In this issue of Blood, Stetka et al report on their examination of the effect of iron status in polycythemia vera (PV) on megakaryopoiesis and erythropoiesis.1 They first focused on the well-recognized but not fully understood association of iron deficiency (ID) and reactive thrombocytosis. ID is frequent in PV and is virtually universally present in phlebotomized PV patients. Although it was previously suspected that ID increases the number of common megakaryocyte/erythroblast progenitors that augment platelet production,2 this work conclusively proved it by a series of elegant experiments in mouse models expressing different amounts of JAK2 V617F mutation and a mouse model with the activating JAK2 exon 12 mutation. The authors demonstrate that ID directly stimulates production of megakaryocyte/erythroblast progenitors, resulting in increased megakaryopoiesis and thrombocytosis, which normalizes with correction of ID. The authors further show in their mouse models that the activating JAK2 exon 12 mutation does not stimulate megakaryopoiesis and thrombocytosis as much as JAK2 V617F, which also has clinical implications for platelet count of PV patients with JAK2 exon 12 mutations.
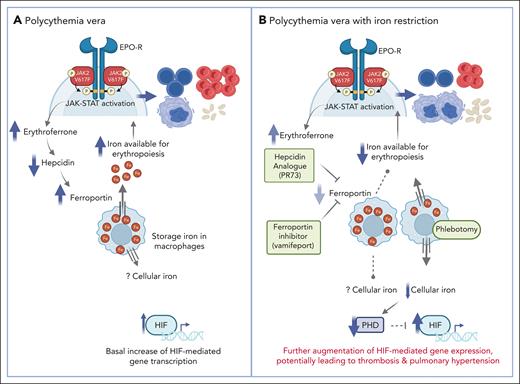
The mouse models with low and high JAK2 V617F allelic burdens phenocopy human essential thrombocythemia (ET) and PV, respectively. The higher JAK2 V617F allelic burden in PV is the result of uniparental disomy3 resulting in homozygosity, whereas JAK2 V617F clones in ET are largely heterozygous.4 Homozygous JAK2 V617F erythroid progenitors have augmented erythropoiesis. Abundant iron delivery is required to sustain erythropoiesis. The master regulator of iron homeostasis is hepcidin, which degrades the iron exporter ferroportin, resulting in inhibition of the efflux of iron from macrophage stores and reduction of iron absorption.5 Erythroferrone produced from erythroblasts suppresses hepcidin production from hepatocytes, augmenting iron delivery from macrophage iron stores.5 Increased erythroferrone causing downregulation of hepcidin is generally associated with increased erythropoietin (EPO) levels.5 However, in this report, “hepcidin and erythropoietin levels were decreased yet erythroferrone increased in the PV JAK2 V617F and JAK2 exon 12 mutated mice.” A similar association of low EPO and high erythroferrone has been reported in primary familial and congenital polycythemia caused by gain-of-function EPOR mutations,5 indicating the central role of augmented erythropoiesis rather than EPO in down-regulation of hepcidin levels (see figure).
(A) In polycythemia vera, erythropoietin-independent JAK-STAT activation results in increased erythroferrone production to support iron availability for enhanced erythropoiesis. There is also basally increased hypoxia inducible factor (HIF). (B) Iron restriction in polycythemia vera biases the pre-megakaryocytic/erythroid progenitor toward megakaryopoiesis, reducing hemoglobin/hematocrit and increasing platelet count. Reduced cellular iron induced by phlebotomy decreases PHDs, which are the primary negative regulators of HIFs, resulting in further augmentation of HIF activity. Created with BioRender.com.
(A) In polycythemia vera, erythropoietin-independent JAK-STAT activation results in increased erythroferrone production to support iron availability for enhanced erythropoiesis. There is also basally increased hypoxia inducible factor (HIF). (B) Iron restriction in polycythemia vera biases the pre-megakaryocytic/erythroid progenitor toward megakaryopoiesis, reducing hemoglobin/hematocrit and increasing platelet count. Reduced cellular iron induced by phlebotomy decreases PHDs, which are the primary negative regulators of HIFs, resulting in further augmentation of HIF activity. Created with BioRender.com.
This article also confirms previously reported ID augmentation of already increased activity of HIFs in PV,6 as the authors showed by measurements of transcription of HIF-regulated genes such as EPO, Vegfa, Igf2, and Glut1 (Slc2a1) in the kidney. This was expected as HIF levels are posttranscriptionally regulated by prolyl hydroxylase (PHD) enzymes, which leads to the proteasomal destruction of HIFs’ alpha subunits. PHDs require iron as a cofactor; hence ID stabilizes HIFs, leading to increased HIF activity.
The authors confirmed the central role of iron availability to PV erythrocytosis by using a hepcidin analogue, PR73, that inhibits ferroportin, thereby impairing the export of storage iron. This manipulation normalized the elevated hemoglobin and dramatically decreased plasma iron and transferrin saturation. In keeping with this finding, another hepcidin analogue, rusfertide, is being used in ongoing human PV trials and results in rapid correction of erythrocytosis. Next, the authors directly inhibited ferroportin, which resulted in similar reductions in hemoglobin and hematocrit and markedly decreased plasma iron and transferrin saturation, validating the central role of iron availability in PV erythrocytosis.
Although we agree with the conclusion of the authors that these iron metabolism modifiers may substitute for use of phlebotomies in PV, it is unlikely that these manipulations would have beneficial effects on the principal cause of PV morbidity and mortality, thrombosis. Iron-restricting manipulations do not address leukocytosis that is common in PV and is correlated with thrombotic complications in multivariate analysis.7 Furthermore, the role of elevated hemoglobin and hematocrit is contentious as a cause of morbidity and mortality in erythrocytoses.7 In most types of erythrocytoses, such as Eisenmenger complex, pulmonary disorders, or congenital disorders from inheritance of high hemoglobin oxygen affinity mutations, the hematocrit may be as high as or even higher than in PV but thromboses are not increased. However, in the first described disorder of congenital augmentation of HIFs, Chuvash erythrocytosis, thromboses are even more common than in PV and increase further with ID induced by phlebotomies.8 Patients with Chuvash erythrocytosis also have vascular abnormalities and pulmonary hypertension. Indeed, HIF-1 augments transcription of a wide array of genes, including several prothrombotic factors including transcription and activity of tissue factor.6,9 We and others have reported that HIF-1 activity is also increased in PV and ET.6,9
Hepcidin levels are also increased in inflammation and in cancer, wherein anemia is common yet ample iron stores exist. High hepcidin levels in these disorders restrict iron availability not only to erythropoiesis but likely to other tissues, resulting in functional iron deficiency. Although it remains to be shown whether the consequences of true ID (resulting from therapeutic phlebotomy) differ from functional iron deficiency, it is possible that pharmacological hepcidin augmentation or ferroportin inhibition as described in this article decreases iron availability and cellular iron in both erythroid and nonerythroid cells. If so, the increased HIF activity because of functional ID may have detrimental pathophysiological consequences including thrombosis, pulmonary hypertension, etc (see figure panel B). This merits additional evaluation of the potential risks of increased HIFs in therapeutic applications.
Conflict-of-interest disclosure: J.T.P. declares no competing financial interests. B.N.R. has received honoraria from BMS, Incyte, PharmaEssentia, MorphoSYS, and CTI BioPharma, and has an educational grant from CSL Behring.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal