains a data supplement.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal